A Comprehensive Survey of Phenolic Constituents Reported in Monofloral Honeys around the Globe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Tabulation and Representation
3. Results and Discussion
3.1. Botanical Classification
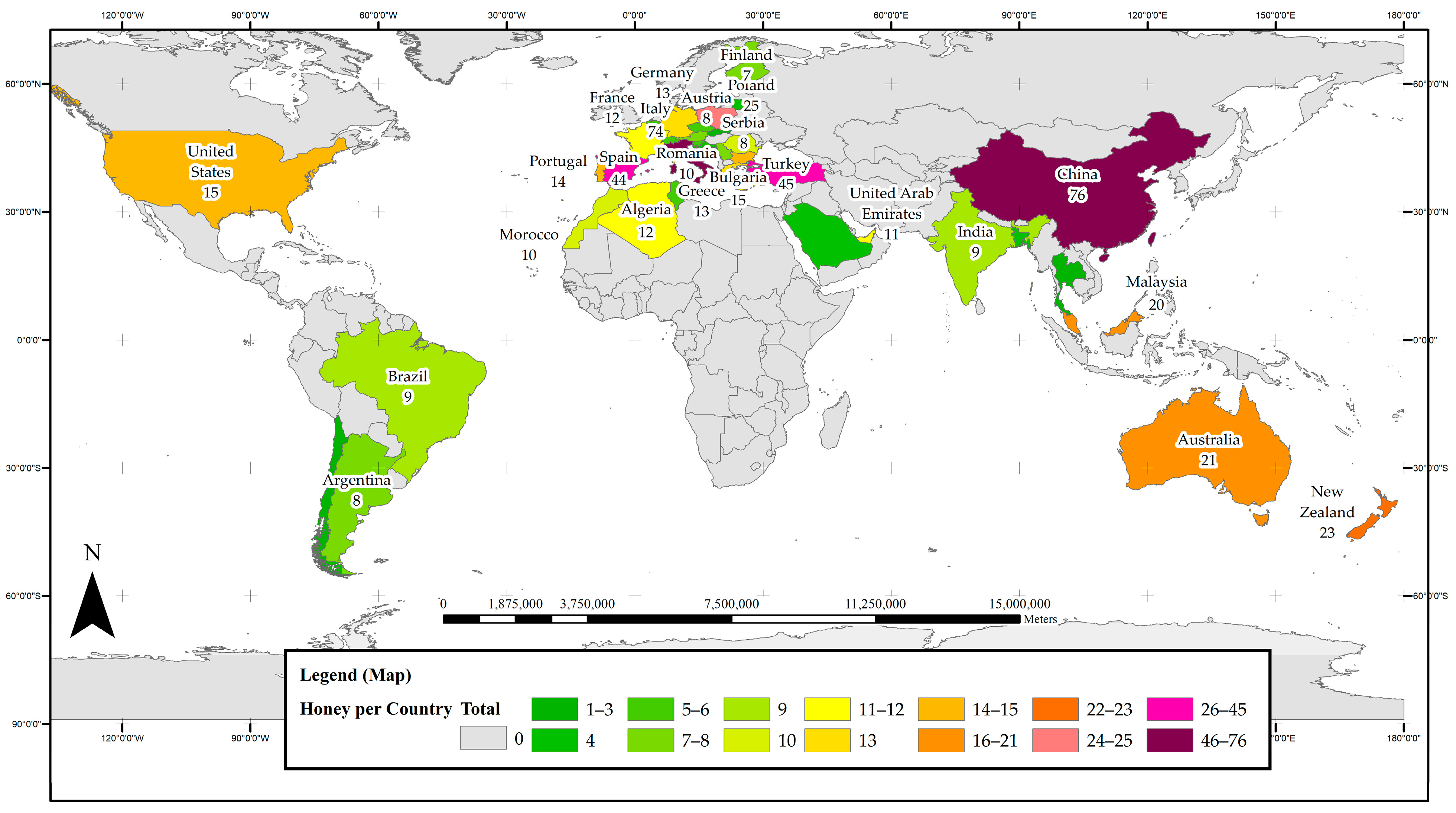
3.2. Global Hotspots of Honey Phenolics Research
3.3. Regional Hotspots of Honey Phenolics Research
3.3.1. Australia and New Zealand
3.3.2. Asia
3.3.3. The Americas
3.3.4. Africa and Europe
3.4. Phenolic Honey Constituents
3.4.1. Flavonoids
Flavones
Flavonols
Flavanones
Flavanonols
Flavan-3-ols
Isoflavonoids
Aurones and Chalcones
3.4.2. Hydroxycinnamic Acid and Its Derivatives
3.4.3. Hydroxybenzoic Acid and Its Derivatives
3.4.4. Miscellaneous and ‘Other’ Phenolics
3.4.5. Non-Phenolic Compounds
3.5. Analytical Methods Used in Compound Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pattamayutanon, P.; Angeli, S.; Thakeow, P.; Abraham, J.; Disayathanoowat, T.; Chantawannakul, P. Biomedical Activity and Related Volatile Compounds of Thai Honeys from 3 Different Honeybee Species. J. Food Sci. 2015, 80, M2228–M2240. [Google Scholar] [CrossRef]
- Shen, Y.-B.; Tian, H.-X.; Chen, C. Research on identification methods of varieties of honey. Shipin Gongye 2016, 37, 251–254. [Google Scholar]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and Its Role in Relieving Multiple Facets of Atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, S.; Gunnoo, J.; Marques Passos, T.; Stout, J.C.; White, B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; Silva, W.P.D.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT—Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M.B.P.P. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules 2017, 22, 1338. [Google Scholar] [CrossRef] [Green Version]
- Sant’ana, L.D.O.; Buarque Ferreira, A.B.; Lorenzon, M.C.A.; Berbara, R.L.L.; Castro, R.N. Correlation of Total Phenolic and Flavonoid Contents of Brazilian Honeys with Colour and Antioxidant Capacity. Int. J. Food Prop. 2014, 17, 65–76. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.; Tulipani, S.; Romandini, S.; Vidal, A.; Battino, M. Methodological Aspects about Determination of Phenolic Compounds and In Vitro Evaluation of Antioxidant Capacity in the Honey: A Review. Curr. Anal. Chem. 2009, 5, 293–302. [Google Scholar] [CrossRef]
- Ciulu, M.; Spano, N.; Pilo, M.I.; Sanna, G. Recent Advances in the Analysis of Phenolic Compounds in Unifloral Honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef]
- Gomez-Caravaca, A.M.; Gomez-Romero, M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Advances in the analysis of phenolic compounds in products derived from bees. J. Pharm. Biomed. Anal. 2006, 41, 1220–1234. [Google Scholar] [CrossRef]
- Pascual-Maté, A.; Osés, S.M.; Fernández-Muiño, M.A.; Sancho, M.T. Analysis of Polyphenols in Honey: Extraction, Separation and Quantification Procedures. Sep. Purif. Rev. 2017, 47, 142–158. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Gasic, U.M.; Milojkovic-Opsenica, D.M.; Tesic, Z.L. Polyphenols as Possible Markers of Botanical Origin of Honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Kaskoniene, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martinez Florez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, M.B.; Abdullah, W.Z.; Sulaiman, S.A.; Suen, A.B. A review of molecular mechanisms of the anti-leukemic effects of phenolic compounds in honey. Int. J. Mol. Sci. 2012, 13, 15054–15073. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, S.K.; Mandal, M. Antiproliferative effects of honey and of its polyphenols: A review. J. Biomed. Biotechnol. 2009, 2009, 830616. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Mandal, M.; Supriyanto, E. Honey and its Phytochemicals: Plausible Agents in Combating Colon Cancer through its Diversified Actions. J. Food Biochem. 2016, 40, 613–629. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Cianciosi, D.; Pistollato, F.; Ansary, J.; Pacetti, M.; Amici, A.; Reboredo-Rodríguez, P.; Simal-Gandara, J.; Quiles, J.L.; et al. Strawberry tree honey as a new potential functional food. Part 1: Strawberry tree honey reduces colon cancer cell proliferation and colony formation ability, inhibits cell cycle and promotes apoptosis by regulating EGFR and MAPKs signaling pathways. J. Funct. Foods 2019, 57, 439–452. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [Green Version]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Abbas, N. Chapter 4—Chemistry of Himalayan Phytochemicals. In Himalayan Phytochemicals; Jan, S., Abbas, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–166. [Google Scholar]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzand, K. Flavonoids: Chemistry, Biochemistry and Antioxidant activity. J. Pharm. Res. 2012, 5, 37. [Google Scholar]
- Marais, J.P.J.; Deavours, B.; Dixon, R.A.; Ferreira, D. The Stereochemistry of Flavonoids. In The Science of Flavonoids; Grotewold, E., Ed.; Springer: New York, NY, USA, 2006; pp. 1–46. [Google Scholar]
- Deadman, B.J. The Flavonoid Profile of New Zealand Manuka Honey. Master of Science (MSc) Thesis, The University of Waikato, Hamilton, New Zealand, 2009; pp. 1–270. Available online: https://hdl.handle.net/10289/5443 (accessed on 1 March 2022).
- Alvarez-Suarez, J.M.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Battino, M. Antioxidant Characterization of Native Monofloral Cuban Honeys. J. Agric. Food Chem. 2010, 58, 9817–9824. [Google Scholar] [CrossRef]
- Souza do Nascimento, K.; Sattler, J.A.G.; Macedo, L.F.L.; Gonzalez, C.V.S.; Pereira de Melo, I.L.; Araujo, E.D.S.; Granato, D.; Sattler, A.; de Almeida-Muradian, L.B. Phenolic compounds, antioxidant capacity and physicochemical properties of Brazilian Apis mellifera honeys. LWT—Food Sci. Technol. 2018, 91, 85–94. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Identification and Quantification of Antioxidant Components of Honeys from Various Floral Sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT—Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Imtara, H.; Kmail, A.; Touzani, S.; Khader, M.; Hamarshi, H.; Saad, B.; Lyoussi, B. Chemical Analysis and Cytotoxic and Cytostatic Effects of Twelve Honey Samples Collected from Different Regions in Morocco and Palestine. Evid. Based Complement. Altern. Med. 2019, 2019, 8768210. [Google Scholar] [CrossRef]
- Istasse, T.; Jacquet, N.; Berchem, T.; Haubruge, E.; Nguyen, B.K.; Richel, A. Extraction of Honey Polyphenols: Method Development and Evidence of Cis Isomerization. Anal. Chem. Insights 2016, 11, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Chen, X.; Liu, T.; Zhuo, Q.; Zhang, S.-Q. Evaluation of cellular antioxidant components of honeys using UPLC-MS/MS and HPLC-FLD based on the quantitative composition-activity relationship. Food Chem. 2019, 293, 169–177. [Google Scholar] [CrossRef]
- Marshall, S.M.; Schneider, K.R.; Cisneros, K.V.; Gu, L. Determination of antioxidant capacities, α-dicarbonyls, and phenolic phytochemicals in florida varietal honeys using HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2014, 62, 8623–8631. [Google Scholar] [CrossRef]
- Aljadi, A.; Mohd Yusoff, K. Isolation and Identification of Phenolic Acids in Malaysian Honey with Antibacterial Activities. Turk. J. Med. Sci. 2003, 33, 229–236. [Google Scholar]
- Petretto, G.L.; Cossu, M.; Alamanni, M.C. Phenolic content, antioxidant and physico-chemical properties of Sardinian monofloral honeys. Int. J. Food Sci. Technol. 2015, 50, 482–491. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Ahmed, Y.B.H.; Alothman, Z.A.; Obbed, M.S.; Al-Harbi, N.M.; Al-Turki, T.M. Ultra high performance liquid chromatography with mass spectrometry method for the simultaneous determination of phenolic constituents in honey from various floral sources using multiwalled carbon nanotubes as extraction sorbents. J. Sep. Sci. 2015, 38, 2597–2606. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Santos-Buelga, C.; Era, B.; González-Paramás, A.M.; Tuberoso, C.I.G.; Medda, R.; Pintus, F.; Fais, A. Sardinian honeys as sources of xanthine oxidase and tyrosinase inhibitors. Food Sci. Biotechnol. 2018, 27, 139–146. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Rastrelli, L. Determination of phenolic compounds in honey using dispersive liquid–liquid microextraction. J. Chromatogr. A 2014, 1334, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, F.B.; Lira, A.F.; Rumjanek, V.M.; Castro, R.N. Phenolic Composition and Antioxidant Properties of Brazilian Honeys. Quim. Nova 2014, 37, 821–826. [Google Scholar] [CrossRef]
- Andrade, P.; Ferreres, F.; Amaral, M.T. Analysis of Honey Phenolic Acids by HPLC, Its Application to Honey Botanical Characterization. J. Liq. Chromatogr. Rel. Technol. 1997, 20, 2281–2288. [Google Scholar] [CrossRef]
- Soler, C.; Gil, M.I.; García-Viguera, C.; Tomás-Barberán, F.A. Flavonoid patterns of French honeys with different floral origin. Apidologie 1995, 26, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Trautvetter, S.; Koelling-Speer, I.; Speer, K. Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS. Apidologie 2009, 40, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Barberán, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138, 148–156. [Google Scholar] [CrossRef]
- Truchado, P.; Ferreres, F.; Tomas-Barberan, F.A. Liquid chromatography-tandem mass spectrometry reveals the widespread occurrence of flavonoid glycosides in honey, and their potential as floral origin markers. J. Chromatogr. A 2009, 1216, 7241–7248. [Google Scholar] [CrossRef]
- Martos, I.; Cossentini, M.; Ferreres, F.; Tomas-Barberan, F.A. Flavonoid Composition of Tunisian Honeys and Propolis. J. Agric. Food Chem. 1997, 45, 2824–2829. [Google Scholar] [CrossRef]
- Kıvrak, Ş.; Kıvrak, İ. Assessment of phenolic profile of Turkish honeys. Int. J. Food Prop. 2016, 20, 864–876. [Google Scholar] [CrossRef] [Green Version]
- Kečkeš, S.; Gašić, U.; Veličković, T.Ć.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. The determination of phenolic profiles of Serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry. Food Chem. 2013, 138, 32–40. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Zhang, J.; Li, Y.; Chen, L.; Zhao, W.; Zhou, J.; Jin, Y. Characterization of Chinese Unifloral Honeys Based on Proline and Phenolic Content as Markers of Botanical Origin, Using Multivariate Analysis. Molecules 2017, 22, 735. [Google Scholar] [CrossRef] [Green Version]
- Mattonai, M.; Parri, E.; Querci, D.; Degano, I.; Ribechini, E. Development and validation of an HPLC-DAD and HPLC/ESI-MS 2 method for the determination of polyphenols in monofloral honeys from Tuscany (Italy). Microchem. J. 2016, 126, 220–229. [Google Scholar] [CrossRef]
- Joerg, E.; Sontag, G. Multichannel coulometric detection coupled with liquid chromatography for determination of phenolic esters in honey. J. Chromatogr. 1993, 635, 137–142. [Google Scholar] [CrossRef]
- Yaoa, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acids in Australian Melaleuca, Guioa, Lophostemon, Banksia and Helianthus honeys and their potential for floral authentication. Food Res. Int. 2005, 38, 651–658. [Google Scholar] [CrossRef]
- Dimitrova, B.; Gevrenova, R.; Anklam, E. Analysis of phenolic acids in honeys of different floral origin by solid-phase extraction and high-performance liquid chromatography. Phytochem. Anal. 2007, 18, 24–32. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Gruyaert, S.; Poliwoda, A.; Kafarski, P. Chemical Profiling of Polyfloral Belgian Honey: Ellagic Acid and Pinocembrin as Antioxidants and Chemical Markers. J. Chem. 2017, 2017, 5393158. [Google Scholar] [CrossRef] [Green Version]
- Di Marco, G.; Gismondi, A.; Panzanella, L.; Canuti, L.; Impei, S.; Leonardi, D.; Canini, A. Botanical influence on phenolic profile and antioxidant level of Italian honeys. J. Food Sci. Technol. 2018, 55, 4042–4050. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Zhuo, Q.; Chen, X.; Liu, T.; Zhang, S.Q. Quantitative and discriminative evaluation of contents of phenolic and flavonoid and antioxidant competence for chinese honeys from different botanical origins. Molecules 2018, 23, 1110. [Google Scholar] [CrossRef] [Green Version]
- Ciucure, C.T.; Geana, E.-I. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 2019, 30, 481–492. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Wang, J.; Li, X.; Wang, W.; Huang, Z. Sugaring-out assisted liquid-liquid extraction coupled with high performance liquid chromatography-electrochemical detection for the determination of 17 phenolic compounds in honey. J. Chromatogr. A 2019, 1601, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Orsák, M.; Hejtmánková, A.; Kovářová, E. Evaluation of antioxidant activity and total phenolics of selected Czech honeys. LWT—Food Sci. Technol. 2010, 43, 52–58. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Kovacik, J.; Gruz, J.; Biba, O.; Hedbavny, J. Content of metals and metabolites in honey originated from the vicinity of industrial town Kosice (eastern Slovakia). Environ. Sci. Pollut. Res. 2016, 23, 4531–4540. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, N.; Xue, X.; Wu, L.; Zhu, X.; Cao, W. Chromatographic ECD fingerprints combined with a chemometric method used for the identification of three light-coloured unifloral honeys. Anal. Methods 2015, 7, 8393–8401. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Yung, A.C.; Azlan, S.A.B.M.; Sulaiman, S.A.; Rao, P.V.; Hawlader, M.N.I.; Gan, S.H. Identification of phenolic acids and flavonoids in monofloral honey from Bangladesh by high performance liquid chromatography: Determination of antioxidant capacity. Biomed. Res. Int. 2014, 2014, 737490. [Google Scholar] [CrossRef]
- Sergiel, I.; Pohl, P.; Biesaga, M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014, 145, 404–408. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.; Du, X.; Cheng, N.; Chen, L.; Zhao, J.; Zheng, J.; Cao, W. Identification of Acacia Honey Adulteration with Rape Honey Using Liquid Chromatography–Electrochemical Detection and Chemometrics. Food Anal. Methods 2014, 7, 2003–2012. [Google Scholar] [CrossRef]
- Scripca, L.A.; Norocel, L.; Amariei, S. Comparison of Physicochemical, Microbiological Properties and Bioactive Compounds Content of Grassland Honey and other Floral Origin Honeys. Molecules 2019, 24, 2932. [Google Scholar] [CrossRef] [Green Version]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT—Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Truchado, P.; Tourn, E.; Gallez, L.M.; Moreno, D.A.; Ferreres, F.; Tomás-Barberán, F.A. Identification of botanical biomarkers in argentinean diplotaxis honeys: Flavonoids and glucosinolates. J. Agric. Food Chem. 2010, 58, 12678–12685. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Mohd Yusof, Y.A. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules 2011, 16, 6378–6395. [Google Scholar] [CrossRef]
- Ismail, N.I.; Abdul Kadir, M.R.; Mahmood, N.H.; Singh, O.P.; Iqbal, N.; Zulkifli, R.M. Apini and Meliponini foraging activities influence the phenolic content of different types of Malaysian honey. J. Apic. Res. 2016, 55, 137–150. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Amrah Sulaiman, S.; Gan, S.H. Phenolic Acid and Flavonoid Composition of Malaysian Honeys. J. Food Biochem. 2016, 41, e12282. [Google Scholar] [CrossRef]
- Tenore, G.C.; Ritieni, A.; Campiglia, P.; Novellino, E. Nutraceutical potential of monofloral honeys produced by the Sicilian black honeybees (Apis mellifera ssp. sicula). Food Chem. Toxicol. 2012, 50, 1955–1961. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Gonzalez-Paramas, A.M.; Damiani, E.; Astolfi, P.; Martinez-Sanchez, G.; Bompadre, S.; Quiles, J.L.; Santos-Buelga, C.; Battino, M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem. Toxicol. 2012, 50, 1508–1516. [Google Scholar] [CrossRef]
- Velásquez, P.; Montenegro, G.; Leyton, F.; Ascar, L.; Ramirez, O.; Giordano, A. Bioactive compounds and antibacterial properties of monofloral Ulmo honey. CyTA—J. Food 2020, 18, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Daniels, B.J.; Middleditch, M.J.; Furkert, D.P.; Brimble, M.A.; Bong, J.; Stephens, J.M.; Loomes, K.M. Utility of the Leptospermum scoparium Compound Lepteridine as a Chemical Marker for Manuka Honey Authenticity. ACS Omega 2020, 5, 8858–8866. [Google Scholar] [CrossRef] [Green Version]
- Weston, R.J.; Brocklebank, L.K.; Lu, Y. Identification and quantitative levels of antibacterial components of some New Zealand honeys. Food Chem. 2000, 70, 427–435. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Poliwoda, A.; Dereń, M.; Kafarski, P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012, 131, 1149–1156. [Google Scholar] [CrossRef]
- Stanek, N.; Jasicka-Misiak, I. HPTLC Phenolic Profiles as Useful Tools for the Authentication of Honey. Food Anal. Methods 2018, 11, 2979–2989. [Google Scholar] [CrossRef] [Green Version]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Natural Occurrence of Abscisic Acid in Heather Honey and Floral Nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Canini, A.; Pichichero, E.; Alesian, D.; Canuti, L.; Leonardi, D. Nutritional and botanical interest of honey collected from protected natural areas. Plant Biosyst. Int. J. Deal. All. Asp. Plant Biol. 2009, 143, 62–70. [Google Scholar] [CrossRef]
- Aygul, I.; Yaylaci Karahalil, F.; Supuran, C.T. Investigation of the inhibitory properties of some phenolic standards and bee products against human carbonic anhydrase I and II. J. Enzyme Inhib. Med. Chem. 2016, 31, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Can, Z. Determination of in-vitro antioxidant, anti-urease, anti-hyaluronidase activities by phenolic rich bee products from different region of Turkey. Fresenius Environ. Bull. 2018, 27, 6858–6866. [Google Scholar]
- Huttunen, S.; Riihinen, K.; Kauhanen, J.; Tikkanen-Kaukanen, C. Antimicrobial activity of different Finnish monofloral honeys against human pathogenic bacteria. APMIS 2013, 121, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Oelschlaegel, S.; Koelling-Speer, I.; Speer, K. Determination of the floral origin of honey by secondary plant metabolites. Dtsch. Lebensm.-Rundsch. 2012, 108, 415–418. [Google Scholar]
- Silici, S.; Sarioglu, K.; Dogan, M.; Karaman, K. HPLC-DAD Analysis to Identify the Phenolic Profile of Rhododendron Honeys Collected from Different Regions in Turkey. Int. J. Food Prop. 2014, 17, 1126–1135. [Google Scholar] [CrossRef]
- Salonen, A.; Julkunen-Tiitto, R. Characterisation of two unique unifloral honeys from the boreal coniferous zone: Lingonberry and mire honeys. Agric. Food Sci. 2012, 21, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Ramanauskiene, K.; Stelmakiene, A.; Briedis, V.; Ivanauskas, L.; Jakštas, V. The quantitative analysis of biologically active compounds in Lithuanian honey. Food Chem. 2012, 132, 1544–1548. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Tan, T.T.E. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 9, 313798. [Google Scholar] [CrossRef]
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Bioactive components, antioxidant and DNA damage inhibitory activities of honeys from arid regions. Food Chem. 2014, 153, 28–34. [Google Scholar] [CrossRef]
- Guo, P.; Deng, Q.; Lu, Q. Anti-alcoholic effects of honeys from different floral origins and their correlation with honey chemical compositions. Food Chem. 2019, 286, 608–615. [Google Scholar] [CrossRef]
- Pichichero, E.; Canuti, L.; Canini, A. Characterisation of the phenolic and flavonoid fractions and antioxidant power of Italian honeys of different botanical origin. J. Sci. Food Agric. 2009, 89, 609–616. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A comparative study on phenolic profile, vitamin C content and antioxidant activity of Italian honeys of different botanical origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Gambacorta, E.; Simonetti, A.; Garrisi, N.; Intaglietta, I.; Perna, A. Antioxidant properties and phenolic content of sulla (Hedysarum spp.) honeys from Southern Italy. Int. J. Food Sci. Technol. 2014, 49, 2260–2268. [Google Scholar] [CrossRef]
- Iurlina, M.; Saiz, A.; Fritz, R.; Manrique, G. Major flavonoids of Argentinean honeys. Optimisation of the extraction method and analysis of their content in relationship to the geographical source of honeys. Food Chem. Food Chem. 2009, 115, 1141–1149. [Google Scholar] [CrossRef]
- Ciappini, M.C. Polyhenolic profile of floral honeys in correlation with their pollen spectrum. J. Apic. Res. 2019, 58, 772–779. [Google Scholar] [CrossRef]
- Isla, M.I.; Craig, A.; Ordonez, R.; Zampini, C.; Sayago, J.; Bedascarrasbure, E.; Alvarez, A.; Salomon, V.; Maldonado, L. Physico chemical and bioactive properties of honeys from Northwestern Argentina. LWT—Food Sci. Technol. 2011, 44, 1922–1930. [Google Scholar] [CrossRef]
- Sun, C.; Tan, H.; Zhang, Y.; Zhang, H. Phenolics and abscisic acid identified in acacia honey comparing different SPE cartridges coupled with HPLC-PDA. J. Food Compost. Anal. 2016, 53, 91–101. [Google Scholar] [CrossRef]
- Daher, S.; Gülaçar, F.O. Analysis of Phenolic and Other Aromatic Compounds in Honeys by Solid-Phase Microextraction Followed by Gas Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 5775–5780. [Google Scholar] [CrossRef] [PubMed]
- Marghitas, L.A.; Dezmirean, D.S.; Pocol, C.B.; Ilea, M.; Bobis, O.; Gergen, I. The development of a biochemical profile of acacia honey by identifying biochemical determinants of its quality. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 84–90. [Google Scholar]
- Kenjerić, D.; Mandić, M.L.; Primorac, L.; Bubalo, D.; Perl, A. Flavonoid profile of Robinia honeys produced in Croatia. Food Chem. 2007, 102, 683–690. [Google Scholar] [CrossRef]
- Rostislav, H.; Petr, T.; Sanja Ćavar, Z. Characterisation of phenolics and other quality parameters of different types of honey. Czech J. Food Sci. 2016, 34, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Gismondi, A.; De Rossi, S.; Canuti, L.; Novelli, S.; Di Marco, G.; Fattorini, L.; Canini, A. From Robinia pseudoacacia L. nectar to Acacia monofloral honey: Biochemical changes and variation of biological properties. J. Sci. Food Agric. 2018, 98, 4312–4322. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Polak, T.; Kropf, U.; Korosec, M.; Golob, T. LC-DAD-ESI/MS analysis of flavonoids and abscisic acid with chemometric approach for the classification of Slovenian honey. Food Chem. 2011, 127, 296–302. [Google Scholar] [CrossRef]
- Stanek, N.; Kafarski, P.; Jasicka-Misiak, I. Development of a high performance thin layer chromatography method for the rapid qualification and quantification of phenolic compounds and abscisic acid in honeys. J. Chromatogr. A 2019, 1598, 209–215. [Google Scholar] [CrossRef]
- Stephens, J.M.; Schlothauer, R.C.; Morris, B.D.; Yang, D.; Fearnley, L.; Greenwood, D.R.; Loomes, K.M. Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chem. 2010, 120, 78–86. [Google Scholar] [CrossRef]
- Lin, B.; Loomes, K.M.; Prijic, G.; Schlothauer, R.; Stephens, J.M. Lepteridine as a unique fluorescent marker for the authentication of manuka honey. Food Chem. 2017, 225, 175–180. [Google Scholar] [CrossRef]
- Kolayli, S.; Can, Z.; Yildiz, O.; Sahin, H.; Karaoglu, S.A. A comparative study of the antihyaluronidase, antiurease, antioxidant, antimicrobial and physicochemical properties of different unifloral degrees of chestnut (Castanea sativa Mill.) honeys. J. Enzyme Inhib. Med. Chem. 2016, 31, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Cavazza, A.; Corradini, C.; Musci, M.; Salvadeo, P. High-performance liquid chromatographic phenolic compound fingerprint for authenticity assessment of honey. J. Sci. Food Agric. 2013, 93, 1169–1175. [Google Scholar] [CrossRef]
- Ronsisvalle, S.; Lissandrello, E.; Fuochi, V.; Petronio Petronio, G.; Straquadanio, C.; Crasci, L.; Panico, A.; Milito, M.; Cova, A.M.; Tempera, G.; et al. Antioxidant and antimicrobial properties of Casteanea sativa Miller chestnut honey produced on Mount Etna (Sicily). Nat. Prod. Res. 2019, 33, 843–850. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019, 67, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Güneş, M.E.; Şahin, S.; Demir, C.; Borum, E.; Tosunoğlu, A. Determination of phenolic compounds profile in chestnut and floral honeys and their antioxidant and antimicrobial activities. J. Food Biochem. 2017, 41, e12345. [Google Scholar] [CrossRef]
- Çol Ayvaz, M.; Ömür, B.; Ertürk, Ö.; Kabakçi, D. Phenolic profiles, antioxidant, antimicrobial, and DNA damage inhibitory activities of chestnut honeys from Black Sea Region of Turkey. J. Food Biochem. 2018, 42, e12502. [Google Scholar] [CrossRef]
- Sarikaya, A.L.I.; Ulusoy, E.; Öztürk, N.; Tuncel, M.; Kolayli, S. Antioxidant activity and phenolic acid constituents of chestnut (Castania Sativa Mill.) Honey and Propolis. J. Food Biochem. 2009, 33, 470–481. [Google Scholar] [CrossRef]
- Kolayli, S.; Can, Z.; Cakir, H.E.; Okan, O.T.; Yildiz, O. An investigation on Trakya region Oak (Quercus spp.) honeys of Turkey: Their physico-chemical, antioxidant and phenolic compounds properties. Turk. J. Biochem. 2018, 43, 362–374. [Google Scholar] [CrossRef]
- Stanek, N.; Teper, D.; Kafarski, P.; Jasicka-Misiak, I. Authentication of phacelia honeys (Phacelia tanacetifolia) based on a combination of HPLC and HPTLC analyses as well as spectrophotometric measurements. LWT—Food Sci. Technol. 2019, 107, 199–207. [Google Scholar] [CrossRef]
- Nayik, G.A.; Nanda, V. A chemometric approach to evaluate the phenolic compounds, antioxidant activity and mineral content of different unifloral honey types from Kashmir, India. LWT 2016, 74, 504–513. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison With Important Commercial Honeys. Front. Microbiol. 2019, 10, 263. [Google Scholar] [CrossRef]
- Gyergyák, K.; Boros, B.; Marton, K.; Felinger, A.; Papp, N.; Farkas, Á. Bioactive constituents and antioxidant activity of some carpathian basin honeys. Nat. Prod. Commun. 2016, 11, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Román, D.; Gómez-Caravaca, A.M.; Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of phenolic compounds in rosemary honey using solid-phase extraction by capillary electrophoresis-electrospray ionization-mass spectrometry. J. Pharm. Biomed. Anal. 2006, 41, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Ferreres, F.; Ortiz, A.; Subra, E.; Tomas-Barberan, F.A. Plant Phenolic Metabolites and Floral Origin of Rosemary Honey. J. Agric. Food Chem. 1995, 43, 2833–2838. [Google Scholar] [CrossRef]
- Gašić, U.M.; Natić, M.M.; Mišić, D.M.; Lušić, D.V.; Milojković-Opsenica, D.M.; Tešić, Ž.L.; Lušić, D. Chemical markers for the authentication of unifloral Salvia officinalis L. honey. J. Food Compost. Anal. 2015, 44, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Kenjerić, D.; Mandić, M.L.; Primorac, L.; Čačić, F. Flavonoid pattern of sage (Salvia officinalis L.) unifloral honey. Food Chem. 2008, 110, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Zekić, M.; Radonić, A.; Tuberoso, C.I.G. Screening of Satureja subspicata Vis. honey by HPLC-DAD, GC-FID/MS and UV/VIS: Prephenate derivatives as biomarkers. Molecules 2016, 21, 377. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, I.K.; Vavoura, M.V.; Badeka, A.; Kontakos, S.; Kontominas, M.G. Differentiation of Greek Thyme Honeys According to Geographical Origin Based on the Combination of Phenolic Compounds and Conventional Quality Parameters Using Chemometrics. Food Anal. Methods 2014, 7, 2113–2121. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Dimitriou, E.; Kontakos, S.; Kontominas, M.G. Phenolic profile, colour intensity, and radical scavenging activity of Greek unifloral honeys. Eur. Food Res. Technol. 2016, 242, 1201–1210. [Google Scholar] [CrossRef]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef] [Green Version]
- Tsiapara, A.V.; Jaakkola, M.; Chinou, I.; Graikou, K.; Tolonen, T.; Virtanen, V.; Moutsatsou, P. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: Profile analysis of extracts. Food Chem. 2009, 116, 702–708. [Google Scholar] [CrossRef]
- Zhao, J.; Du, X.; Cheng, N.; Chen, L.; Xue, X.; Zhao, J.; Wu, L.; Cao, W. Identification of monofloral honeys using HPLC-ECD and chemometrics. Food Chem. 2016, 194, 167–174. [Google Scholar] [CrossRef]
- Gašić, U.; Šikoparija, B.; Tosti, T.; Trifković, J.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. Phytochemical fingerprints of lime honey collected in Serbia. J. AOAC Int. 2014, 97, 1259–1267. [Google Scholar] [CrossRef]
- Devi, A.; Jangir, J.; Anu-Appaiah, K.A. Chemical characterization complemented with chemometrics for the botanical origin identification of unifloral and multifloral honeys from India. Food Res. Int. 2018, 107, 216–226. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Yao, L.; D’Arcy, B.; Caffin, N.; Tomas-Barberan, F.A. Flavonoids in Monospecific Eucalyptus Honeys from Australia. J. Agric. Food Chem. 2000, 48, 4744–4748. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Y.; Singanusong, R.; Datta, N.; Raymont, K. Phenolic acids and abscisic acid in Australian Eucalyptus honeys and their potential for floral authentication. Food Chem. 2004, 86, 169–177. [Google Scholar] [CrossRef]
- Bong, J.; Loomes, K.M.; Lin, B.; Stephens, J.M. New approach: Chemical and fluorescence profiling of NZ honeys. Food Chem. 2018, 267, 355–367. [Google Scholar] [CrossRef]
- Beitlich, N.; Koelling-Speer, I.; Oelschlaegel, S.; Speer, K. Differentiation of manuka honey from kanuka honey and from jelly bush honey using HS-SPME-GC/MS and UHPLC-PDA-MS/MS. J. Agric. Food Chem. 2014, 62, 6435–6444. [Google Scholar] [CrossRef]
- Yao, L.; Datta, N.; Tomas-Barberan, F.A.; Ferreres, F.; Martos, I.; Singanusong, R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003, 81, 159–168. [Google Scholar] [CrossRef]
- Hempattarasuwan, P.; Settachaimongkon, S.; Duangmal, K. Impact of botanical source and processing conditions on physicochemical properties and antioxidant activity of honey in the northern part of Thailand. Int. J. Food Sci. Technol. 2019, 54, 3185–3195. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 1: Enhancement of cellular viability, regulation of cellular apoptosis and improvement of mitochondrial functionality. Food Chem. Toxicol. 2018, 121, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic Acid Composition and Antioxidant Properties of Malaysian Honeys. J. Food Sci. 2011, 76, C921–C928. [Google Scholar] [CrossRef] [PubMed]
- Rückriemen, J.; Henle, T. Pilot study on the discrimination of commercial Leptospermum honeys from New Zealand and Australia by HPLC–MS/MS analysis. Eur. Food Res. Technol. 2018, 244, 1203–1209. [Google Scholar] [CrossRef]
- Stephens, J.M.; Loomes, K.M.; Braggins, T.J.; Bong, J.; Lin, B.; Prijic, G. Fluorescence: A Novel Method for Determining Manuka Honey Floral Purity. In Honey Analysis; IntechOpen: London, UK, 2017. [Google Scholar]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res. 2010, 30, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Pasini, F.; Gardini, S.; Marcazzan, G.L.; Caboni, M.F. Buckwheat honeys: Screening of composition and properties. Food Chem. 2013, 141, 2802–2811. [Google Scholar] [CrossRef]
- Cheng, N.; Wu, L.; Zheng, J.; Cao, W. Buckwheat Honey Attenuates Carbon Tetrachloride-Induced Liver and DNA Damage in Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 987385. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Li, P.; Cheng, N.; Gao, H.; Wang, B.; Wei, Y.; Cao, W. Protective effects of buckwheat honey on DNA damage induced by hydroxyl radicals. Food Chem. Toxicol. 2012, 50, 2766–2773. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, W.; Chen, W.; Xiao, X.-H.; Zheng, J.-B. Simultaneous determination of four phenolic components in citrus honey by high performance liquid chromatography using electrochemical detection. Food Chem. 2009, 114, 1537–1541. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Using flavonoids, phenolic compounds and headspace volatile profile for botanical authentication of lemon and orange honeys. Food Res. Int. 2011, 44, 1504–1513. [Google Scholar] [CrossRef]
- Lianda, R.; Sant’Ana, L.; Echevarria, A.; Nora Castro, R. Antioxidant Activity and Phenolic Composition of Brazilian Honeys and their Extracts. J. Braz. Chem. Soc. 2012, 23, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Giordano, A.; Retamal, M.; Leyton, F.; Martinez, P.; Bridi, R.; Velasquez, P.; Montenegro, G. Bioactive polyphenols and antioxidant capacity of Azara petiolaris and Azara integrifolia Honeys. CyTA—J. Food 2018, 16, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Jerković, I.; Kuś, P.M.; Tuberoso, C.I.G.; Šarolić, M. Phytochemical and physical-chemical analysis of Polish willow (Salix spp.) honey: Identification of the marker compounds. Food Chem. 2014, 145, 8–14. [Google Scholar] [CrossRef]
- Mesbahi, M.A.; Ouahrani, M.R.; Rebiai, A.; Amara, D.G.; Chouikh, A. Characterization of Zygophyllum album L Monofloral Honey from El-Oued, Algeria. Curr. Nutr. Food Sci. 2019, 15, 476–483. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Koes, R.E.; Quattrocchio, F.; Mol, J.N.M. The flavonoid biosynthetic pathway in plants: Function and evolution. Bioessays 1994, 16, 123–132. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.H.L.N.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, Biosynthesis and Chemical Ecology. In Flavonoids—From Biosynthesis to Human Health; IntechOpen: London, UK, 2017. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Barberán, F.A.; Truchado, P.; Ferreres, F. Flavonoids in Stingless-Bee and Honey-Bee Honeys. In Pot-Honey: A Legacy of Stingless Bees; Vit, P., Pedro, S.R.M., Roubik, D., Eds.; Springer New York: New York, NY, USA, 2013; pp. 461–474. [Google Scholar]
- Tapas, D.A.; Sakarkar, D.M.; Kakde, R. Flavonoids as Nutraceuticals: A Review. Trop. J. Pharm. Res. 2008, 7, 1089–1099. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Jankun, J.; Selman, S.H.; Swiercz, R.; Skrzypczak-Jankun, E. Why drinking green tea could prevent cancer. Nature 1997, 387, 561. [Google Scholar] [CrossRef]
- Garbisa, S.; Biggin, S.; Cavallarin, N.; Sartor, L.; Benelli, R.; Albini, A. Tumor invasion: Molecular shears blunted by green tea. Nat. Med. 1999, 5, 1216. [Google Scholar] [CrossRef] [PubMed]
- Afroz, R.; Tanvir, E.M.; Paul, S.; Bhoumik, N.C.; Gan, S.H.; Khalil, M.D.I. DNA Damage Inhibition Properties of Sundarban Honey and its Phenolic Composition. J. Food Biochem. 2016, 40, 436–445. [Google Scholar] [CrossRef]
- Smith, M.A.; Perry, G.; Richey, P.L.; Sayre, L.M.; Anderson, V.E.; Beal, M.F.; Kowall, N. Oxidative damage in Alzheimer’s. Nature 1996, 382, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary Intakes of Flavonols, Flavones and Isoflavones by Japanese Women and the Inverse Correlation between Quercetin Intake and Plasma LDL Cholesterol Concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Kootstra, A. Protection from UV-B-induced DNA damage by flavonoids. Plant Mol. Biol. 1994, 26, 771–774. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Kurhekar, J.V. Chapter 17—Antimicrobial lead compounds from marine plants. In Phytochemicals as Lead Compounds for New Drug Discovery; Egbuna, C., Kumar, S., Ifemeje, J.C., Ezzat, S.M., Kaliyaperumal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 257–274. [Google Scholar]
- Zuiter, A.S. Proanthocyanidin: Chemistry and Biology: From Phenolic Compounds to Proanthocyanidins. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol Profile Is a Reliable Indicator to Assess Canopy Architecture and the Exposure of Red Wine Grapes to Solar Radiation. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Das, A.B.; Goud, V.V.; Das, C. 9—Phenolic Compounds as Functional Ingredients in Beverages. In Value-Added Ingredients and Enrichments of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 285–323. [Google Scholar]
- Duodu, K.G.; Awika, J.M. Chapter 8—Phytochemical-Related Health-Promoting Attributes of Sorghum and Millets. In Sorghum and Millets, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; AACC International Press: Sawston, UK, 2019; pp. 225–258. [Google Scholar]
- Awika, J.M. Chapter 3—Sorghum: Its Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains; Taylor, J.R.N., Awika, J.M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 21–54. [Google Scholar]
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Chapter 2—Sweet Cherry Phenolic Compounds: Identification, Characterization, and Health Benefits. In Studies in Natural Products Chemistry; Atta, U.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 31–78. [Google Scholar]
- Mena, P.; Domínguez-Perles, R.; Gironés-Vilaplana, A.; Baenas, N.; García-Viguera, C.; Villaño, D. Flavan-3-ols, anthocyanins, and inflammation. IUBMB Life 2014, 66, 745–758. [Google Scholar] [CrossRef]
- Murkovic, M. Phenolic Compounds: Occurrence, Classes, and Analysis. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 346–351. [Google Scholar]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. 4.08—Extraction Techniques for the Determination of Phenolic Compounds in Food. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 159–180. [Google Scholar]
- Shrinet, K.; Singh, R.K.; Chaurasia, A.K.; Tripathi, A.; Kumar, A. Chapter 17—Bioactive compounds and their future therapeutic applications. In Natural Bioactive Compounds; Sinha, R.P., Häder, D.-P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 337–362. [Google Scholar]
- Popova, A.V.; Bondarenko, S.P.; Frasinyuk, M.S. Aurones: Synthesis and Properties. Chem. Heterocycl. Compd. 2019, 55, 285–299. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Raut, N.A.; Dhore, P.W.; Saoji, S.D.; Kokare, D.M. Chapter 9—Selected Bioactive Natural Products for Diabetes Mellitus. In Studies in Natural Products Chemistry; Atta, U.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 287–322. [Google Scholar]
- Liwa, A.C.; Barton, E.N.; Cole, W.C.; Nwokocha, C.R. Chapter 15—Bioactive Plant Molecules, Sources and Mechanism of Action in the Treatment of Cardiovascular Disease. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 315–336. [Google Scholar]
- Szeleszczuk, Ł.; Pisklak, D.M.; Zielińska-Pisklak, M.; Wawer, I. Effects of structural differences on the NMR chemical shifts in cinnamic acid derivatives: Comparison of GIAO and GIPAW calculations. Chem. Phys. Lett. 2016, 653, 35–41. [Google Scholar] [CrossRef]
- Martinez, K.B.; Mackert, J.D.; McIntosh, M.K. Chapter 18—Polyphenols and Intestinal Health. In Nutrition and Functional Foods for Healthy Aging; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 191–210. [Google Scholar]
- Dileep, K.V.; Remya, C.; Cerezo, J.; Fassihi, A.; Pérez-Sánchez, H.; Sadasivan, C. Comparative studies on the inhibitory activities of selected benzoic acid derivatives against secretory phospholipase A2, a key enzyme involved in the inflammatory pathway. Mol. Biosyst. 2015, 11, 1973–1979. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Clifford, M.N. Dietary hydroxybenzoic acid derivative—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1024–1032. [Google Scholar] [CrossRef]

















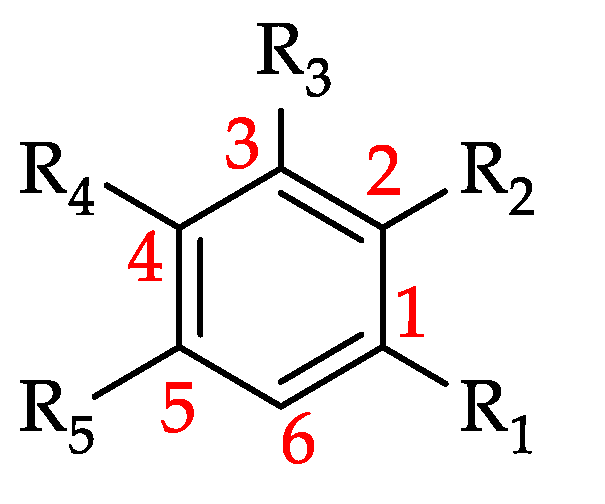


| No | Plant Family | Scientific Name/s | Common Name/s | Country (Research Location) | No. of Study/s | Flavonoids | HCAD | HBAD | Misc./Other Phenolics | NP | Total | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Acanthaceae | Avicennia germinans Jacq. | Black Mangrove | Italy | 1 | Isor, Kaem, Kaem-8-ME, Quer, Quer rham, | CA, p-CouA | SyrA, VA | N.I. | N.I. | 9 | [30] |
| 2. | Anacardiaceae | Schinus terebinthifolius | Mastic, Hawaiian Christmas berry | Brazil, USA | 2 | Quer | t-CA | GA | N.I. | N.I. | 3 | [31,32] |
| 3. | Annarrhinum sp. | Annarrhinum sp. | Annarrhinum | Algeria | 1 | Api, Chr, Lut, Gal, Isor, Kaem, Kaemf, Quer, Pinoc, Pinob, Dai, Gene | CA, p-CouA | BenA, ProA, p-HBA, SyrA, VA | N.I. | N.I. | 19 | [33] |
| 4. | Apiaceae | Ammi visnaga L. | Bochnikha | Morocco | 1 | N.I. | CA, FA, p-CouA, RosA | GA, SyrA | N.I. | N.I. | 6 | [34] |
| 5. | Apiaceae | Apiaceae sp. | Apiaceae | Algeria | 1 | Api, Chr, Lut, Gal, Isor, Kaem, Quer, Quer rham, Hest, Isosak, Pinoc, Pinob, Gene | CA, FA, p-CouA, t-CA | BenA, ProA, p-HBA, VA, SyrA | 3,4-DHPAA, p-HPAA | N.I. | 24 | [33] |
| 6. | Apiaceae | Daucus sp. | Wild Carrot | Belgium | 1 | Chr, Pinoc | CA, p-CouA | N.I. | N.I. | N.I. | 4 | [35] |
| 7. | Apiaceae | Eryngium campestre L. | Common Eryngo | Turkey | 1 | N.I. | CA, FA, p-CouA, | p-HBA | N.I. | N.I. | 4 | [36] |
| 8. | Apiaceae | Foeniculum vulgare | Fennel | China | 1 | Chr, Lut, Vit, Fis, Gal, Isor, Kaem, Quer, Hest, Hesd, Nar, Pinoc, Sak, Pinob, Tax, Form, | CA, ChloA, CChloA, FA, IfA, p-CouA, SinA, | GA, ProA, p-HBA, SalA, SyrA | N.I. | N.I. | 28 | [37] |
| 9. | Aquifoliaceae | Ilex sp. | Gallberry | USA | 1 | Chr, Gal Kaem, Quer, Rut, Hest, Pinoc | p-CouA | N.I. | N.I. | N.I. | 8 | [38] |
| 10. | Arecaceae | Cocos nucifera | Coconut | Malaysia | 1 | N.I. | CA | BenA, GA | N.I. | N.I. | 3 | [39] |
| 11. | Arecaceae | Cynara cardunculus | Cardoon | Italy | 1 | Api, Gal Quer Pinob, Pinoc | N.I. | SyrA | N.I. | N.I. | 6 | [40] |
| 12. | Arecaceae | Serenoa repens | Palmetto | USA | 1 | Chr, Lut, Gal, Kaem, Quer, Rut, Hest, Pinoc | p-CouA | N.I. | N.I. | N.I. | 9 | [38] |
| 13. | Asphodelaceae | Aloe vera barbadensis | Aloe | Saudi Arabia | 1 | Chr, Lut, Gal, Kaem Myr, Quer, Nar | CA, ChloA, p-CouA | GA, p-HBA, SyrA | p-HPAA | N.I. | 14 | [41] |
| 14. | Asphodelaceae | Asphodelus sp., A. microcarpus Salzm. and Viv. | Asphodel, Asphodelus | Italy | 2 | Api, Gal, Quer, Pinoc, Pinob | FA | MS, SyrA, | N.I. | PhAn, Tyr | 10 | [40,42] |
| 15. | Asteraceae | Cardus sp. | Thistle | Italy | 1 | N.I. | N.I. | N.I. | DL-β-PLA | Lum, PhAn, Tyr | 4 | [42] |
| 16. | Asteraceae | Centaurea dumulosa | Morar | Morocco | 1 | N.I. | CA, FA, p-CouA, RosA | GA, SyrA | N.I. | N.I. | 6 | [34] |
| 17. | Asteraceae | Chrysanthemum sp. | Chrysanthemum | China | 1 | N.I. | CA | GA, p-HBA, SyrA | Prod | N.I. | 5 | [43] |
| 18. | Asteraceae | Cirsium discolor | Cardo | Italy | 1 | Api, Chr, Lut, Gal, Kaem, Myr, Quer, Pinoc, Pinob | CA, FA, p-CouA, | SyrA, VA | N.I. | N.I. | 14 | [44] |
| 19. | Asteraceae | Conyza bonariensis | Rabat | Morocco | 1 | N.I. | CA, FA | SyrA | N.I. | N.I. | 3 | [34] |
| 20. | Asteraceae | Echinops spinosissimus | Morar Akhdar | Morocco | 1 | N.I. | CA, RosA | N.I. | N.I. | N.I. | 2 | [34] |
| 21. | Asteraceae | Gochnatia sp. | Cambara | Brazil | 1 | Chr, Gal, Nar | t-CA, m-MCA, m-CouA | BenA, GA, SyrA | N.I. | AbsA | 10 | [45] |
| 22. | Asteraceae | Helianthus annuus L. | Sunflower | Australia, Austria, Belgium, Bulgaria, China, France, Germany, Italy, Portugal, Romania, Serbia, Spain, Tunisia, Turkey | 15 | Aca, Api, Chr, Lut, Tec, Gal, Gal-3-ME, Isor, Kaem, Kaem-8-ME, Kaem-3-O-(6”-acetyl)-β-Gluc, Mor, Myr, Myr-3,7,4′5′-TeME, Quer, Quer-3,3-DME, Quer-3,7-DME, Quer-3-ME, Rut, Hest, Nar, Pinoc, Pinos, Pinob, Pinob-3-O-ace, CG | CA, CADAE, CAPE, ChloA, FA, m-CouA, MF, o-CouA, p-CouA, SinA, t-CA, t-p-CouAME, | BenA, ElA, GA, GenA, M-4-HBz, MS, ProA, p-HBA, ResA, SalA, SyrA, VA, VAME, | 3,4-DHPAA, HGA, MandA, PAA, p-HPAA, DL-β-PLA, 3-PPA, PhlA, 4-MPC, Prod, Van, | AbsA | 63 | [35,46,47,48,49,50,51,52,53,54,55,56,57,58,59] |
| 23. | Asteraceae | Pluchea Sagittalis | Quitoco | Brazil | 1 | Quer | N.I. | GA | N.I. | N.I. | 2 | [31] |
| 24. | Asteraceae | Solidago virgaurea L. | Goldenrod | China, Poland, Serbia | 3 | Api, Chr, Lut, Gal, Kaem, Myr, Quer, Rut, Hest, Nar, Pinoc, EC, Gene | CA, ChloA, FA, p-CouA, t-CA | 2,3,4-THBA, ElA, GA, GenA, ProA, p-HBA, VA | Prod | N.I. | 26 | [43,54,60] |
| 25. | Asteraceae | Taraxacum officinalis | Taraxacum, Dandelion | Austria, Italy, Spain | 3 | Aca, Api, Chr, Lut, Tec, Gal, Isor, Kaem, Kaem-8-ME, Myr, Quer, Quer-3-ME, Isosak, Pinoc, Pinob | CAPE, FA, MF, p-CouA | MS, VAME | N.I | N.I | 21 | [51,57,61], |
| 26. | Asteraceae | Vernonia sp. | Assa peixe | Brazil | 1 | Chr | ChloA, FA, t-CA | BenA, GA, PAA, ProA | N.I | AbsA | 9 | [45] |
| 27. | Boraginaceae | Borago officinalis | Blue borage | China | 1 | Api, Chr, Tang, Hest, Nar | CA, ChloA | 2,3,4-THBA, GA, p-HBA, SyrA | Prod | N.I. | 12 | [43] |
| 28. | Boraginaceae | Echium plantagineum | Echium | Bulgaria | 1 | N.I. | CA, FA, o-CouA, p-CouA, t-CA | BenA, ProA | PAA, DL-p-HPLA, DL-β-PLA, | N.I. | 10 | [59] |
| 29. | Brassicaceae | Brassica sp., B. campestri, B. campestris L., B. napus, B. napus L., B. napus oleifera, B. nigra, B. rapa, B. napus L. var. oleifera Metzger | Oilseed, Rape, Rapeseed, Canola, Mustard flower | Austria, Bangladesh, Bulgaria, China, Czech Republic, France, Germany, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Tunisia | 24 | Aca, Api, Bai, Chr, Lut, Tec, Vit, Gal Gal-3-ME, Isor, Kaem, Kaem-8-ME, Kaem-3-O-(6”-acetyl)-β-Gluc, Mor, Myr, Myr-3,7,4′5′-TeME, Quer, Quer-3,7-DME, Quer-3-ME, Querc, Rham, Rut, Erio, Hest, Hesd, Isosak, Nar, Pinoc, Pinos, Pinob, Pinob-3-O-ace, Ono | CA, CADAE, CAPE, ChloA, FA, IfA, o-CouA, p-CouA, p-MCA, RosA, SinA, t-CA | BenA, ElA, GA, GenA, M-4-HBz, MS, m-HBA, PAA, ProA, p-HBA, ResA, SalA, SyrA, VA, VAME | 3,4-DHPAA, HGA, MandA, PAA, p-HPAA, DL-β-PLA, 3-PPA, PhlA, 4-MPC | AbsA | 69 | [46,47,48,49,51,52,54,55,57,59,62,63,64,65,66,67,68,69,70,71,72,73] |
| 30. | Brassicaceae | Diplotaxis tenuifolia | Diplotaxis | Argentina | 1 | Chr, Tec, Isor, Isor-4′-diGlc, Isor-4′-gent, isor-4′-Glc, Isor-3-Glc-4′-gent, Kaem, Kaem-3-diGlc isomer, Kaem-3-soph, Kaem-4′-Glc, Quer, Quer-3,3′,4′-triGlc, Quer-3,4′-diGlc, Quer-3-soph, Pinoc, Pinos, Pinob | CADAE, ChloA, FA, p-CouA | N.I. | N.I. | N.I. | 22 | [74] |
| 31. | Bromeliaceae | Ananas comosus | Nenas, Pineapple | Malaysia | 3 | Chr, Kaem, Myr, Quer, Rut, Hest, Nar | CA, ChloA, FA, p-CouA | BenA, GA, SyrA, ElA | N.I. | N.I. | 15 | [75,76,77] |
| 32. | Cactaceae | Opuntia | Prickly pear | Italy | 1 | Chr, Kaem, Myr, Quer, Rut, Pinoc, EC | FA, SinA | GA | N.I. | N.I. | 10 | [78] |
| 33. | Campanulaceae | Codonopsis pilosula (Franch.) Nannf. | Codonopsis | China | 2 | Api, Bai, Chr, Lut, Vit, Kaem, Myr, Quer, Querc, Rut, Hest, Hesd, Nar, Pinoc, EGC, Cal, Form, Gene, Ono, Cal-7-O-β-D-gluc | CA, CAPE, ChloA, FA, IfA, p-CouA, SinA | BenA, GA, m-HBA, ProA, p-HBA, SalA, SyrA | N.I. | AbsA | 35 | [55,62] |
| 34. | Capparaceae | Capparis sp., Capparis spinosa | Capparis, Caper, Kabbar | Algeria, Morocco | 2 | Api, Chr, Lut, Gal, Isor, Kaem, Kaemf, Myr, Quer, Quer-rham, Pinoc, Pinob, EC, Dai, Gene | CA, FA, p-CouA, RosA, t-CA | BenA, ProA, GA, p-HBA, SyrA, VA | 3,4-DHPAA, HVA, p-HPAA | N.I. | 29 | [33,34] |
| 35. | Caprifoliaceae | Lonicera sp. | Honeysuckle | China | 1 | Lut | N.I. | N.I. | N.I. | N.I. | 1 | [43] |
| 36. | Cistaceae | Cistus L. | Cistus | Italy | 1 | Api, Lut, Gal, Kaem, Pinoc, Pinob | t-CA | SyrA | N.I. | N.I. | 8 | [40] |
| 37. | Convolvulaceae | Ipomoea triloba L. | Morning Glory | Italy | 1 | Isor, Kaem, Kaem-8-ME, Kaem-7-O-rham, Quer | CA, FA, p-CouA, | SyrA, VA | N.I. | N.I. | 10 | [30] |
| 38. | Convolvulaceae | Turbina corymbosa (L.) Raf | Christmas Vine | Italy | 2 | Isor, Kaem, Kaem-8-ME, Kaem-7-O-rham, Quer | CA, FA, p-CouA | VA | N.I | N.I | 9 | [30,79] |
| 39. | Cucurbitaceae | Cucumis melo | Honeydew | China | 1 | Chr, Kaem, Querc, Hest, | CA | p-HBA, ProA | Prod | N.I. | 8 | [43] |
| 40. | Cucurbitaceae | Cucurbita sp. | Squash Blossoms | Turkey | 1 | Api, Chr, Lut, Kaem, Rut, Hest, Nar, CG | CA, FA, o-CouA, | ElA, GA, GenA, ProA, p-HBA, SyrA, VA | HGA, Prod, Van | N.I. | 21 | [53] |
| 41. | Cunoniaceae | Eucryphia cordifolia Cav. | Ulmo | Chile | 1 | Api, Chr, Quer, Pinoc | CA, ChloA, p-CouA | GA, m-HBA, | N.I. | AbsA | 10 | [80] |
| 42. | Cunoniaceae | Weinmannia racemosa | Kamahi | China, New Zealand | 2 | Api, Lut, Gal, Kaem, Quer, Querc, Hest, Nar | CA, ChloA | 2,3,4-THBA, GA, GenA, SyrA | Prod, Van | Leptd | 17 | [43,81] |
| 43. | Ericaceae | Arbutus unedo L., Arbutus unedo | Strawberry Tree, Arbousie, Arbutus | Italy, Morocco | 4 | Api, Lut, Gal, Isor, Kaem, Myr, Quer, Rut, Pinoc, Pinob, EC | CA, FA, p-CouA, RosA, t-CA, | GA, p-HBA, SyrA | HGA | AbsAa | 21 | [22,34,40,42] |
| 44. | Ericaceae | Calluna, Heather, Erica, Bell Heather, Ling Heather | Algeria, Bulgaria, Finland, France, Germany, Italy, New Zealand, Poland, Portugal, Spain, Turkey | 24 | Api, Chr, Lut, Gal, Isor, Kaem, Kaem-8-ME, Kaemf, Myr, Myr-3-ME, Quer, Quer-rham, Querc, Rham, Rut, Hest, Isosak, Nar, Pinoc, Pinob, C, CG, Dai, Gene | CA, CADAE, CAPE, ChloA, FA, m-CouA, o-CouA, p-CouA, RosA, t-CA | BenA, ElA, GA, GenA, MS, m-HBA, ProA, p-HBA, ResA, SalA, SyrA, VA | 3,4-DHPAA, HGA, HVA, PAA, p-HPAA, DL-p-HPLA, DL-β-PLA, 3-PPA, Prod, Van | AbsA, Lum | 58 | [33,36,46,47,49,53,56,59,61,70,82,83,84,85,86,87,88,89,90] | |
| 45. | Ericaceae | Oxydendrum arboretum | Sourwood | Malaysia | 1 | Hest | CA | N.I. | N.I. | N.I. | 2 | [77] |
| 46. | Ericaceae | Rhododendron ponticum | Rhododendron | France, Italy, Portugal, Spain, Turkey | 9 | Aca, Api, Chr, Lut, Isor, Kaem, Kaem-8-ME, Quer, Rut, Hest, Nar, Pinoc, Pinob, C, EC, Gene | CA, CADAE, CAPE, ChloA, FA, m-CouA, o-CouA, p-CouA, t-CA | BenA, GA, GenA, ProA, p-HBA, ResA, SyrA, VA | HGA, Prod | N.I. | 35 | [36,46,47,51,53,61,87,88,91] |
| 47. | Ericaceae | Vaccinium sp. V. vitis-idaea | Mire Lingonberry | Finland | 3 | Aca, Kaem-7-O-rham, Rham, Nar-ME, GC | ChloA, FA, p-CouA, t-CA | BenA, ProA, p-HBA, VA | 3-PPA | N.I | 14 | [89,92] |
| 48. | Euphorbiaceae | Croton sp. | Morrão de Candeia | Brazil | 1 | Gal, Nar | ChloA, m-CouA | BenA, GA, p-HBA, SyrA | N.I. | AbsA | 9 | [45] |
| 49. | Euphorbiaceae | Euphorbia sp. | Euphorbia, Spurge, Daghmos | Morocco, Turkey | 2 | Api, Chr, Lut, Kaem, Rut, Hest, Nar, CG, EC | CA, FA, o-CouA, p-CouA | ElA, GA, GenA, ProA, p-HBA, SyrA, VA | HGA, Prod | N.I. | 22 | [34,53] |
| 50. | Euphorbiaceae | Hevea brasiliensis | Rubber Tree | Malaysia | 1 | Myr, C | CA | BenA, GA | N.I. | N.I. | 5 | [77] |
| 51. | Fabaceae | Acacia catechu | Acacia | Lithuania | 1 | N.I. | CA, ChloA, FA, RosA, t-CA, | N.I. | N.I. | N.I. | 5 | [93] |
| 52. | Fabaceae | Acacia ehrenbergiana | Acacia | Saudi Arabia | 1 | Api, Chr, Lut, Gal, Quer | CA, ChloA, p-CouA | GA, p-HBA, SyrA | p-HPAA | 12 | [41] | |
| 53. | Fabaceae | Acacia mangium | Acacia | Malaysia | 5 | Kaem, Quer, Rut, Hest, Nar, C | CA, ChloA, FA, p-CouA, t-CA | BenA, ElA, PGG, SyrA | N.I. | N.I. | 15 | [76,77,94] |
| 54. | Fabaceae | Acacia sp. | Acacia, Acacia Flower | China | 3 | Lut, Quer Querc, Hest, Narg | N.I. | 2,3,4-THBA, GA, GenA, p-HBA | Prod | N.I. | 10 | [43] |
| 55. | Fabaceae | Acacia tortilis | Acacia, Wild Mountain, Oman Same, Rasul Khaima Samar, Doany Samer, Marya Herba, Ashab Marya Samer | UAE, Saudi Arabia | 5 | Api, Chr, Gal, Kaem, Myr, Quer, Rut, Nar, Narg, C, EC | CA, ChloA, FA, p-CouA, t-CA | GA, p-HBA, SyrA, VA | p-HPAA | N.I. | 21 | [41,95] |
| 56. | Fabaceae | Astragalus membranaceus (Fisch.) Bunge, A.microcephalus Willd., A. sinicus | Astragalus | China, Turkey | 3 | Api, Bai, Chr, Lut, Vit, Kaem, Myr, Quer, Querc, Rut, Hest, Hesd, Nar, Pinoc, Cal, Form, Gene, Ono, Cal-7-O-β-D-gluc | CA, ChloA, FA, IfA, p-CouA, SinA | BenA, SalA, m-HBA, p-HBA, ProA, VA, GA, SyrA | N.I. | AbsA | 34 | [36,62,96] |
| 57. | Fabaceae | Ceratonia siliqua | Carob | Turkey | 1 | Api, Chr, Lut, Kaem Rut, Hest, Nar, Gene | CA, FA, o-CouA, p-CouA | GA, GenA, ProA, p-HBA, SyrA, VA | HGA, Van, Prod | N.I. | 21 | [53] |
| 58. | Fabaceae | Glycine max | Soybean | USA | 1 | N.I. | p-CouA, t-CA, | p-HBA | N.I. | N.I. | 3 | [32] |
| 59. | Fabaceae | Hedysarum sp., H. coronarium, H. coronarium, L. | Hedysarum Sulla, Fior Di Sulla | Algeria, Bulgaria Italy | 10 | Api, Chr, Lut, Gal, Isor, Kaem, Myr, Quer, Rut, Hest, Hesd, Isosak, Nar, Pinoc, Pinob, C, EC | CA, ChloA, FA, o-CouA, p-CouA, t-CA, | BenA, GA, GenA, ProA, p-HBA, SalA, SyrA, VA | PAA, p-HPAA, DL-p-HPLA, 5,7-DMCoum | AbsA, PhAn, Tyr | 38 | [33,42,44,56,59,61,86,97,98,99] |
| 60. | Fabaceae | Lotus sp. | Lotus | Argentina, Algeria | 2 | Api, Chr, Lut, Gal Isor, Kaem, Myr, Quer, Pinob | CA, FA, p-CouA | BenA, p-HBA, SyrA, VA | p-HPAA | N.I. | 17 | [33,100] |
| 61. | Fabaceae | Lysiloma latisiquum (L.) Benth | Singing Bean | Italy | 1 | Isor, Kaem, Kaem-7-O-rham, Kaem 8-ME, Myr, Quer, Quer-diGlc, Quer-rham | CA, p-CouA | VA | N.I. | N.I. | 11 | [30] |
| 62. | Fabaceae | Medicago sativa | Alfalfa, Lucerne | Argentina, Spain | 2 | Aca, Api, Chr, Gal, Isor, Kaem, Quer, Quer-3-ME, Isosak, Pinoc, Pinob | CA, CAPE, FA, o-CouA, | ElA, SyrA | N.I. | N.I. | 17 | [51,101] |
| 63. | Fabaceae | Melilotus officinalis L., Melilotus sp. | Melilotus, Yellow Sweet Clover, Clover | Algeria, Poland, USA | 3 | Api, Chr, Lut, Gal, Isor Kaem, Mor, Myr, Quer, Pinoc, Pinob, Diadzein, Gene, C | CA, FA, p-CouA, RosA, t-CA | BenA, ElA, GA, m-HBA, p-HBA, SyrA, VA | p-HPAA | N.I. | 27 | [32,33,60] |
| 64. | Fabaceae | Prosopis nigra, P. juliflora | Algarrobo, Ghaf | Argentina, UAE | 2 | Chr, Hest, Pinoc | FA, p-CouA, t-CA | SyrA | N.I. | N.I. | 7 | [95,102] |
| 65. | Fabaceae | Robinia pseudoacacia L. | Acacia, Black Locust, Acacia grove, Robinia | Austria, Belgium, Bulgaria, China, Croatia, Czech Republic, Italy, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Switzerland, Turkey, USA | 36 | Aca, Api, Bai, Chr, Chr-2′-ME, Genk, Lut, Tec, Vit, Fis, Gal, Isor, Kaem, Kaem-8-ME, Kaem-7-O-rham, Kaem-3-O-(6”-acetyl)-β-Gluc, kaem-3-O-(hexoxyl) rob-7-O-rham, kaem-3-O-(hexoxyl) robi, kaem-3-O-hex-7-O-rham, kaem-3-O-rob-7-O-rham, kaem-3-O-rob, Mor, Myr, Quer, Quer-3,3-DME, Quer-3,7-DME, Quer-3-ME, Querc, Rham, Rut, Alp, Erio, Hest, Hesd, Isosak, Nar, Pinoc, Pinos, Sak, Pinob, Pinob-3-O-ace, Pinob-5-ME, Tax, CG, EGC, GC, Cal, Form, Gene, Geni, Ono, Cal-7-O-β-D-gluc, Pinob Chal | 3,4-DMCA, CA, CABE, CADAE, CAPE, ChloA, CChloA, FA, IfA, m-CouA, o-CouA, p-CouA, RosA, SinA, t-CA | BenA, CuA, ElA, GA, GenA, MS, m-HBA, ProA, p-HBA, SalA, SyrA, VA | HGA, PAA, DL-β-PLA, 3-PPA, 5-Phenylpent-4-enoic acid, 2-M-4-VP, 2,3,5-TMP, 2-MBd, Prod, Van, 5,7-DMCoum, DBZO | AbsA | 93 | [32,35,36,37,44,46,49,50,51,53,54,56,57,59,61,62,63,64,66,67,70,71,72,86,96,97,103,104,105,106,107,108,109,110] |
| 66. | Fabaceae | Trifolium repems, Trifolium sp., Trifolium pratense | Clover, Trifolium, Trefoils, 45° South clover | Algeria, Argentina, Austria, China, Germany, Italy, New Zealand, Turkey | 12 | Api, Chr, Lut, Gal, Isor, Kaem, Quer, Rut, Hest, Isosak, Nar, Pinoc, Pinob, Gene | CA, ChloA, FA, o-CouA, p-CouA, SinA, t-CA | 3,4,5-TMBA, BenA, ElA, GA, GenA, M-4-HBz, MS, OAA, PAA, ProA, p-HBA, SalA, SyrA, VA, VAME | 3,4-DHPAA, HGA, HVA, PAA, p-HPAA, 4-mPLA, DL-β-PLA, 3-PPA, PhlA, 4-MPC, Prod, Van | AbsA, Leptd | 50 | [33,36,43,48,53,56,57,81,82,101,111,112] |
| 67. | Fabaceae | Vicia dichroantha, V. villosa Roth | Vicia | China | 2 | Gal, Kaem, Quer, Rut, GC | CA, ChloA, FA, p-CouA, RosA | BenA, p-HBA, ProA, GA, SyrA, ElA | N.I | N.I | 16 | [68,96] |
| 68. | Fagaceae | Castanea sativa Mill., C. sativa Miller | Chestnut | Austria, Belgium, Bulgaria, France, Germany, Italy, Portugal, Slovenia, Spain, Switzerland, Turkey | 28 | Aca, Api, Chr, Chr-2′-ME, Chr-6-ME, Genk, Lut, Tec, Gal, Gal-5-ME, Isor, Kaem, Kaem-ME, Kaem-8-ME, Myr, Quer, Quer-3,3-DME, Quer-3,7-DME, Quer-3-ME, Querc, Rham, Rut, Hest, Nar, Pinoc, Pinob, pinob-3-O-pent, Pinob-5-ME, C, CG, EC, Gene, Leptosin, Pinob Chal | 3,4-DMCA, CA, CAIPE, CAPE, ChloA, FA, m-CouA, o-CouA, p-CouA, SinA, t-CA | BenA, CuA, ElA, GA, GenA, M-4-HBz, MS, m-HBA, OAA, ProA, p-HBA, ResA, SalA, SyrA, VA, VAME | HGA, PAA, DL-p-HPLA, DL-β-PLA, 3-PPA, 5-Phenylpent-4-enoic acid, 2-M-4-VP, 2-MBd, Prod, Van, 1-(2-Aminophenyl)butan-1-one, 5,7-DMCoum, DBZO | KyA, Lum | 76 | [35,36,40,46,47,49,51,53,56,57,59,61,86,87,88,90,97,98,104,109,113,114,115,116,117,118,119] |
| 69. | Fagaceae | Fagus sp. | Beech Forest | China | 1 | Api, Lut, Quer, Querc | N.I. | 2,3,4-THBA, GA, GenA, ProA, p-HBA | Van, Prod | N.I. | 11 | [43] |
| 70. | Fagaceae | Quercus sp., Q. robur L. | Oak | Switzerland, Turkey | 4 | Aca, Chr, Chr-2′-ME, Genk, Tec, Gal, Quer, Rut, Pinoc, Pinob-5-ME, EC, Pinob Chal | 3,4-DMCA, CA, FA, p-CouA, t-CA, | BenA, CuA, GA, MS, ProA, p-HBA, SalA, SyrA | 3-PPA, 5-Phenylpent-4-enoic acid, 2-M-4-VP, 2-MBd, DBZO, | N.I. | 30 | [36,88,104,120] |
| 71. | Hydrophyllaceae | Phacelia tanacetifolia | Phacelia | Poland | 1 | Api, Chr, Gal, Kaem, Myr, Quer Nar, Pinoc, EC | CA, ChloA, FA, p-CouA, t-CA, | GA, p-HBA | N.I. | AbsA | 17 | [121] |
| 72. | Hypericaceae | Hypericum sp. | Hypericum | China | 1 | Lut, Kaem, Quer, Narg | CA, ChloA, | GA, p-HBA | Prod | N.I. | 9 | [43] |
| 73. | Iridaceae | Crocus sativus | Saffron | India | 1 | Api, Kaem, Myr, Quer, Nar, Pinob, C | CA, ChloA, FA, p-CouA | GA, ElA | N.I. | N.I. | 13 | [122] |
| 74. | Lamiaceae | Agastache sp. | Agastache | Australia | 1 | Kaem, Quer, Rut, Hest | CA, ChloA, FA, p-CouA, SinA, t-CA, | MS, ProA, p-HBA, ResA, SyrA, VA, | PAA, DL-β-PLA | N.I. | 18 | [123] |
| 75. | Lamiaceae | Lavandula sp. L. stoeclias | Lavender | Bulgaria, France, Hungary, Italy, Portugal, Spain, Switzerland, Turkey | 10 | Aca, Api, Chr, Chr-2′-ME, Genk, Lut, Tec, Gal, Isor, Kaem, Kaem-8-ME, Myr, Quer, Quer-3-ME, Rut, Erio, Hest, Nar, Pinoc, Pinob, Pinob-5-ME, C, CG, EC, Pinob Chal | 3,4-DMCA, CA, CADAE, CAPE, ChloA, FA, m-CouA, o-CouA, p-CouA, RosA, t-CA | BenA, CuA, ElA, GA, GenA, MS, ProA, p-HBA, SalA, SyrA, VA | HGA, PAA, DL-p-HPLA, DL-β-PLA, 3-PPA, 5-Phenylpent-4-enoic acid,2-M-4-VP, 2-MBd, Prod, Emo, n-β-L, DBZO | AbsA | 60 | [36,40,46,47,49,51,53,59,104,124] |
| 76. | Lamiaceae | Leonurus cardiaca | Motherwort | China | 1 | N.I. | GA, p-HBA | N.I. | Prod, Van | N.I. | 4 | [43] |
| 77. | Lamiaceae | Ocimum basilicum | Basil | Serbia | 1 | Api, Chr, Lut, Gal, Kaem, Quer, Rut, Pinoc | CA, ChloA | GA, ProA | N.I. | N.I. | 12 | [54] |
| 78. | Lamiaceae | Phlomis armeniaca Willd. | Jerusalem Tea | Turkey | 1 | Api, Quer | ChloA, FA, p-CouA | p-HBA, VA | N.I. | N.I. | 7 | [36] |
| 79. | Lamiaceae | Plectranthus rugosus | Wild Bush | India | 1 | Api, Kaem, Myr, Quer, Nar, Pinob, C | CA, ChloA, FA, p-CouA | ElA, GA | N.I. | N.I. | 13 | [122] |
| 80. | Lamiaceae | Prunella vulgaris | Prunella | China | 1 | Api, Chr, Gal, Quer, Nar, Pinob | CA, ChloA, FA, p-CouA, RosA, t-CA, | ElA, ProA, SyrA, VA, | N.I. | N.I. | 16 | [125] |
| 81. | Lamiaceae | Rosmarinus officinalis L. | Rosemary | Bulgaria, Czech Republic, France, Italy, Portugal, Spain, Tunisia | 13 | Aca, Api, Chr, Chr-6-ME, Lut, Tec, Gal, Gal-5-ME, Isor, Kaem, Kaem-ME, Kaem-8-ME, Kaemf, Myr Myr-3,7,4′5′-TeME, Quer Quer-3,3-DME, Quer-3,7-DME, Quer-7,3′-DME, Rham, Rut, Erio, Nar, Pinoc, Pinos, Sak, Pinob, Pinob-3-O-butyr, Tax, C, Gene | CA, CAIPE, CAPE, ChloA, FA, IfA, p-CouA, RosA, SinA, t-CA | GA, GenA, m-HBA, ProA, p-HBA, SalA, SyrA, VA | PAA, DL-β-PLA, 5,7-DMCoum | N.I. | 52 | [10,40,46,47,49,51,52,59,86,107,116,126,127] |
| 82. | Lamiaceae | Salvia officinalis L. | Sage | Croatia | 2 | Api, Chr, Lut, Gal, Isor, Kaem, Quer, Hest, Nar, Pinoc, Pinos, Pinob, EC, EGC, EGCG, GC, GCG | CA, ChloA, FA, p-CouA, RosA | GA, GenA, ProA, p-HBA | Resv | N.I. | 27 | [128,129] |
| 83. | Lamiaceae | Satureja hortensis, Satureja subspicata Vis. | Savory, Satureja | Italy, Croatia | 2 | Api, Chr, Gal, Kaem, Quer, Gene | ChloA, p-CouA | BenA, GA, MS | DL-β-PLA | PhAn, Tyr | 14 | [97,130] |
| 84. | Lamiaceae | Sideritis sp. | Sideritis | Turkey | 1 | Api, Chr, Lut, Kaem, Rut, Hest, Nar, CG | CA, FA, o-CouA | ElA, GA, GenA, ProA, p-HBA, SyrA, VA | HGA, Prod, Van | N.I. | 21 | [53] |
| 85. | Lamiaceae | Thymus sp., T. algeriensis, T. capitatus (L.), T. capitatus and T. herba-borona, T. capitatus Hoffgg.e.LK., T. vulgare, T. vulgaris L., T. capitatus | Thyme /Zaˆatar /Zohif | Belgium, China, Greece, Hungary, Italy, Morocco, Portugal, Tunisia, Turkey | 16 | Api, Chr, Chr-6-ME, Lut, Gal, Gal-5-ME, Isor Kaem, Kaem-ME, Kaem-8-ME, kaem-3-O-neoh, Myr, Myr 3,7,4′5′-TeME, Quer, Quer-3,3-DME, Quer-3,7-DME, Quer-3-ME, Querc, Rham, Rut, Erio, Hest, Nar, Pinoc, Pinos, Pinob, CG, EC, Gene | CA, CADAE, CAPE, ChloA, FA, Gene, o-CouA, p-CouA, RosA, t-CA | ElA, GA, GenA, ProA, p-HBA, ResA, SyrA, VA | HGA, Prod, Van | KyA | 51 | [34,35,40,43,46,52,53,61,97,116,124,131,132,133,134] |
| 86. | Lamiaceae | Vitex agnus-castus L. | Chaste | China, Turkey | 3 | Api, Chr, Lut, Gal, Kaem, Myr, Quer, Rut, Pinoc, C | 3,4-DMCA, CA, ChloA, FA, p-CouA, RosA, SinA, t-CA | ElA, GA, ProA, p-HBA, ResA, VA | N.I. | N.I. | 24 | [36,64,135] |
| 87. | Lamiaceae | Vitex negundo var. heterophylla Rehd. | Vitex | China, Turkey | 3 | Api, Bai, Chr, Lut, Vit, Fis, Gal, Isor, Kaem, Mor, Quer, Querc, Rut, Hest, Hesd, Isosak, Nar, Pinoc, Sak, Pinob, Tax, EGC Form, Gene, Geni, Ono, | CA, ChloA, CChloA, FA, IfA, o-CouA, p-CouA, SinA | GA, GenA, m-HBA, ProA, p-HBA, SalA, SyrA, VA | HGA, Prod, Van | AbsA | 46 | [37,53,62] |
| 88. | Lauraceae | Persea americana | Avocado | Spain | 1 | Chr, Gal, Gal-5-ME, Isor, Kaem, Kaem-3-O-neoh, Quer, Quer-3,7-DME, Rut, Pinoc, Pinob, Pinob-5-ME | N.I. | ElA | N.I. | N.I. | 13 | [116] |
| 89. | Malvaceae | Gaya macrantha | Field Flower | Brazil | 1 | N.I. | N.I. | GA | N.I. | N.I. | 1 | [31] |
| 90. | Malvaceae | Gossypium hirsutum L. | Cotton | Turkey | 1 | Api, Chr, Lut, Kaem, Rut, Hest, Nar, CG | CA, FA, o-CouA | ElA, GA, GenA, ProA, p-HBA, SyrA, VA | HGA, Prod, | N.I. | 20 | [53] |
| 91. | Malvaceae | Tilia sp., T. amurensis Rupr., T. argentea, T. cordata, T. cordata L., T. europa, T. europaea, T. scop, T. platyphyllos | Linden, Tilia, Lime Tree, Lime, Lime-blossom, Linden tree, Linden blossom | Austria, Bulgaria, China, Czech Republic, France, Germany, Italy, Poland, Romania, Serbia, Slovenia, Spain, Turkey | 25 | Aca, Api, Bai, Chr, Lut, Tec, Vit, Fis, Gal, Isor, Kaem, Kaem-8-ME, Kaemf, Mor, Myr, Quer, Quer-3-ME, Querc, Rham, Rut, Hest, Hesd, Isosak, Nar, Narg, Pinoc, Sak, Pinob, Pinob-3-O-ace, Tax, C, EGC, Form, Gene, Ono | CA, CAPE, ChloA, CChloA, FA, IfA, p-CouA, p-MCA, RosA, SinA, t-CA | BenA, ElA, GA, GenA, M-4-HBz, MS, m-HBA, ProA, p-HBA, SalA, SyrA, VA, VAME | 3,4-DHPAA, HGA, MandA, PAA, p-HPAA, DL-β-PLA, 3-PPA, 4-MPC, Prod | AbsA | 69 | [36,37,43,47,48,49,50,51,54,57,59,61,62,65,66,68,70,72,107,109,110,114,136] |
| 92. | Meliaceae | Azadiractha indica | Neem | India | 1 | Lut, Isor Myr Quer Rut, C, EC | p-CouA, CA, FA, ChloA | ProA, GA, SyrA | N.I. | N.I. | 14 | [137] |
| 93. | Myrtaceae | Eucalyptus camaldulensis | River Red Gum Eucalyptus | Australia, Italy, Spain, Switzerland | 4 | Aca, Api, Chr, Chr-2′-ME, Genk, Lut, Tec, Tri, Gal, Kaem, Myr, Quer, Pinoc, Pinob, Pinob-5-ME, GC | 3,4-DMCA, CA, ChloA, FA, p-CouA, t-CA | BenA, GA, p-HBA, SalA, SyrA | PAA, 2-M-4-VP, 2,3,5-TMP, 2-MBd, 1-(3-methoxy-phenyl) ethanone, DBZO | AbsA | 35 | [40,104,138,139] |
| 94. | Myrtaceae | Eucalyptus crebra | Narrow-leaved Ironbark | Australia | 1 | N.I. | CA, ChloA, FA, p-CouA, | ElA, GA | N.I. | AbsA | 7 | [139] |
| 95. | Myrtaceae | Eucalyptus globoidea | Stringybark | Australia | 1 | N.I. | CA, ChloA, p-CouA | ElA, GA | N.I. | AbsA | 6 | [139] |
| 96. | Myrtaceae | Eucalyptus globulus | Eucalyptus | Lithuania | 1 | N.I. | CA, ChloA, FA, RosA, t-CA | GA, VA | N.I. | N.I. | 7 | [93] |
| 97. | Myrtaceae | Eucalyptus intermedia | Bloodwood | Australia | 1 | N.I. | CA, ChloA, FA, p-CouA | ElA, GA | N.I. | AbsA | 7 | [139] |
| 98. | Myrtaceae | Eucalyptus largiflorens | Blackbox | Australia | 1 | N.I. | CA, ChloA, FA, p-CouA | ElA, GA | N.I. | AbsA | 7 | [139] |
| 99. | Myrtaceae | Eucalyptus marginata | Jarrah | Australia | 1 | Quer, Rut, Hest | CA, ChloA, FA, p-CouA, SinA, t-CA, | GA, MS, ProA, p-HBA, SyrA, VA | DL-β-PLA | N.I. | 16 | [123] |
| 100. | Myrtaceae | Eucalyptus melliodora | Yellow Box | Australia, Spain | 2 | Lut, Tri, Kaem, Myr, Quer, Quer-3-ME | CA, ChloA, FA, p-CouA | ElA, GA | N.I. | AbsA | 13 | [138,139] |
| 101. | Myrtaceae | Eucalyptus moluccana | Gum Top | Australia | 1 | N.I. | CA, ChloA, FA, p-CouA | ElA, GA | N.I. | AbsA | 7 | [139] |
| 102. | Myrtaceae | Eucalyptus nubila | Blue Top Ironbark | Australia | 1 | N.I. | CA, ChloA, FA, p-CouA | ElA, GA | N.I. | AbsA | 7 | [139] |
| 103. | Myrtaceae | Eucalyptus ochrophloia | Yapunyah | Australia | 1 | N.I. | CA, ChloA, FA, p-CouA | GA, ElA | N.I. | AbsA | 7 | [139] |
| 104. | Myrtaceae | Eucalyptus pilligaensus | Mallee | Spain | 1 | Lut, Tri, Myr, Quer, Pinob, Pinoc, GC | N.I. | N.I. | N.I. | N.I. | 7 | [138] |
| 105. | Myrtaceae | Eucalyptus sp. | Eucalyptus | Algeria, Argentina, Belgium, Brazil, Bulgaria, China, Germany, India, Italy, Spain, Tunisia, Turkey | 21 | Api, Chr, Chr-6-ME, Lut, Tec, Tri, Gal, Gal-5-ME, Isor, Kaem, Kaem-8-ME, kaem-3-O-neoh, Kaemf, Myr, Myr-3,7,4′5′-TeME, Quer, Quer-3,3-DME, Quer-3,7-DME, Quer-3-ME, Quer-3-O-hex (1→2) hex, Quer-rham, Rham, Rut, Hest, Isosak, Nar, Narg, Pinoc, Pinos, Pinob, C, EC, Dai, Gene, Leptosin | 3,4-DMCA, CA, ChloA, FA, m-CouA, o-CouA, p-CouA, SinA, t-CA | 2,3,4-THBA, BenA, ElA, GA, GenA, MS, m-HBA, ProA, p-HBA, SalA, SyrA, VA | 3,4-DHPAA, HGA, HVA, PAA, p-HPAA, DL-p-HPLA, DL-β-PLA, Prod, Van, 5,7-DMCoum | KyA, Lum, PhAn, Tyr | 70 | [31,33,35,42,43,49,51,52,53,59,61,64,86,90,98,100,101,114,116,137,138] |
| 106. | Myrtaceae | Kunzea ericoides | Kanuka | Germany, New Zealand | 5 | Leptosin | N.I. | 3,4,5-TMBA, GA, Lepp, MS, OAA, PAA, SyrA | 4-mPLA, DL-p-HPLA, DL-β-PLA, 3,4,5-TMP, p-And, 2′-MAPo | 5-MF-3-CA, AbsA, KojA, Leptd, Lum | 19 | [81,111,112,140,141] |
| 107. | Myrtaceae | Miellerie, Leptospermum lanigerum, and Leptospermum scoparium | Tea tree | Australia | 1 | Kaem, Quer, Rut, Hest | CA, ChloA, FA, p-CouA, SinA, t-CA | p-HBA, ProA, VA, GA, SyrA, ResA, MS | DL-β-PLA | N.I. | 18 | [123] |
| 108. | Myrtaceae | Leptospermum polygalifolium | Jelly Bush | Australia, Germany, New Zealand | 4 | Chr, Lut, Tec, Tri, Isor, Kaem, Kaem-8-ME, Quer, Quer-3,3-DME, Quer-3-ME, Rut, Hest, Pinoc, Pinob, Leptosin | CA, ChloA, FA, p-CouA, SinA, t-CA | GA, MS, OAA, ProA, p-HBA, ResA, SyrA, VA | DL-β-PLA, 3,4,5-TMP, 2’-MAPo | 5-MF-3-CA, KojA, Leptd | 35 | [112,123,141,142] |
| 109. | Myrtaceae | Leptospermum scoparium | Manuka | Australia, China, Germany, Italy, Malaysia, New Zealand, Poland, Spain, Thailand, UAE, USA | 29 | Api, Chr, Chr-6-ME, Lut, Vit, Fis, Gal, Isor, Kaem, Kaem-8-ME, Myr, Quer, Quer-3,3-DME, Quer-3,7-DME, Quer-3-ME, Quer-3-O-hex (1→2) hex, Querc, Rut, Hest, Hesd, Isosak, Nar, Narg, Pinoc, Sak, Pinob, Tax, C, EC, GC, Form, Leptosin | CA, CAPE, ChloA, CChloA, FA, IfA, p-CouA, RosA, SinA, t-CA | 2,3,4-THBA, 3,4,5-TMBA, BenA, ElA, GA, GenA, Lepp, MS, OAA, PAA, ProA, p-HBA, ResA, SalA, SyrA, VA | PAA, p-HPAA, 4-mPLA, DL-p-HPLA, DL-β-PLA, 3,4,5-TMP, p-And, Prod, 2’-HAPo, 2′-MAPo, 3-hydroxy-1-(2-methoxyphenyl)penta-1,4-dione | 2-MBF, 5-MF-3-CA, AbsA, KojA, Leptd, Lum, | 75 | [37,38,43,81,82,84,90,95,111,112,116,123,140,141,142,143,144,145,146,147,148] |
| 110. | Myrtaceae | Lophostemon conferta | Brush Box | Australia | 1 | N.I. | CA, ChloA, FA, p-CouA | ElA, GA, SyrA, | N.I. | AbsA | 8 | [58] |
| 111. | Myrtaceae | Melaleuca cajuputi | Gelam | Malaysia | 7 | Api, Chr, Lut, Kaem, Myr, Quer, Rut, Hest, Nar, Pinob-3-O-prop | CA, ChloA, FA, p-CouA, t-CA | BenA, GA, SyrA, ElA | N.I. | N.I. | 19 | [39,75,76,77,94,149] |
| 112. | Myrtaceae | Melaleuca quinquenervia | Tea Tree | Australia | 1 | N.I. | CA, ChloA, FA, p-CouA | ElA, GA, SyrA | N.I. | AbsA | 8 | [58] |
| 113. | Myrtaceae | Metrosideros robusta | Rata | China | 1 | Api, Chr, Lut, Quer, Querc, Hest | CA, ChloA | 2,3,4-THBA, GA, SyrA | Prod | N.I. | 12 | [43] |
| 114. | Myrtaceae | Myrtaceae sp. | Myrtaceae | Algeria | 1 | Api, Chr, Lut, Gal, Isor, Kaem, Kaemf, Myr, Quer, Isosak, Pinoc, Pinob | t-CA, p-CouA, CA, FA | BenA, GenA, ProA, p-HBA, SyrA, VA | 3,4-DHPAA, p-HPAA | N.I. | 24 | [33] |
| 115. | Myrtaceae | Myrtus communis L. | Myrtus | Italy | 1 | Api, Lut, Gal, Quer, Pinoc, Pinob, C | t-CA | N.I. | N.I. | N.I. | 6 | [40] |
| 116. | Nelumbonaceae | Nelumbo nucifera | Padma Flower | Bangladesh | 1 | Kaem, C | CA, FA, t-CA | N.I. | N.I. | N.I. | 5 | [69] |
| 117. | Nothofagaceae | Nothofagus sp. | Beech | New Zealand | 1 | Chr, Gal, Pinoc, Pinob | p-CouA, t-CA | BenA, MS, p-HBA, SyrA, VA | N.I. | N.I. | 11 | [82] |
| 118. | Nyssaceae | Nyssa aquatica | Tupelo | USA | 2 | Chr, Gal, Kaem, Quer, Hest, Pinoc | p-CouA, t-CA | VA | N.I. | N.I. | 9 | [32,38] |
| 119. | Oleaceae | Osmanthus fragrans | Wild Osmanthus | China | 1 | N.I. | N.I. | 2,3,4-THBA, GenA, SyrA, p-HBA | HGA, Prod | N.I. | 6 | [43] |
| 120. | Onagraceae | Epilobium angustifolium | fireweed willow herb | Finland, USA | 2 | N.I. | p-CouA, t-CA | BenA, p-HBA | 3-PPA | N.I. | 5 | [32,89] |
| 121. | Pedaliaceae | Sesamum indicum | Teel/Sesame | Bangladesh | 1 | Gal, Nar, C | CA, ChloA, FA | GA | N.I. | N.I. | 7 | [69] |
| 122. | Pinaceae | Abies sp., A. alba Mill., A. cephallonica, A. cephalonica Loudon | Fir | France, Greece, Slovenia | 5 | Api, Chr, Lut, Gal, Isor, Kaem, Kaem-8-ME, Myr, Quer, Nar, Pinoc, Pinob | CA, FA, p-CouA | ProA, p-HBA, SyrA, VA | N.I. | N.I. | 19 | [47,109,132,133,134] |
| 123. | Pinaceae | Cedrus libani var. stenocoma | Cedar | Turkey | 1 | Api, Chr, Lut, Kaem, Rut, Hest, Nar, Gene | CA, FA, o-CouA, p-CouA, t-CA | GA, GenA, ProA, p-HBA, SyrA, VA | HGA, Van, Prod | N.I. | 22 | [53] |
| 124. | Pinaceae | Picea abies (L) Karst | Spruce | Slovenia | 1 | Api, Chr, Lut, Gal, Kaem, Myr, Quer, Nar, Pinoc, Pinob | N.I. | N.I. | N.I. | N.I. | 10 | [109] |
| 125. | Pinaceae | Pinus sp., Pinus brutia L. | Forest Fino Pine | Greece, Turkey | 6 | Api, Chr, Lut, Kaem, Myr, Quer, Rut, Hest, Nar, Pinob, C, CG, Gene | o-CouA, p-CouA, CA, FA | p-HBA, ProA, GA, GenA, SyrA, VA | HGA, Prod, Van | N.I. | 26 | [36,53,132,133,134] |
| 126. | Polygonaceae | Fagopyrum esculentum | Buckwheat | China, Finland, Italy, Lithuania, Poland, Serbia, Turkey, USA | 16 | Api, Chr, Lut, Vit, Fis, Gal, Isor, Kaem, Mor, Myr, Quer, Rut, Hest, Hesd, Isosak, Nar, Pinoc, Sak, Pinob, Tax, Form, GC | CA, CAPE, ChloA, CChloA, FA, IfA, RosA, SinA, p-CouA, t-CA | BenA, ElA, GA, m-HBA, ProA, p-HBA, SalA, SyrA, VA, | HGA, 3-PPA | AbsA | 44 | [32,37,54,55,66,70,73,83,84,88,89,93,145,150,151,152] |
| 127. | Proteaceae | Banksia ericifolia | Heath | Australia | 1 | N.I. | CA, ChloA, FA, p-CouA | ElA, GA | N.I. | AbsA | 7 | [58] |
| 128. | Proteaceae | Knightia excelsa | Rewarewa | New Zealand | 2 | N.I. | N.I. | 3,4,5-TMBA, GA, MS, OAA, PAA, SyrA | 4-mPLA, DL-β-PLA | AbsA, Leptd | 10 | [81,111] |
| 129. | Ranunculaceae | Coptis sp. | Mountain Coptis | China | 1 | Lut, Gal, Kaem, Quer | ChloA | p-HBA, GA, SyrA | Prod | N.I. | 9 | [43] |
| 130. | Ranunculaceae | Nigella sativa | Kalijira | Bangladesh | 1 | Nar, C | CA, FA, ChloA | N.I. | N.I. | N.I. | 5 | [69] |
| 131. | Rhamnaceae | Frangula sp. | Alder | France | 1 | Kaem-8-ME | N.I. | N.I. | N.I. | N.I. | 1 | [47] |
| 132. | Rhamnaceae | Gouania polygama (Jack)Urb | Linen vine | Italy | 1 | Isor, Kaem, Kaem-8-ME, Kaem-7-O-rham, Quer Quer-rham | CA, FA, p-CouA | SyrA, VA | N.I. | N.I. | 11 | [30] |
| 133. | Rhamnaceae | Hovenia dulcis | Japanese grape | Brazil | 1 | Quer | p-CouA | GA | N.I. | N.I. | 3 | [31] |
| 134. | Rhamnaceae | Paliurus spina-christi | Marruca | Italy | 1 | Quer, Hesd, Nar | CA, FA, p-CouA | p-HBA, VA | N.I. | N.I. | 8 | [56] |
| 135. | Rhamnaceae | Ziziphus jujuba, Ziziphus jujube Mill | Jujube/Zaohua | China | 4 | Api, Chr, Lut, Vit, Gal, Isor, Kaem, Myr, Quer, Hest, Isosak, Nar, Pinoc, Sak, Pinob, Tax, GC | CA, ChloA, CChloA, FA, IfA, p-CouA, RosA, SinA, t-CA | BenA, ElA, GA, GenA, ProA, p-HBA, ResA, SalA, SyrA, VA | Prod | N.I. | 37 | [37,43,96,135] |
| 136. | Rhamnaceae | Ziziphus spina-csisti | Wild jujube | Morocco, Saudi Arabia UAE | 7 | Api, Chr, Gal, Kaem, Myr, Quer, Rut, Nar, Narg, C, EC | CA, ChloA, FA, p-CouA, RosA, t-CA | GA, p-HBA, SyrA, VA | p-HPAA | N.I. | 22 | [34,41,95] |
| 137. | Rosaceae | Crataegus sp. | Wild hawthorn | China | 1 | N.I. | N.I. | GA, GenA, ProA, p-HBA | Prod | N.I. | 5 | [43] |
| 138. | Rosaceae | Eriobotrya japonica | Loquat | China | 1 | N.I. | N.I. | p-HBA | Prod | N.I. | 2 | [43] |
| 139. | Rosaceae | Malus domestica | Apple | India | 1 | Api, Kaem, Myr, Quer, Nar, Pinob, C | CA, ChloA, FA, p-CouA | ElA, GA | N.I. | N.I. | 13 | [122] |
| 140. | Rosaceae | Mespilus germanica | Medlar | Italy | 1 | Chr, Kaem, Myr, Quer, Rut, Pinoc, EC | CA, ChloA, FA, SinA | GA, VA | N.I. | N.I. | 13 | [78] |
| 141. | Rosaceae | Prunus avium | Cherry blossom | India, Spain | 2 | Aca, Api, Chr, Tec, Gal, Isor, Kaem, Myr Quer, Quer-3-ME, Isosak, Nar, Pinoc, Pinob, C | CA, CAPE, ChloA, FA, p-CouA | ElA, GA | N.I. | N.I. | 24 | [51,122] |
| 142. | Rosaceae | Prunus dulcis L. | Almond | Italy | 1 | Chr, Kaem, Myr, Quer, Rut, Pinoc, EC | CA, ChloA, FA, SinA | GA, SyrA, VA | N.I. | N.I. | 14 | [78] |
| 143. | Rosaceae | Rosa acicularis | Wild rose | China | 1 | Lut, Quer, Hesd | CA, ChloA | GA, p-HBA, SyrA | HGA, Prod | N.I. | 10 | [43] |
| 144. | Rosaceae | Rubus chamaemorus | Clodberry | Finland | 1 | N.I. | N.I. | p-HBA | 3-PPA | N.I. | 2 | [89] |
| 145. | Rosaceae | Rubus idaeus | Raspberry | Czech Republic, Lithuania | 2 | Chr | CA, ChloA, FA, RosA, t-CA | GA, VA | N.I. | N.I. | 8 | [65], [93] |
| 146. | Rutaceae | Citrus bergamia | Bergamot | China | 1 | Api, Chr, Querc | N.I. | GA, GenA, p-HBA | Prod | N.I. | 7 | [43] |
| 147. | Rutaceae | Citrus sp. | Citrus | Algeria, China, Greece, Italy, Portugal, Spain, Turkey, USA | 11 | Api, Chr, Lut, Gal, Isor, Kaem, Myr, Quer, Rut, Hest, Isosak, Nar, Pinoc, Pinob, CG, Gene | CA, ChloA, FA, o-CouA, p-CouA, t-CA | BenA, GA, GenA, ProA, p-HBA, SyrA, VA | HGA, 3,4-DHPAA, Prod | N.I. | 32 | [10,33,38,44,46,49,53,98,114,133,153] |
| 148. | Rutaceae | Citrus sp., C. lemon, C. limon, C. limon Burm | Lemon blossom, Lemon | Argentina, Bulgaria, China, India, Italy, Portugal, Spain | 8 | Api, Chr, Lut, Gal, Kaem, Myr, Quer, Rut, Hest, Hesd, Nar, Pinoc, C, EC | CA, ChloA FA, p-CouA, SinA, t-CA | 2,3,4-THBA, GA, m-HBA, p-HBA, ProA, SyrA, VA | PAA, DL-β-PLA, Prod | N.I. | 30 | [43,46,59,78,102,137,154] |
| 149. | Rutaceae | Citrus sp., C. sinensis, C. aurantium L. | Orange, Orange blossom | Austria, Brazil, Bulgaria, China, France, Greece, Italy, Spain, Switzerland, Tunisia | 14 | Aca, Api, Chr, Chr-2′-ME, Genk, Lut, Tec, Gal, Gal-3-ME, Isor, Kaem, Kaem-8-ME, Myr, Myr-3,7,4′5′-TeME, Quer, Quer-3,7-DME, Quer-3-ME, Rut, Hest, Isosak, Nar, Narg, Pinoc, Pinos, Pinob, Pinob-3-O-ace, Pinob-5-ME, EC, Pinob Chal | 3,4-DMCA, CA, CAPE, ChloA, FA, MF, o-CouA, p-CouA, SinA, t-CA | 2,3,4-THBA, BenA, ElA, GA, M-4-HBz, MS, ProA, p-HBA, SyrA, VA, VAME | PAA, DL-β-PLA, 5-Phenylpent-4-enoic acid, 2-M-4-VP, 2,3,5-TMP, 2-MBd, Prod | N.I. | 57 | [43,47,49,51,52,57,59,61,78,97,104,132,154,155] |
| 150. | Salicaceae | Azara integrifolia, A. petiolaris | Azara | Chile | 2 | Api, Chr, Lut, Quer, Rut, Pinoc | CA, p-CouA | SyrA | N.I. | AbsA | 10 | [156] |
| 151. | Salicaceae | Salix sp. | Willow Polish, Willow | Poland | 2 | N.I. | CA, ChloA, FA, p-CouA | BenA, MS, ProA, p-HBA, VA | N.I. | AbsA, KyA | 11 | [84,157] |
| 152. | Sapindaceae | Dimocarpus longan | Longan | China Thailand Malaysia | 6 | Api, Chr, Lut, Gal Kaem Myr Quer Rut, Nar, Narg, Pinoc, C | 3,4-DMCA, CA, ChloA, FA, p-CouA, RosA, t-CA | BenA, ElA, GA, GenA, ProA, p-HBA, ResA, SyrA, VA | Prod | N.I. | 29 | [43,64,77,135,143] |
| 153. | Sapindaceae | Guioa semiglauca | Crow ash | Australia | 1 | N.I. | ChloA, CA, FA, p-CouA | ElA, GA, SyrA | N.I. | AbsA | 8 | [58] |
| 154. | Sapindaceae | Litchi chinensis | Litchi | China, India | 2 | Api, Chr, Lut, Gal Kaem Myr Quer Rut, Pinoc, C, EC, Proc | 3,4-DMCA, CA, FA, SinA | GA, ProA, p-HBA, VA | 4-MPC | N.I. | 21 | [64,137] |
| 155. | Schisandraceae | Schisandra chinensis | Schisandra | China | 2 | Api, Chr, Lut, Gal, Isor, Kaem, Quer, Rut, Pinoc | 3,4-DMCA, CA, FA, IfA, p-CouA, SinA | BenA, GA, ProA, p-HBA, SyrA, VA | N.I. | N.I. | 21 | [64,96] |
| 156. | Simaroubaceae | Ailanthus altissima | Tree of heaven | Italy | 1 | Api, Chr, Gal, Kaem, Myr, Quer, Gene | CA, ChloA, p-CouA | N.I. | N.I. | N.I. | 10 | [97] |
| 157. | Solanaceae | Lycium sp. | Wolfberry | China | 1 | Quer, Hesd | CA, ChloA | 2,3,4-THBA, GA, GenA, p-HBA | Prod, Van | N.I. | 10 | [43] |
| 158. | Zingiberaceae | Zingeber officinale | Ginger | India | 1 | Kaem, Quer, Rut, C, EC | CA, ChloA, FA | GA, SyrA, VA | Gin | N.I | 12 | [137] |
| 159. | Zygophyllaceae | Zygophyllum album L. | Zygophyllum | Algeria | 1 | Quer, Rut | CA, ChloA, p-CouA | GA, VA | Van | N.I | 8 | [158] |
| R5 | R6 | R7 | R8 | R2′ | R3′ | R4′ | R5′ | R6′ | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -O-H | -H | -O-Me | -H | -H | -H | -O-Me | -H | -H | Acacetin | 1 | 480-44-4 | 15 |
| -O-H | -H | -O-H | -H | -H | -H | -O-H | -H | -H | Apigenin | 2 | 520-36-5 | 74 |
| -O-H | -H | -O-Glc | -H | -H | -H | -H | -H | -H | Baicalin | 3 | 21967-41-9 | 6 |
| -O-H | -H | -O-H | -H | -H | -H | -H | -H | -H | Chrysin | 4 | 480-40-0 | 83 |
| -O-H | -H | -O-H | -H | -O-Me | -H | -O-H | -H | -H | Chrysin-2′-methylether | 5 | 10458-35-2 | 6 |
| -O-H | -O-Me | -O-H | -H | -H | -H | -H | -H | -O-H | Chrysin-6-methylether | 6 | 480-11-5 | 5 |
| -O-H | -H | -O-Me | -H | -H | -H | -O-H | -H | -H | Genkwanin | 7 | 437-64-9 | 6 |
| -O-H | -H | -O-H | -H | -H | -O-H | -O-H | -H | -H | Luteolin | 8 | 491-70-3 | 69 |
| -O-Me | -O-Me | -O-Me | -O-Me | -H | -H | -O-Me | -H | -H | Tangerin | 9 | 481-53-8 | 1 |
| -O-H | -H | -O-Me | -H | -H | -H | -H | -H | -H | Tectochrysin | 10 | 520-28-5 | 16 |
| -O-H | -H | -O-H | -H | -H | -O-H | -O-H | -O-H | -H | Tricetin | 11 | 520-31-0 | 5 |
| -O-H | -H | -O-H | -Glc | -H | -H | -O-H | -H | -H | Vitexin | 12 | 3681-93-4 | 10 |
| R3 | R5 | R7 | R8 | R2′ | R3′ | R4′ | R5′ | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|---|---|---|---|---|---|---|
| -O-H | -H | -O-H | -H | -H | -O-H | -O-H | -H | Fisetin | 13 | 528-48-3 | 6 |
| -O-H | -O-H | -O-H | -H | -H | -H | -H | -H | Galangin | 14 | 548-83-4 | 66 |
| -O-H | -O-Me | -O-H | -H | -H | -H | -H | -H | Galangin-5-methyl ether | 15 | 104594-69-6 | 5 |
| -O-Me | -O-H | -O-H | -H | -H | -H | -H | -H | Galangin-3-methyl ether | 16 | 6665-74-3 | 3 |
| -O-H | -O-H | -O-H | -H | -H | -O-Me | -O-H | -H | Isorhamnetin | 17 | 480-19-3 | 43 |
| -O-H | -O-H | -O-H | -H | -H | -O-Me | -O-diGlc | -H | Isorhamnetin-4′-diglucoside | 18 | N.I. | 2 |
| -O-H | -O-H | -O-H | -H | -H | -O-Me | -O-Gen | -H | Isorhamnetin-4′—gentiobioside | 19 | N.I. | 2 |
| -O-H | -O-H | -O-H | -H | -H | -O-Me | -O-Glc | -H | Isorhamnetin-4′-Glc | 20 | N.I. | 2 |
| -O-Glc | -O-H | -O-H | -H | -H | -O-Me | -O-Gen | -H | isorhamnetin-3-Glc-4’-gentiobioside | 21 | N.I. | 2 |
| -O-H | -O-H | -O-H | -H | -H | -H | -O-H | -H | Kaempferol | 22 | 520-18-3 | 89 |
| -O-H | -O-H | -O-H | -H | -H | -H | -O-H | -H | Kaempferol-*-methylether | 23 | N.I. | 3 |
| -O-H | -O-H | -O-H | -O-Me | -H | -H | -O-H | -H | Kaempferol-8-methylether | 24 | 571-74-4 | 22 |
| -O-H | -O-H | -O-Rham | -H | -H | -H | -O-H | -H | Kaempferol-7-O-rhamnoside | 25 | N.I. | 6 |
| -O-H | -O-H | -O-H | -H | -H | -H | -O-H | -H | Kaempferol3-O-(6”-acetyl)-beta-glucopyranoside | 26 | N.I. | 3 |
| -O-(hexoxyl) rob | -O-H | -O-Rham | -H | -H | -H | -O-H | -H | Kaempferol-3-O-(hexoxyl) robinoside-7-O-rhamnoside | 27 | N.I. | 1 |
| -O-(hexoxyl) rob | -O-H | -O-H | -H | -H | -H | -O-H | -H | Kaempferol-3-O-(hexoxyl)robinoside | 28 | N.I. | 1 |
| -O-hex | -O-H | -O-Rham | -H | -H | -H | -O-H | -H | kaempferol-3-O-hexoside-7-O-rhamnoside | 29 | N.I. | 1 |
| -O-rob | -O-H | -O-Rham | -H | -H | -H | -O-H | -H | Kaempferol-3-O-robinoside-7-O-rhamnoside | 30 | N.I. | 1 |
| -O-Rob | -O-H | -O-H | -H | -H | -H | -O-H | -H | Kaempferol-3-O-robinoside | 31 | N.I. | 1 |
| -O-diGlc | -O-H | -O-H | -H | -H | -H | -O-H | -H | Kaempferol-3-diGlc isomer | 32 | N.I. | 2 |
| -O-Soph | -O-H | -O-H | -H | -H | -H | -O-H | -H | Kaempferol-3-sophoroside | 33 | N.I. | 2 |
| -O-H | -O-H | -O-H | -H | -H | -H | -O-Glc | -H | Kaempferol-4’-Glc | 34 | N.I. | 2 |
| -O-Neoh | -O-H | -O-H | -H | -H | -H | -O-H | -H | Kaempferol-3-O- neohespeidoside | 35 | N.I. | 3 |
| -O-H | -O-H | -O-H | -H | -H | -H | -O-Me | -H | Kaempferide | 36 | 491-54-3 | 7 |
| -O-H | -O-H | -O-H | -H | -O-H | -H | -O-H | -H | Morin | 37 | 480-16-0 | 7 |
| -O-H | -O-H | -O-H | -H | -H | -O-H | -O-H | -O-H | Myricetin | 38 | 529-44-2 | 54 |
| -O-Me | -O-H | -O-H | -H | -H | -O-H | -O-H | -O-H | Myricetin-3-methyl ether | 39 | 1486-67-5 | 1 |
| -O-Me | -O-H | -O-Me | -H | -H | -O-Me | -O-Me | -O-H | Myricetin-3,7,4′5′-tetramethyl ether | 40 | 14290-57-4 | 6 |
| -O-H | -O-H | -O-H | -H | -H | -O-H | -O-H | -H | Quercetin | 41 | 117-39-5 | 102 |
| -O-Me | -O-H | -O-H | -H | -H | -O-Me | -O-H | -H | Quercetin-3,3-dimethyl ether | 42 | 4382-17-6 | 8 |
| -O-Me | -O-H | -O-Me | -H | -H | -O-H | -O-H | -H | Quercetin-3,7-dimethyl ether | 43 | 2068-02-2 | 10 |
| -O-Me | -O-H | -O-H | -H | -H | -O-H | -O-H | -H | Quercetin-3-methyl ether | 44 | 1486-70-0 | 15 |
| -O-Glc | -O-H | -O-H | -H | -H | -O-Glc | -O-Glc | -H | Quercetin-3,3’,4’-triGlc | 45 | N.I. | 2 |
| -O-Glc | -O-H | -O-H | -H | -H | -O-H | -O-Glc | -H | Quercetin-3,4’-diGlc | 46 | N.I. | 2 |
| -O-Soph | -O-H | -O-H | -H | -H | -O-H | -O-H | -H | Quercetin-3-sophoroside | 47 | N.I. | 2 |
| -O-hex (1→2) hex | -O-H | -O-H | -H | -H | -O-H | -O-H | -H | Quercetin-3-O-hex (1→2) hex | 48 | N.I. | 2 |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Quercitin diglycoside | 49 | N.I. | 1 |
| N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Quercitin rhamnoside | 50 | N.I. | 7 |
| -O-H | -O-H | -O-Me | -H | -H | -O-Me | -O-H | -H | Quercetin-7,3’ dimethyl ether | 51 | 552-54-5 | 1 |
| -O-Rham | -O-H | -O-H | -H | -H | -O-H | -O-H | -H | Quercitrin | 52 | 522-12-3 | 16 |
| -O-H | -O-H | -O-Me | -H | -H | -O-H | -O-H | -H | Rhamnetin | 53 | 90-19-7 | 9 |
| -O-Rut | -O-H | -O-H | -H | -H | -O-H | -O-H | -H | Rutin | 54 | 153-18-4 | 58 |
| R5 | R7 | R3′ | R4′ | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|---|---|---|
| -O-Me | -O-H | -H | -H | Alpinetin | 55 | 36052-37-6 | 1 |
| -O-H | -O-H | -O-H | -O-H | Eriodictyol | 56 | 552-58-9 | 5 |
| -O-H | -O-H | -O-H | -O-Me | Hesperitin | 57 | 520-33-2 | 49 |
| -O-H | -O-Rut | -O-H | -O-Me | Hesperidin | 58 | 520-26-3 | 14 |
| -O-H | -O-H | -H | -O-Me | Isosakuranetin | 59 | 480-43-3 | 18 |
| -O-H | -O-H | -H | -O-H | Naringenin | 60 | 67604-48-2 | 54 |
| -O-H | -O-H | -H | -O-H | Naringenin-?-methyl ether | 61 | N.I. | 1 |
| -O-H | -O-Rut | -H | -O-H | Naringin | 62 | 10236-47-2 | 9 |
| -O-H | -O-H | -H | -H | Pinocembrin | 63 | 480-39-7 | 64 |
| -O-H | -O-Me | -H | -H | Pinostrobi | 64 | 480-37-5 | 9 |
| -O-H | -O-Me | -H | -O-H | Sakuranetin | 65 | 2957-21-3 | 8 |
| R3 | R5 | R3′ | R4′ | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|---|---|---|
| -O-H | -O-H | -H | -H | Pinobanksin | 66 | 548-82-3 | 49 |
| -O-Ace | -O-H | -H | -H | Pinobanksin-3-O-acetate | 67 | 52117-69-8 | 5 |
| -O-But | -O-H | -H | -H | Pinobanksin-3-O-butyrate | 68 | 126394-71-6 | 1 |
| -O-Pent | -O-H | -H | -H | Pinobanksin-3-O-pentenoate | 69 | N.I. | 1 |
| -O-Prop | -O-H | -H | -H | Pinobanksin-3-O-propionate | 70 | 126394-70-5 | 1 |
| -O-H | -O-Me | -H | -H | Pinobanksin-5-methyl ether | 71 | 87620-04-0 | 7 |
| -O-H | -O-H | -O-H | -O-H | Taxifolin | 72 | 480-18-2 | 8 |
| R3 | R5′ | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|---|
| -O-H | -H | Catechin | 73 | 154-23-4 | 29 |
| -O-Gall | -H | Catechin gallate | 74 | 130405-40-2 | 12 |
| -O-H | -H | Epicatechin | 75 | 490-46-0 | 24 |
| -O-H | -O-H | Epigallocatechin | 76 | 970-74-1 | 5 |
| -O-Gall | -O-H | Epigallocatechin gallate | 77 | 989-51-5 | 1 |
| -O-H | -O-H | Gallocatechin | 78 | 970-73-0 | 9 |
| -O-Gall | -O-H | Gallocatechin gallate | 79 | 4233-96-9 | 1 |
| N/A | N/A | ‘Procyanidin’ # | 80 | 4852-22-6 | 1 |
| R5 | R7 | R3′ | R4′ | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|---|---|---|
| -H | -O-H | -O-H | -O-Me | Calycosin | 81 | 20575-57-9 | 3 |
| -H | -O-Glc | -O-H | -O-Me | Calycosin-7-O-β-D-glucoside | 82 | 20633-67-4 | 3 |
| -H | -O-H | -O-H | -H | Daidzein | 83 | 486-66-8 | 5 |
| -H | -O-H | -H | -O-Me | Formononetin | 84 | 485-72-3 | 8 |
| -O-H | -O-H | -H | -O-H | Genistein | 85 | 446-72-0 | 23 |
| -O-H | -O-Glc | -H | -O-H | Genistin | 86 | 529-59-9 | 2 |
| -H | -O-Glc | -H | -O-Me | Ononin | 87 | 486-62-4 | 6 |
| Subclass | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|
| Aurone | Leptosin | 88 | 486-23-7 | 5 |
| Chalcone | Pinobanksin chalcone | 89 | N.I. | 6 |
| OR1 | R5 | R6 | R7 | R8 | Name | Code | CAS No. | No of Honeys |
|---|---|---|---|---|---|---|---|---|
| -H | -H | -O-Me | -O-Me | -H | 3,4 Dimethoxycinnamic acid | 90 | 2316-26-9 | 11 |
| -H | -H | -O-H | -O-H | -H | Caffeic acid | 91 | 331-39-5 | 117 |
| -benzyl | -H | -O-H | -O-H | -H | Caffeic acid benzyl ester | 92 | 107843-77-6 | 1 |
| -dimethylallyl | -H | -O-H | -O-H | -H | Caffeic acid dimethylallyl ester | 93 | 100884-13-7 | 8 |
| -isoprenyl | -H | -O-H | -O-H | -H | Caffeic acid isoprenyl ester | 94 | N.I. | 2 |
| -phenyl | -H | -O-H | -O-H | -H | Caffeic acid phenethyl ester | 95 | 104594-70-9 | 17 |
| -QA (3) | -H | -O-H | -O-H | -H | Chlorogenic acid | 96 | 327-97-9 | 85 |
| -QA (4) | -H | -O-H | -O-H | -H | Cryptochlorogenic acid | 97 | 905-99-7 | 7 |
| -H | -H | -O-Me | -O-H | -H | Ferulic acid | 98 | 537-98-4 | 102 |
| -H | -H | -O-H | -O-Me | -H | Isoferulic acid | 99 | 537-73-5 | 12 |
| -H | -H | -O-H | -H | -H | m-Coumaric acid | 100 | 14755-02-3 | 10 |
| -H | -H | -O-Me | -H | -H | m-Methoxycinnamic acid | 101 | 6099-04-3 | 1 |
| -Me | -H | -O-Me | -O-H | -H | Methyl ferulate | 102 | 2309-07-1 | 3 |
| -H | -O-H | -H | -H | -H | o-Coumaric acid | 103 | 614-60-8 | 23 |
| -H | -H | -H | -O-H | -H | p-Coumaric acid | 104 | 501-98-4 | 103 |
| -H | -H | -H | -O-Me | -H | p-Methoxycinnamic acid | 105 | 830-09-1 | 2 |
| -3,4-DHPLA | -H | -O-H | -O-H | -H | Rosmarinic acid | 106 | 20283-92-5 | 25 |
| -H | -H | -O-Me | -O-H | -Me | Sinapic acid | 107 | 530-59-6 | 27 |
| -H | -H | -H | -H | -H | t-Cinnamic acid | 108 | 140-10-3 | 56 |
| -Me | -H | -H | -O-H | -H | trans-p-Coumaric acid methyl ester | 109 | 19367-38-5 | 1 |
| OR1 | R3 | R4 | R5 | R6 | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|---|---|---|---|
| -H | -O-H | -O-H | -O-H | -H | 2,3,4 Trihydrobenzoic acid | 110 | 610-02-6 | 12 |
| -H | -H | -O-Me | -O-Me | -O-Me | 3,4,5-Trimethoxybenzoic acid | 111 | 118-41-2 | 4 |
| -H | -H | -H | -H | -H | Benzoic acid | 112 | 65-85-0 | 43 |
| -H | -H | -H | -Isopropyl | -H | Cuminic acid | 113 | 536-66-3 | 4 |
| See Figure 16 | Ellagic acid | 114 | 476-66-4 | 45 | ||||
| -H | -H | -O-H | -O-H | -O-H | Gallic acid | 115 | 149-91-7 | 105 |
| -H | -O-H | -H | -H | -O-H | Gentisic acid | 116 | 490-79-9 | 35 |
| -Me | -H | -O-Me | -O-gent | -O-Me | Leptosperin | 117 | N.I | 2 |
| -Me | -H | -H | -O-H | -H | Methyl 4-hydroxybenzoate | 118 | 99-76-3 | 6 |
| -Me | -H | -O-Me | -O-H | -O-Me | Methyl syringate | 119 | 884-35-5 | 23 |
| -H | -H | -O-H | -H | -H | m-Hydroxybenzoic acid | 120 | 99-06-9 | 14 |
| -H | -O-Me | -H | -H | -H | o-Anisic acid | 121 | 579-75-9 | 6 |
| -H | -H | -H | -O-Me | -H | p-Anisic acid | 122 | 100-09-4 | 6 |
| See Figure 16 | Penta-O-galloyl-β-D-glucose (PGG) | 123 | 14937-32-7 | 1 | ||||
| -H | -H | -O-H | -O-H | -H | Protocatechuic acid | 124 | 99-50-3 | 57 |
| -H | -H | -H | -O-H | -H | p-Hydroxybenzoic acid | 125 | 99-96-7 | 79 |
| -H | -O-H | -O-H | -H | -H | Resorcylic acid | 126 | 303-38-8 | 13 |
| -H | -O-H | -H | -H | -H | Salicylic acid | 127 | 69-72-7 | 20 |
| -H | -H | -O-Me | -O-H | -O-Me | Syringic acid | 128 | 530-57-4 | 85 |
| -H | -H | -O-Me | -O-H | -H | Vanillic acid | 129 | 121-34-6 | 66 |
| -Me | -H | -O-Me | -O-H | -H | Vanillic acid methyl ester | 130 | 3943-74-6 | 7 |
| R2b | R5 | R6 | R7 | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|---|---|---|
| -H | -H | -O-H | -O-H | 3,4-Dihydroxyphenylacetic acid | 131 | 102-32-9 | 10 |
| -H | -O-H | -H | -O-H | Homogentisic acid | 132 | 451-13-8 | 24 |
| -H | -O-Me | -O-H | -H | Homovanillic acid | 133 | 306-08-1 | 4 |
| -O-H | -H | -H | -H | Mandelic acid | 134 | 90-64-2 | 3 |
| -H | -H | -H | -H | Phenylacetic acid | 135 | 103-82-2 | 17 |
| -H | -H | -O-H | -H | p-Hydroxyphenylacetic acid | 136 | 156-38-7 | 17 |
| R2b | R7 | Subclass | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|---|---|
| -O-H | -O-H | HPLAD | DL-p hydroxyphenyllactic acid | 137 | 306-23-0 | 4 |
| -O-H | -O-Me | HPLAD | 4-Methoxyphenyllactic acid | 138 | N.I. | 7 |
| -O-H | -H | HPLAD | DL-β-Phenyllactic acid | 139 | 828-01-3 | 23 |
| -H | -H | HPPAD | 3-Phenyl propionic acid | 140 | 501-52-0 | 13 |
| -H | -O-H | HPPAD | Phloretic acid | 141 | 501-97-3 | 3 |
| Subclass | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|
| HPPeA | 5-Phenylpent-4-enoic acid | 142 | 306-23-0 | 5 |
| R1 | R2 | R3 | R4 | R5 | Subclass | Name | Code | CAS No. | No. of Honeys |
| -O-H | -O-Me | -H | -vinyl | -H | AMPh | 2-Methoxy-4-vinylphenol | 143 | 7786-61-0 | 6 |
| -O-H | -Me | -Me | -H | -Me | APh | 2,3,5-Trimethyl phenol | 144 | 697-82-5 | 3 |
| -O-H | -H | -Me | -Me | -Me | APh | 3,4,5-Trimethyl phenol | 145 | 527-54-8 | 3 |
| -O-H | -O-H | -H | -H | -Me | APh | 4-Methylpyrocatechol | 146 | 452-86-8 | 5 |
| -C(=O)H | -Me | -H | -H | -H | HBzd | 2-Methylbenzaldehyde | 147 | 529-20-4 | 6 |
| -C(=O)H | -H | -H | -O-Me | -H | HBzd | p-Anisaldehyde | 148 | 123-11-5 | 2 |
| -C(=O)H | -H | -O-H | -O-H | -H | HBzd | Protocatechualdehyde | 149 | 139-85-5 | 40 |
| -C(=O)H | -H | -O-Me | -O-H | -H | HBzd | Vanillin | 150 | 121-33-5 | 18 |
| -ethanone | -H | -O-Me | -H | -H | HAPhn | 1-(3-Methoxy-phenyl)-ethanone | 151 | 586-37-8 | 1 |
| - ethanone | -O-H | -H | -H | -H | HAPhn | 2’-Hydroxyacetophenone | 152 | 118-93-4 | 1 |
| - ethanone | -O-Me | -H | -H | -H | HAPhn | 2’-Methoxyacetophenone | 153 | 579-74-8 | 3 |
| -5-hydroxydeca-3-one | -H | -O-Me | -O-H | -H | Guaiacol | Gingerol | 154 | 23513-14-6 | 1 |
| - butan-1-one | -NH2 | -H | -H | -H | Others | 1-(2-Aminophenyl)butan-1-one | 155 | 2034-40-4 | 1 |
| -3-hydroxy-penta-1,4-dione | -O-Me | -H | -H | -H | Others | 3-Hydroxy-1-(2-methoxyphenyl)penta-1,4-dione | 156 | N.I. | 1 |
| Subclass | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|
| Hydroxycoumarin | 5,7-Dimethoxycoumarin | 157 | 487-06-9 | 5 |
| Anthraquinone | Emodin | 158 | 518-82-1 | 1 |
| Naphthoquinone | Nor-β-lapachone | 159 | 52436-88-1 | 1 |
| Stilbenes | Resveratrol | 160 | 501-36-0 | 1 |
| Oxalate ester | Dibenzyl oxalate | 161 | 7579-36-4 | 5 |
| Subclass | Name | Code | CAS No. | No. of Honeys |
|---|---|---|---|---|
| Non-Phenolics | 2-Methylbenzofuran | 162 | 4265-25-2 | 1 |
| Non-Phenolics | 5-Methylfuran-3-carboxylic acid | 163 | 21984-93-0 | 3 |
| Non-Phenolics | Absiscic acid | 164 | 21293-29-8 | 36 |
| Non-Phenolics | Kojic acid | 165 | 501-30-4 | 3 |
| Non-Phenolics | Kynurenic acid | 166 | 492-27-3 | 4 |
| Non-Phenolics | Lepteridine | 167 | N.I. | 6 |
| Non-Phenolics | Lumichrome | 168 | 1086-80-2 | 6 |
| Non-Phenolics | Phenylalanine | 169 | 63-91-2 | 5 |
| Non-Phenolics | Tyrosin | 170 | 556-03-6 | 5 |
| Method | No. of Reports |
|---|---|
| Fluorescence Spectroscopy | 1 |
| GC–MS | 6 |
| HPLC (UV, UV–UV, UV–Vis, DAD/PDA, DAD–UV, ECD, ECD–UV) | 373 |
| HPLC–DAD and LC–MS | 23 |
| HPLC–DAD and LC–MS and GC–MS | 3 |
| HPTLC | 6 |
| LC–MS | 136 |
| N.I. * | 8 |
| Total | 556 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawag, I.L.; Lim, L.-Y.; Joshi, R.; Hammer, K.A.; Locher, C. A Comprehensive Survey of Phenolic Constituents Reported in Monofloral Honeys around the Globe. Foods 2022, 11, 1152. https://doi.org/10.3390/foods11081152
Lawag IL, Lim L-Y, Joshi R, Hammer KA, Locher C. A Comprehensive Survey of Phenolic Constituents Reported in Monofloral Honeys around the Globe. Foods. 2022; 11(8):1152. https://doi.org/10.3390/foods11081152
Chicago/Turabian StyleLawag, Ivan Lozada, Lee-Yong Lim, Ranee Joshi, Katherine A. Hammer, and Cornelia Locher. 2022. "A Comprehensive Survey of Phenolic Constituents Reported in Monofloral Honeys around the Globe" Foods 11, no. 8: 1152. https://doi.org/10.3390/foods11081152
APA StyleLawag, I. L., Lim, L.-Y., Joshi, R., Hammer, K. A., & Locher, C. (2022). A Comprehensive Survey of Phenolic Constituents Reported in Monofloral Honeys around the Globe. Foods, 11(8), 1152. https://doi.org/10.3390/foods11081152









