Screening of Estrogenic-Disrupting Compounds in Dairy Products Based on the Estrogen Receptor Cocktail
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
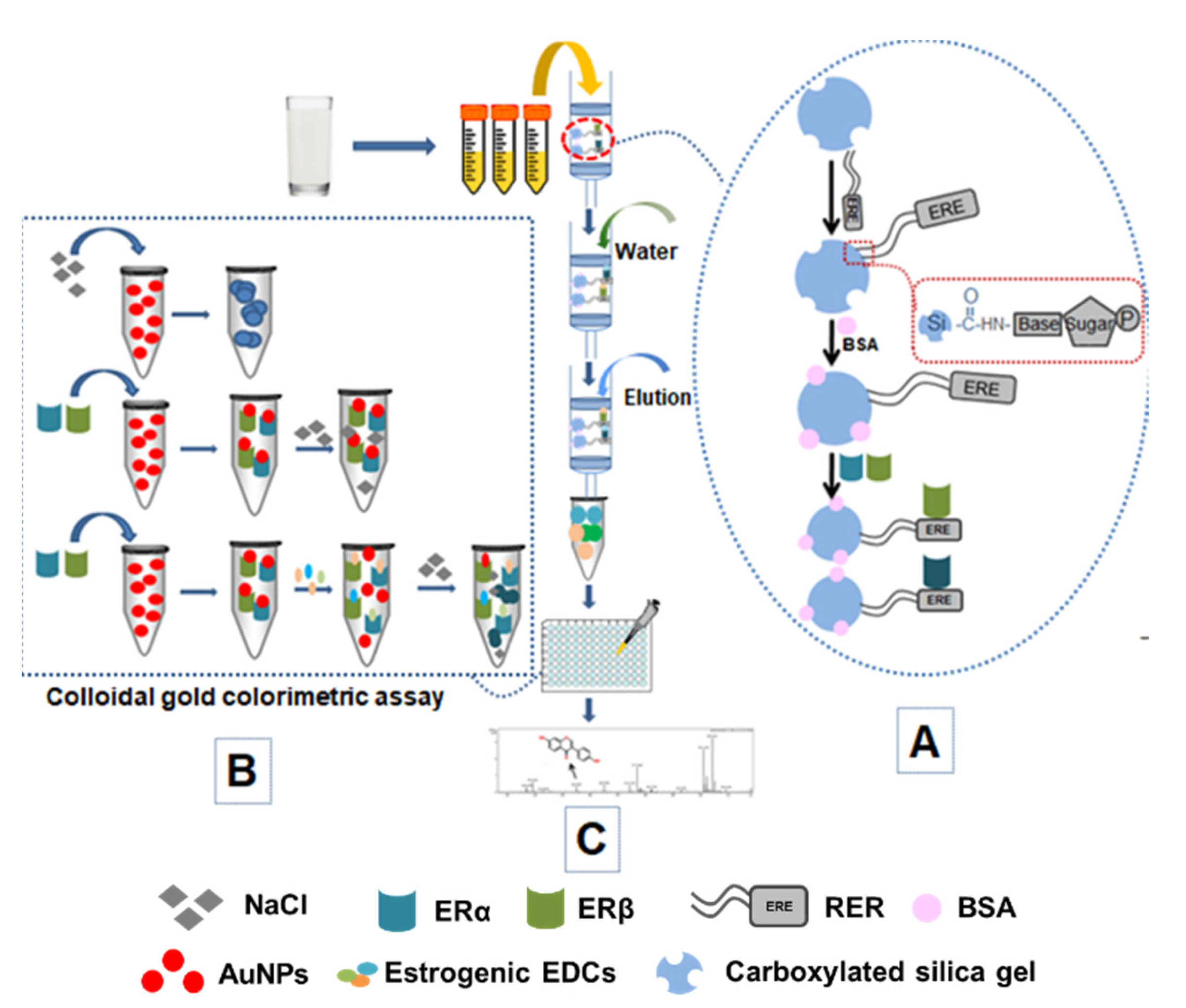
2.2. Preparation of SPE Column
2.3. Enrichment of EDCs with SPE Column
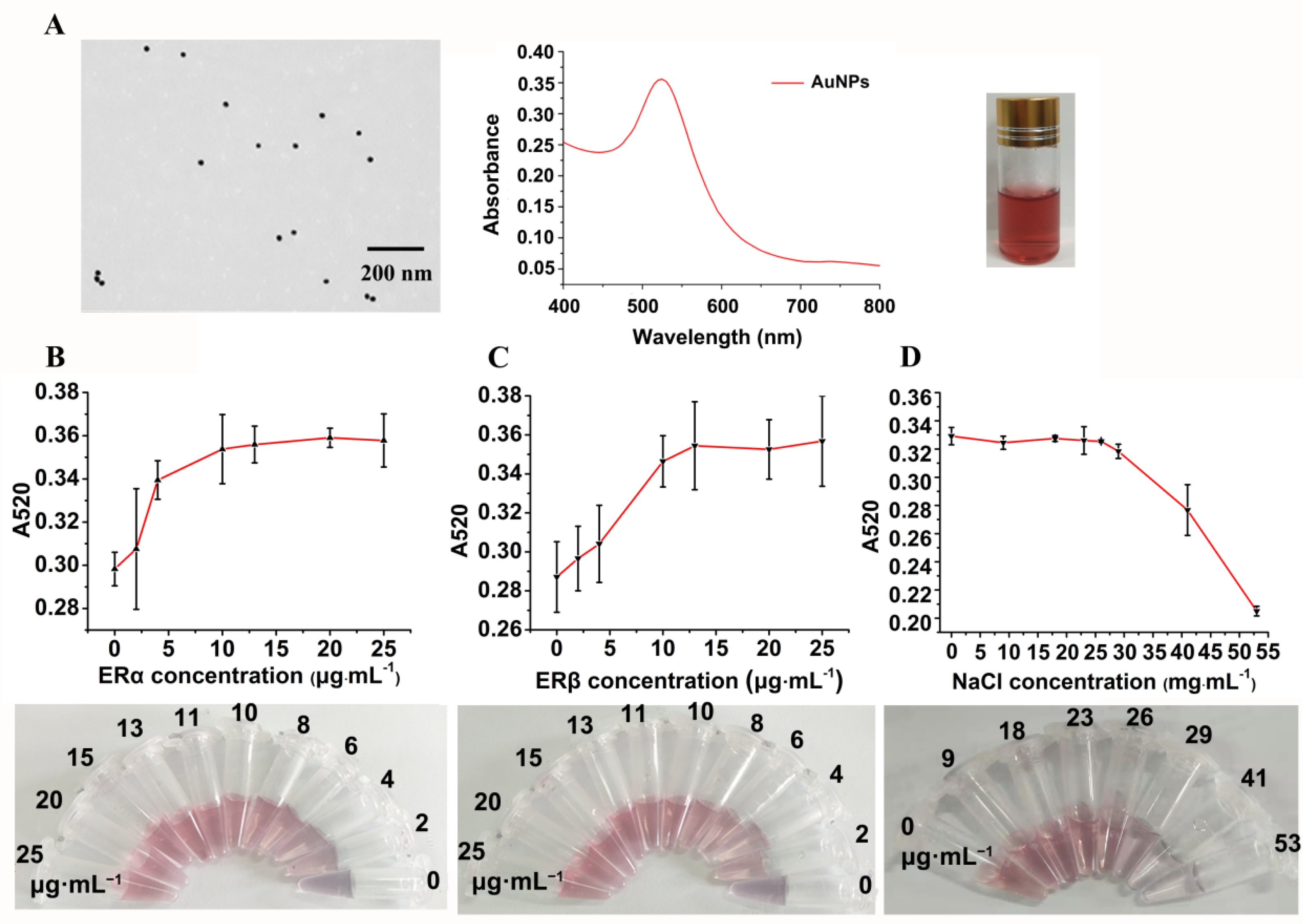
2.4. Preparation of AuNPs
2.5. Optimization of the Concentrations of ERα and ERβ
2.6. Preparation of ER Cocktail and Optimization of NaCl Concentration
2.7. Determination of EDCs by AuNPs-ERα/ERβ/ER Cocktail Colorimetric Assay
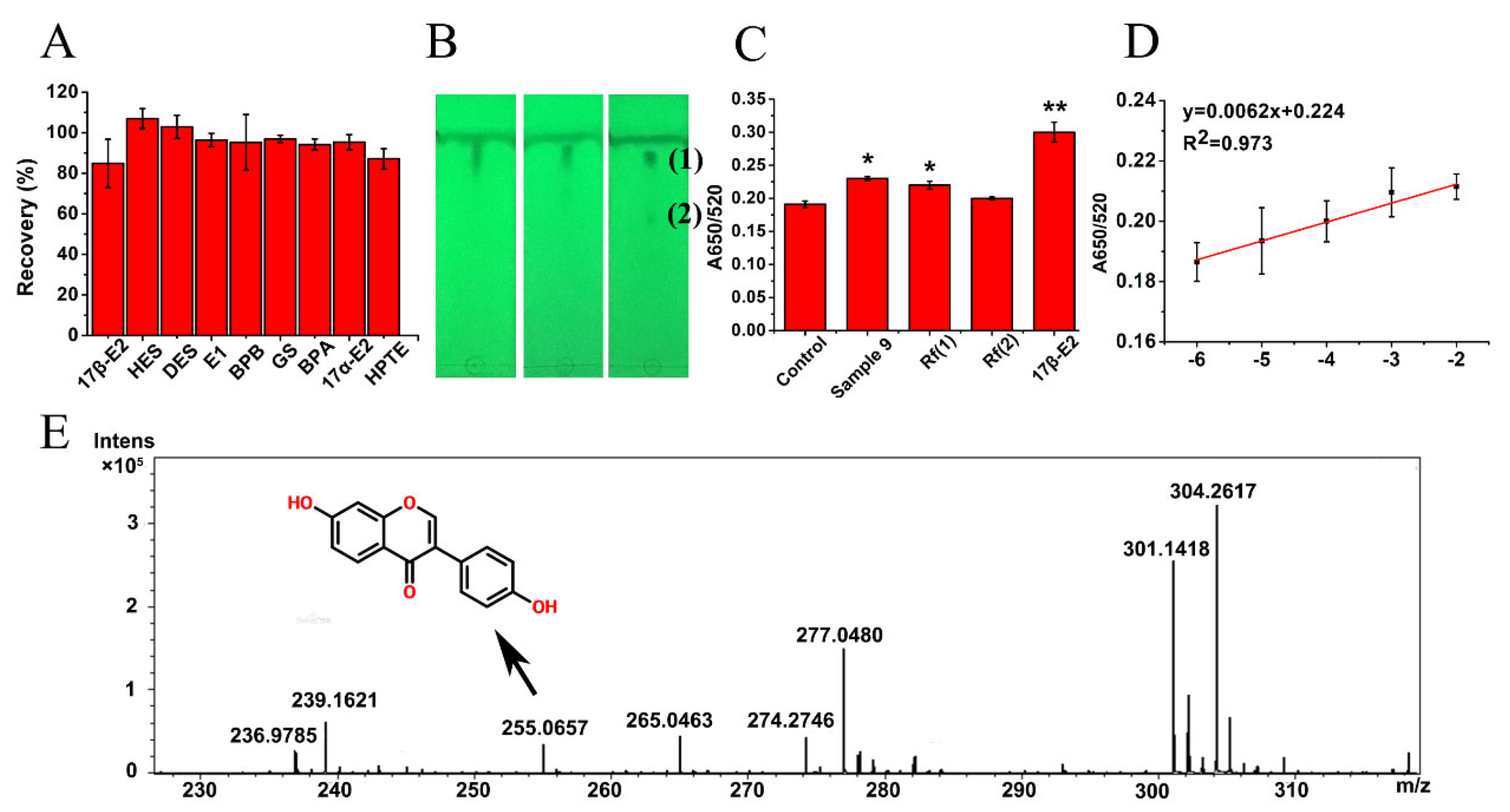
2.8. Enrichment and Detection of EDCs in Dairy Products
2.9. Isolation and Identification of EDCs
2.10. Data Analysis
3. Results and Discussion
3.1. Establishment of SPE Column for Sample Pretreatment
3.2. Characterization of Prepared AuNPs
3.3. Establishment of AuNPs-ER Cocktail Colorimetric Assay
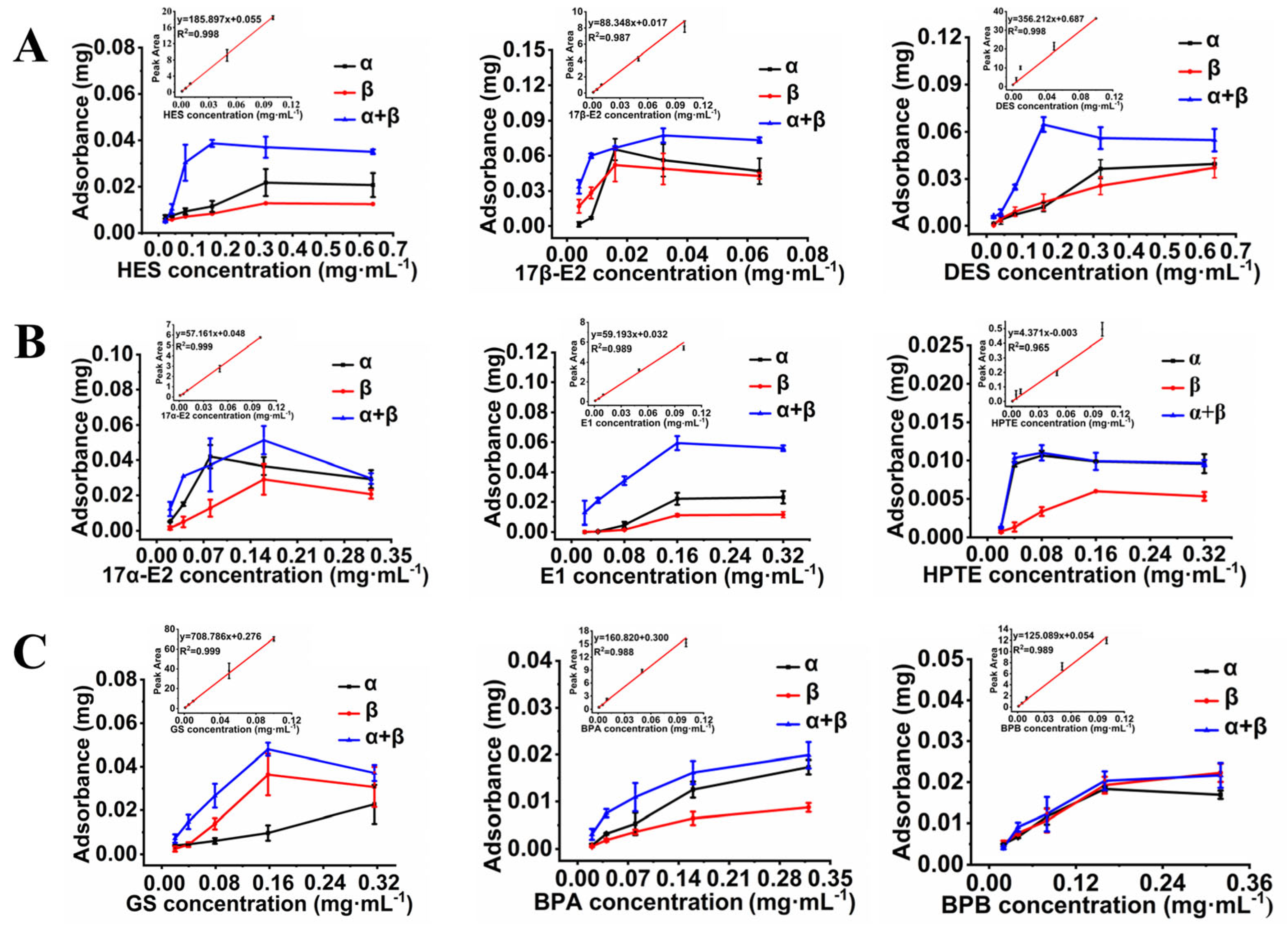
3.4. The Efficiency of AuNPs-ER Cocktail Colorimetric Assay for EDC Detection
3.5. Performance Assessment of the AuNPs-ER Cocktail Colorimetric Assay Equipped with an ER Cocktail SPE Column
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDCs | estrogenic-disrupting compounds |
| AuNPs | gold nanoparticles |
| ER | estrogen receptor |
| SPE | solid phase extraction |
| E1 | estrone |
| GS | genistein |
| ELRA | enzyme-linked receptor assay |
| ELISA | enzyme-linked immunosorbent assay |
| HPLC | high-performance liquid chromatography |
| ERE | estrogen response element |
| DES | diethylstilbestrol |
| 17α-E2 | 17α-estradiol |
| HES | hexestrol |
| 17β-E2 | 17β-estradiol |
| HPTE | 2,2-bis (4-hydroxyphenyl)-1,1,1-trichloroethane |
| BPA | bisphenol A |
| BSA | bovine serum albumin |
| TLC | thin layer chromatography |
| SE | standard error |
| SI | soybean isoflavones |
References
- Roy, J.R.; Chakraborty, S.; Chakraborty, T.R. Estrogen-like endocrine disrupting chemicals affecting puberty in humans—A review. Med. Sci. Monit. 2009, 15, 137–145. [Google Scholar] [CrossRef]
- Pinto, P.I.; Estêvão, M.D.; Power, D.M. Effects of estrogens and estrogenic disrupting compounds on fish mineralized tissues. Mar. Drugs 2014, 12, 4474–4494. [Google Scholar] [CrossRef] [Green Version]
- Socas-Rodriguez, B.; Lankova, D.; Urbancova, K.; Krtkova, V.; Hernandez Borges, J.; Angel Rodriguez Delgado, M.; Pulkrabova, J.; Hajslova, J. Multiclass analytical method for the determination of natural/synthetic steroid hormones, phytoestrogens, and mycoestrogens in milk and yogurt. Anal. Bioanal. Chem. 2017, 409, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, H.B.; Huang, Z.B.; Sun, D.W.; Fu, H.H. Recent advances in the detection of 17β-estradiol in food matrices: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2144–2157. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.D.; Lee, H.A.; Morgan, M.R.A. A nonisotopic estrogen receptor–based assay to detect estrogenic compounds. Nat. Biotechnol. 1999, 17, 1219–1222. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, S.H.; Kim, D.; Cho, S.W.; Son, A.; Yoon, M.Y. Detection of nonylphenol with a gold-nanoparticle-based small-molecule sensing system using an ssDNA aptamer. Int. J. Mol. Sci. 2019, 27, 208. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.Y.; Zhang, S.Q.; Du, S.Y.; Pang, S.X.; Liu, X.Y.; Zhang, H.Y. A high throughput screening method for endocrine disrupting chemicals in tap water and milk samples based on estrogen receptor α and gold nanoparticles. Anal. Methods 2020, 12, 200–204. [Google Scholar] [CrossRef]
- Song, W.; Zhao, L.; Sun, Z.; Yang, X.; Zhou, Q.; Jian, G. A novel high throughput screening assay for binding affinities of perfluoroalkyl iodide for estrogen receptor alpha and beta isoforms. Talanta 2017, 175, 413–420. [Google Scholar] [CrossRef]
- Mayer-Wrangowski, S.C.; Rauh, D. Monitoring ligand-induced conformational changes for the identification of estrogen receptor agonists and antagonists. Angew. Chem. Int. Ed. Engl. 2015, 54, 4379–4382. [Google Scholar] [CrossRef]
- Brennan, J.C.; Bassal, A.; He, G.; Denison, M.S. Development of a recombinant human ovarian (BG1) cell line containing estrogen receptor alpha and beta for improved detection of estrogenic/antiestrogenic chemicals. Environ. Toxicol. Chem. 2016, 36, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangkak, G.; Bhattar, R.; Mittal, A.; Yadav, S.S.; Tomar, V.; Yadav, A.; Mehta, J. Immunohistochemical analysis of estrogen receptors in prostate and clinical correlation in men with benign prostatic hyperplasia. Investig. Clin. Urol. 2017, 58, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.H.; Zang, L.G.; Wang, T.; Wang, X.W.; Jia, M.; Zhang, D.; Zhang, H.Y. A solid-phase extraction method for estrogenic disrupting compounds based on the estrogen response element. Food Chem. 2020, 333, 127529. [Google Scholar] [CrossRef] [PubMed]
- Busayapongchai, P.; Siri, S. Sensitive detection of estradiol based on ligand binding domain of estrogen receptor and gold nanoparticles. Anal. Biochem. 2016, 518, 60–68. [Google Scholar] [CrossRef]
- Mcfarland, A.D.; Haynes, C.L.; Mirkin, C.A.; Van Duyne, R.P.; Godwin, H.A. Color My Nanoworld. J. Chem. Educ. 2004, 81, 544A. [Google Scholar] [CrossRef]
- Cheng, S.C.; Huang, M.Z.; Shiea, J. Thin layer chromatography mass spectrometry. J. Chromatogr. A 2011, 1218, 2700–2711. [Google Scholar] [CrossRef]
- Zhao, F.; Ye, N.; Qiu, X.H.; Qian, J.; Wang, D.H.; Yue, W.J.; Zuo, Z.C.; Chen, M.J. Identification and comparison of oligopeptides during withering process of White tea by ultra-high pressure liquid chromatography coupled with quadrupole-orbitrap ultra-high resolution mass spectrometry. Food Res. Int. 2019, 121, 825–834. [Google Scholar] [CrossRef]
- Seungkuk, A.; Christophe, O.C.; Herdeline, A.M.A.; Grant, M.G.; Patrick, H.C.; Kevin, K.P. Biomimetic and estrogenic fibers promote tissue repair in mice and human skin via estrogen receptor β. Biomaterials 2020, 55, 120149. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S.U.S.; Jang, S.P.; Lee, S.Y. Production of aqueous spherical gold nanoparticles using conventional ultrasonic bath. Nanoscale Res. Lett. 2012, 7, 420. [Google Scholar] [CrossRef] [Green Version]
- Sergio, D.M.; Jan, B.; Jana, O.; Christy, F.L.; Stephan, L. Adsorption of a Protein Monolayer via Hydrophobic Interactions Prevents Nanoparticle Aggregation under Harsh Environmental Conditions. ACS Sustain. Chem. Eng. 2013, 1, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; Mcneil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomedicine 2009, 5, 106–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterni, I.; Granchi, C.; Minutolo, F. Risks and benefits related to alimentary exposure to xenoestrogens. Crit. Rev. Food Sci. Nutr. 2017, 57, 3384–3404. [Google Scholar] [CrossRef] [PubMed]
- Sartippour, M.; Rao, J.; Apple, S.; Wu, D.; Henning, S.; Wang, H.; Elashoff, R.; Rubio, R.; Heber, D.; Brooks, M. A pilot clinical study of short-term isoflavone supplements in breast cancer patients. Nutr. Cancer 2004, 49, 59–65. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Du, S.; Tian, F.; Wang, M.; Wang, Y.; Zhang, H.; Zang, L. Screening of Estrogenic-Disrupting Compounds in Dairy Products Based on the Estrogen Receptor Cocktail. Foods 2022, 11, 1178. https://doi.org/10.3390/foods11091178
Li X, Du S, Tian F, Wang M, Wang Y, Zhang H, Zang L. Screening of Estrogenic-Disrupting Compounds in Dairy Products Based on the Estrogen Receptor Cocktail. Foods. 2022; 11(9):1178. https://doi.org/10.3390/foods11091178
Chicago/Turabian StyleLi, Xiaoqi, Shuyuan Du, Fangyuan Tian, Minglu Wang, Ying Wang, Hongyan Zhang, and Liguo Zang. 2022. "Screening of Estrogenic-Disrupting Compounds in Dairy Products Based on the Estrogen Receptor Cocktail" Foods 11, no. 9: 1178. https://doi.org/10.3390/foods11091178





