Polyphenols Recovery from Thymus serpyllum Industrial Waste Using Microwave-Assisted Extraction–Comparative RSM and ANN Approach for Process Optimization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Chemicals
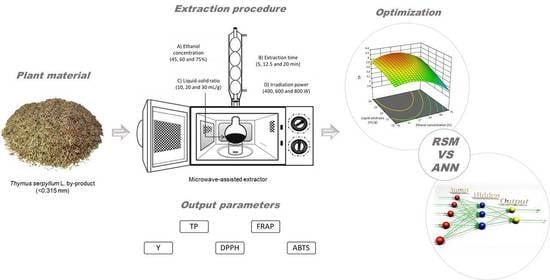
2.3. Microwave-Assisted Extraction (MAE)
2.4. Determination of Y and TP
2.5. Antioxidant Activity of Extracts
2.6. Q Exactive Hybrid Quadrupole-Orbitrap LC-MS/MS Analysis
2.7. Design of Experiments and Statistical Methods
2.7.1. Response Surface Methodology (RSM)
2.7.2. Artificial Neural Network (ANN)
3. Results and Discussion
3.1. Total Extraction Yield (Y) and Total Phenols Yield (TP)
3.2. Antioxidant Activity of T. serpyllum Extracts
3.3. RSM versus ANN Model Fitting
3.4. Process Optimization and Experimental Verification
3.5. Polyphenols Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid.-Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Ivasenko, S.; Orazbayeva, P.; Skalicka–wozniak, K.; Ludwiczuk, A.; Marchenko, A.; Ishmuratova, M.; Poleszak, E.; Korona-Glowniak, I.; Akhmetova, S.; Karilkhan, I.; et al. Antimicrobial Activity of Ultrasonic Extracts of Two Chemotypes of Thymus serpyllum L. of Central Kazakhstan and their Polyphenolic Profiles. Open Access Maced. J. Med. Sci. 2021, 9, 61–67. [Google Scholar] [CrossRef]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Naffati, A.; Vladić, J.; Pavlić, B.; Radosavljević, R.; Gavarić, A.; Vidović, S. Recycling of filter tea industry by-products: Application of subcritical water extraction for recovery of bioactive compounds from A. uva-ursi herbal dust. J. Supercrit. Fluids 2017, 121, 197–206. [Google Scholar] [CrossRef]
- Pavlić, B.; Naffati, A.; Hojan, T.; Vladić, J.; Zeković, Z.; Vidović, S. Microwave-assisted extraction of wild apple fruit dust—production of polyphenol-rich extracts from filter tea factory by-products. J. Food Process Eng. 2017, 40, e12508. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Vidaković, A.; Vidović, S.; Velićanski, A.; Versari, A.; Radosavljević, R.; Zeković, Z. Sage processing from by-product to high quality powder: I. Bioactive potential. Ind. Crops Prod. 2017, 107, 81–89. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Yahya, N.A.; Attan, N.; Wahab, R.A. An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds. Food Bioprod. Process. 2018, 112, 69–85. [Google Scholar] [CrossRef]
- Sparr Eskilsson, C.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Eskilsson, C.S.; Björklund, E.; Mathiasson, L.; Karlsson, L.; Torstensson, A. Microwave-assisted extraction of felodipine tablets. J. Chromatogr. A 1999, 840, 59–70. [Google Scholar] [CrossRef]
- Akhtar, I.; Javad, S.; Yousaf, Z.; Iqbal, S.; Jabeen, K. Microwave assisted extraction of phytochemicals an efficient and modern approach for botanicals and pharmaceuticals. Pak. J. Pharm. Sci. 2019, 32, 223–230. [Google Scholar]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabet, N.; Gilbert-López, B.; Madani, K.; Herrero, M.; Ibáñez, E.; Mendiola, J.A. Optimization of microwave-assisted extraction recovery of bioactive compounds from Origanum glandulosum and Thymus fontanesii. Ind. Crop. Prod. 2019, 129, 395–404. [Google Scholar] [CrossRef]
- Pavlić, B.; Kaplan, M.; Bera, O.; Oktem Olgun, E.; Canli, O.; Milosavljević, N.; Antić, B.; Zeković, Z. Microwave-assisted extraction of peppermint polyphenols—Artificial neural networks approach. Food Bioprod. Process. 2019, 118, 258–269. [Google Scholar] [CrossRef]
- Zeković, Z.; Pintać, D.; Majkić, T.; Vidović, S.; Mimica-Dukić, N.; Teslić, N.; Versari, A.; Pavlić, B. Utilization of sage by-products as raw material for antioxidants recovery—Ultrasound versus microwave-assisted extraction. Ind. Crops Prod. 2017, 99, 49–59. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of Total Phenolics with Phosphomolybdic- Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrkonjić, Ž.; Rakić, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Optimization of antioxidants recovery from wild thyme (Thymus serpyllum L.) by ultrasound-assisted extraction: Multi-response approach. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100333. [Google Scholar] [CrossRef]
- Simic, V.M.; Rajkovic, K.M.; Stojic, S.S.; Velic, D.T.; Nada, C.; Simic, V.M.; Karabegovic, I.T.; Lazic, M.L.; Simić, V.M.; Rajković, K.M.; et al. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Mrkonjić, Ž.; Rakić, D.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Pressurized-liquid extraction as an efficient method for valorization of Thymus serpyllum herbal dust towards sustainable production of antioxidants. Molecules 2021, 26, 2548. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Garofulić, I.E.; Jukić, M.; Penić, M.; Dent, M. The Influence of Microwave-Assisted Extraction on the Isolation of Sage (Salvia officinalis L.) Polyphenols. Food Technol. Biotechnol. 2012, 50, 377–383. [Google Scholar]
- Ranic, M.; Nikolic, M.; Pavlovic, M.; Buntic, A.; Siler-Marinkovic, S.; Dimitrijevic-Brankovic, S. Optimization of microwave-assisted extraction of natural antioxidants from spent espresso coffee grounds by response surface methodology. J. Clean. Prod. 2014, 80, 69–79. [Google Scholar] [CrossRef]
- Teslić, N.; Bojanić, N.; Rakić, D.; Takači, A.; Zeković, Z.; Fišteš, A.; Bodroža-Solarov, M.; Pavlić, B. Defatted wheat germ as source of polyphenols—Optimization of microwave-assisted extraction by RSM and ANN approach. Chem. Eng. Process. Process Intensif. 2019, 143, 107634. [Google Scholar] [CrossRef]
- Sharma, R. Polyphenols in health and disease: Practice and mechanisms of benefits. In Polyphenols in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 757–778. [Google Scholar]
- Đukić, D.; Mašković, P.; Moračanin, S.V.; Kurćubić, V.; Milijašević, M.; Babić, J. Conventional and unconventional extraction methods applied to the plant, Thymus serpyllum L. In Proceedings of the 59th International Meat Industry Conference, Zlatibor, Serbia, 1–4 October 2017; Volume 85, p. 12064. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer Science+ Business Media: New York, NY, USA, 2012; pp. 15–52. [Google Scholar]
- Bu, X.; Xu, Y.; Zhao, M.; Li, D.; Zou, J.; Wang, L.; Bai, J.; Yang, Y. Simultaneous extraction of polysaccharides and polyphenols from blackcurrant fruits: Comparison between response surface methodology and artificial neural networks. Ind. Crops Prod. 2021, 170, 113682. [Google Scholar] [CrossRef]
- Kashyap, P.; Riar, C.S.; Jindal, N. Optimization of ultrasound assisted extraction of polyphenols from Meghalayan cherry fruit (Prunus nepalensis) using response surface methodology (RSM) and artificial neural network (ANN) approach. J. Food Meas. Charact. 2021, 15, 119–133. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Gan, R.Y.; Zhang, D.; Ge, Y.Y.; Cheng, L.Z.; Corke, H. Optimization of kidney bean antioxidants using RSM & ANN and characterization of antioxidant profile by UPLC-QTOF-MS. LWT 2019, 114, 108321. [Google Scholar] [CrossRef]
- Boros, B.; Jakabová, S.; Dörnyei, Á.; Horváth, G.; Pluhár, Z.; Kilár, F.; Felinger, A. Determination of polyphenolic compounds by liquid chromatography-mass spectrometry in Thymus species. J. Chromatogr. A 2010, 1217, 7972–7980. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef]


| Run | Input Parameters | Responses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A: Ethanol Concentration (%) | B: Extraction Time (min) | C: Liquid–Solid Ratio (mL/g) | D: Irradiation Power (W) | Y (%) | TP (g GAE/100 g DW) | DPPH (mM TE/g) | FRAP (mM Fe2+/g) | ABTS (mM TE/g) | |||||
| 1 | 45 | −1 | 5 | −1 | 10 | −1 | 400 | −1 | 15.21 | 3.8200 | 0.2027 | 0.5945 | 0.4570 |
| 2 | 75 | 1 | 5 | −1 | 10 | −1 | 400 | −1 | 10.69 | 3.9155 | 0.1861 | 0.5763 | 0.4373 |
| 3 | 45 | −1 | 20 | 1 | 10 | −1 | 400 | −1 | 15.00 | 4.2637 | 0.2145 | 0.6395 | 0.5018 |
| 4 | 75 | 1 | 20 | 1 | 10 | −1 | 400 | −1 | 13.25 | 3.2721 | 0.1836 | 0.3590 | 0.3857 |
| 5 | 45 | −1 | 5 | −1 | 30 | 1 | 400 | −1 | 14.06 | 3.2983 | 0.1734 | 0.5445 | 0.3789 |
| 6 | 75 | 1 | 5 | −1 | 30 | 1 | 400 | −1 | 14.50 | 4.1299 | 0.2057 | 0.6792 | 0.4208 |
| 7 | 45 | −1 | 20 | 1 | 30 | 1 | 400 | −1 | 11.54 | 2.8903 | 0.1567 | 0.4678 | 0.3377 |
| 8 | 75 | 1 | 20 | 1 | 30 | 1 | 400 | −1 | 15.66 | 4.0896 | 0.2033 | 0.6032 | 0.4970 |
| 9 | 45 | −1 | 5 | −1 | 10 | −1 | 800 | 1 | 15.54 | 4.1036 | 0.2150 | 0.6932 | 0.4163 |
| 10 | 75 | 1 | 5 | −1 | 10 | −1 | 800 | 1 | 14.86 | 4.0438 | 0.2208 | 0.7071 | 0.5023 |
| 11 | 45 | −1 | 20 | 1 | 10 | −1 | 800 | 1 | 11.72 | 3.5056 | 0.1666 | 0.5372 | 0.4318 |
| 12 | 75 | 1 | 20 | 1 | 10 | −1 | 800 | 1 | 15.10 | 4.0288 | 0.2148 | 0.6319 | 0.4942 |
| 13 | 45 | −1 | 5 | −1 | 30 | 1 | 800 | 1 | 12.85 | 3.0775 | 0.1613 | 0.4854 | 0.3707 |
| 14 | 75 | 1 | 5 | −1 | 30 | 1 | 800 | 1 | 15.62 | 3.9141 | 0.2091 | 0.5634 | 0.3918 |
| 15 | 45 | −1 | 20 | 1 | 30 | 1 | 800 | 1 | 11.90 | 3.6965 | 0.1806 | 0.5713 | 0.4237 |
| 16 | 75 | 1 | 20 | 1 | 30 | 1 | 800 | 1 | 12.20 | 3.8593 | 0.1845 | 0.5768 | 0.4393 |
| 17 | 45 | −1 | 12.5 | 0 | 20 | 0 | 600 | 0 | 13.64 | 3.5796 | 0.1874 | 0.7182 | 0.3438 |
| 18 | 75 | 1 | 12.5 | 0 | 20 | 0 | 600 | 0 | 14.82 | 4.2085 | 0.2382 | 0.4889 | 0.4634 |
| 19 | 60 | 0 | 5 | −1 | 20 | 0 | 600 | 0 | 15.39 | 4.1064 | 0.2208 | 0.7109 | 0.5120 |
| 20 | 60 | 0 | 20 | 1 | 20 | 0 | 600 | 0 | 15.06 | 3.6245 | 0.2199 | 0.6465 | 0.4009 |
| 21 | 60 | 0 | 12.5 | 0 | 10 | −1 | 600 | 0 | 15.58 | 3.8154 | 0.2197 | 0.4470 | 0.3991 |
| 22 | 60 | 0 | 12.5 | 0 | 30 | 1 | 600 | 0 | 16.84 | 3.9876 | 0.2125 | 0.4525 | 0.4960 |
| 23 | 60 | 0 | 12.5 | 0 | 20 | 0 | 400 | −1 | 14.86 | 4.0288 | 0.2208 | 0.4416 | 0.4453 |
| 24 | 60 | 0 | 12.5 | 0 | 20 | 0 | 800 | 1 | 16.90 | 3.6371 | 0.2218 | 0.5167 | 0.3284 |
| 25 | 60 | 0 | 12.5 | 0 | 20 | 0 | 600 | 0 | 17.60 | 3.6558 | 0.2346 | 0.5725 | 0.3855 |
| 26 | 60 | 0 | 12.5 | 0 | 20 | 0 | 600 | 0 | 15.21 | 3.8706 | 0.2253 | 0.5196 | 0.4193 |
| 27 | 60 | 0 | 12.5 | 0 | 20 | 0 | 600 | 0 | 15.15 | 3.5922 | 0.1936 | 0.5549 | 0.3429 |
| 28 | 60 | 0 | 12.5 | 0 | 20 | 0 | 600 | 0 | 15.64 | 3.7930 | 0.2280 | 0.4882 | 0.4570 |
| 29 | 60 | 0 | 12.5 | 0 | 20 | 0 | 600 | 0 | 17.19 | 3.8580 | 0.2348 | 0.5786 | 0.4000 |
| Coefficients | Y | TP | DPPH | FRAP | ABTS |
|---|---|---|---|---|---|
| p-Value | |||||
| Linear | |||||
| X1-Ethanol concentration | <0.0001 * | 0.0001 * | <0.0001 * | 0.3235 | 0.0616 |
| X2-Extraction time | 0.0448 ** | 0.1554 | 0.0014 * | 0.6279 | 0.2024 |
| X3-Liquid–solid ratio | 0.0004 * | <0.0001 * | 0.6736 | 0.0339 | <0.0001 * |
| X4-Irradiation power | 0.2989 | 0.8638 | 0.5279 | 0.0545 | 0.5151 |
| Cross-product | |||||
| X1X2 | - | 0.1157 | 0.6323 | - | - |
| X1X3 | - | 0.0321 ** | 0.0161 ** | - | - |
| X1X4 | - | - | 0.5840 | - | 0.3010 |
| X2X3 | 0.0191 ** | - | 0.6873 | - | - |
| X2X4 | 0.2665 | 0.8809 | - | 0.2744 | - |
| X3X4 | - | 0.6695 | 0.2751 | - | - |
| Quadratic | |||||
| X12 | 0.0377 ** | 0.0090 * | 0.1226 | - | 0.0216 ** |
| X22 | 0.0729 | 0.7068 | 0.6754 | - | - |
| X32 | 0.2946 | 0.4842 | 0.0311 ** | - | - |
| X42 | 0.1989 | 0.8243 | - | 0.0098 * | 0.2836 |
| Response | Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Y | Model | 79.21 | 10 | 7.92 | 21.94 | <0.0001 |
| Residual | 6.5 | 18 | 0.361 | |||
| Lack of Fit | 5.86 | 14 | 0.4187 | 2.64 | 0.1804 | |
| Pure Error | 0.6355 | 4 | 0.1589 | |||
| Cor Total | 85.71 | 28 | ||||
| R2 = 0.9242 | ||||||
| CV = 4.11% | ||||||
| TP | Model | 2.66 | 12 | 0.2218 | 7.48 | 0.0002 |
| Residual | 0.4744 | 16 | 0.0296 | |||
| Lack of Fit | 0.2434 | 12 | 0.0203 | 0.3513 | 0.9284 | |
| Pure Error | 0.231 | 4 | 0.0577 | |||
| Cor Total | 3.14 | 28 | ||||
| R2 = 0.8487 | ||||||
| CV = 4.55% | ||||||
| DPPH | Model | 0.0135 | 12 | 0.0011 | 15.68 | <0.0001 |
| Residual | 0.0011 | 16 | 0.0001 | |||
| Lack of Fit | 0.0006 | 12 | 0.0001 | 0.383 | 0.9114 | |
| Pure Error | 0.0005 | 4 | 0.0001 | |||
| Cor Total | 0.0146 | 28 | ||||
| R2 = 0.9216 | ||||||
| CV = 4.14% | ||||||
| FRAP | Model | 0.1038 | 6 | 0.0173 | 3.29 | 0.0182 |
| Residual | 0.1155 | 22 | 0.0052 | |||
| Lack of Fit | 0.1123 | 18 | 0.0062 | 7.8 | 0.0298 | |
| Pure Error | 0.0032 | 4 | 0.0008 | |||
| Cor Total | 0.2193 | 28 | ||||
| R2 = 0.4733 | ||||||
| CV = 12.57% | ||||||
| ABTS | Model | 0.0535 | 7 | 0.0076 | 5.99 | 0.0006 |
| Residual | 0.0268 | 21 | 0.0013 | |||
| Lack of Fit | 0.0188 | 17 | 0.0011 | 0.5563 | 0.8241 | |
| Pure Error | 0.008 | 4 | 0.002 | |||
| Cor Total | 0.0803 | 28 | ||||
| R2 = 0.6661 | ||||||
| CV = 8.44% |
| Response | Model Equation |
|---|---|
| Y | |
| TP | |
| DPPH | |
| FRAP | |
| ABTS |
| Input Parameters | RSM | ANN | ||
| Optimal Parameters | ||||
| Ethanol concentration [%] | 52.19 | 45 | ||
| Extraction time [min] | 20 | 5 | ||
| Liquid–solid ratio [mL/g] | 23.64 | 30 | ||
| Irradiation power [W] | 400 | 400 | ||
| Output Parameters | Predicted Values | Experimental Values | Predicted Values | Experimental Values |
| Y [%] | 16.07 | 16.89 | 15.68 | 17.46 |
| TP [g GAE/100 g] | 4.16 | 4.86 ± 0.1830 | 4.54 | 4.36 ± 0.1556 |
| DPPH [mM TE/g] | 0.23 | 0.26 ± 0.0039 | 0.23 | 0.20 ± 0.0020 |
| ABTS [mM TE/g] | 0.49 | 0.53 ± 0.0142 | 0.58 | 0.57 ± 0.0185 |
| Retention Time [min] | Compound | Sample MAE-CP | Sample MAE-OPT |
|---|---|---|---|
| Measured Mass [m/z]/Error [mDa] | |||
| 12.67 | Monogalloyl-glucose | 331.07/0.48 | 331.07/0.58 |
| 14.14 | Gallic acid | 169.01/0.01 | 169.01/0.46 |
| 16.15 | Vanillic acid | 167.03/0.14 | 167.03/−0.04 |
| 19.28 | Protocatechuic acid | 153.02/0.12 | 153.02/0.32 |
| 37.61 | 3-p-Coumaroylquinic acid | 337.09/0.92 | 337.09/0.92 |
| 37.61 | 4-p-Coumaroylquinic acid | 337.09/0.92 | 337.09/0.92 |
| 39.33 | (+)-Catechin | ND | 289.07/0.69 |
| 39.33 | (−)-Epicatechin | ND | 289.07/0.69 |
| 39.53 | Coumaric acid hexoside isomer-1 | 325.09/0.30 | 325.09/0.30 |
| 39.53 | Coumaric acid hexoside isomer-2 | 325.09/0.30 | 325.09/0.30 |
| 39.53 | Coumaric acid hexoside isomer-3 | 325.09/0.30 | 325.09/0.30 |
| 39.53 | p-Coumaric acid | 163.04/−0.19 | 163.04/−0.19 |
| 43.32 | Dihydroxycoumarin | 177.02/0.36 | 177.02/−0.07 |
| 44.46 | Caffeic acid | 179.03/0.13 | 179.03/0.05 |
| 52.08 | cis-Coutaric acid | 295.05/0.17 | ND |
| 52.08 | trans-Coutaric acid | 295.05/0.17 | ND |
| 66.28 | Quercetin hexoside isomer-1 | 463.09/−0.14 | 463.09/0.98 |
| 66.28 | Quercetin hexoside isomer-2 | 463.09/−0.14 | 463.09/0.98 |
| 66.28 | Quercetin-3-O-galactoside | 463.09/−0.14 | 463.09/0.98 |
| 66.28 | Quercetin-3-O-glucoside | 463.09/−0.14 | 463.09/0.98 |
| 66.61 | Kaempferol-3-rutinoside | 593.15/−0.11 | 593.15/−0.22 |
| 72.90 | Naringenin-7-O-glucoside | 433.11/−0.01 | 433.11/0.34 |
| 76.06 | Quercetin-3-O-rutinoside | 609.15/−0.09 | 609.15/0.01 |
| 76.37 | Quercetin glucuronide | 477.07/1.37 | 477.07/1.50 |
| 76.45 | Kaempferol-3-galactoside | 447.09/−0.59 | 447.09/0.03 |
| 76.45 | Kaempferol-3-glucoside | 447.09/−0.59 | 447.09/0.03 |
| 76.52 | Isorhamnetin-3-O-galactoside | 477.10/−0.64 | 477.10/−0.13 |
| 76.83 | Eriodictyol | 287.06/0.57 | 287.06/0.68 |
| 77.27 | Naringenin | ND | 271.06/1.42 |
| 78.10 | Kaempferol | 285.04/0.24 | 285.04/0.45 |
| 78.10 | Luteolin | 285.04/0.24 | 285.04/0.45 |
| 78.74 | Quercetin pentoside isomer-1 | 433.08/0.30 | ND |
| 78.74 | Quercetin pentoside isomer-2 | 433.08/0.30 | ND |
| 78.84 | Quercetin | 301.03/−1.45 | ND |
| 78.90 | Ellagic acid | 301.00/−0.80 | 301.00/−0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrkonjić, Ž.; Rakić, D.; Takači, A.; Kaplan, M.; Teslić, N.; Zeković, Z.; Lazarević, I.; Pavlić, B. Polyphenols Recovery from Thymus serpyllum Industrial Waste Using Microwave-Assisted Extraction–Comparative RSM and ANN Approach for Process Optimization. Foods 2022, 11, 1184. https://doi.org/10.3390/foods11091184
Mrkonjić Ž, Rakić D, Takači A, Kaplan M, Teslić N, Zeković Z, Lazarević I, Pavlić B. Polyphenols Recovery from Thymus serpyllum Industrial Waste Using Microwave-Assisted Extraction–Comparative RSM and ANN Approach for Process Optimization. Foods. 2022; 11(9):1184. https://doi.org/10.3390/foods11091184
Chicago/Turabian StyleMrkonjić, Živan, Dušan Rakić, Aleksandar Takači, Muammer Kaplan, Nemanja Teslić, Zoran Zeković, Ivana Lazarević, and Branimir Pavlić. 2022. "Polyphenols Recovery from Thymus serpyllum Industrial Waste Using Microwave-Assisted Extraction–Comparative RSM and ANN Approach for Process Optimization" Foods 11, no. 9: 1184. https://doi.org/10.3390/foods11091184
APA StyleMrkonjić, Ž., Rakić, D., Takači, A., Kaplan, M., Teslić, N., Zeković, Z., Lazarević, I., & Pavlić, B. (2022). Polyphenols Recovery from Thymus serpyllum Industrial Waste Using Microwave-Assisted Extraction–Comparative RSM and ANN Approach for Process Optimization. Foods, 11(9), 1184. https://doi.org/10.3390/foods11091184