Sociocultural Factors Influencing Human Streptococcus suis Disease in Southeast Asia
Abstract
:1. Introduction
2. Human S. suis Serotypes and Sequence Types in Southeast Asia
3. Burden and Impact of S. suis Infection in Southeast Asia
4. Contamination of S. suis in the Pork Supply Chain
5. Sociocultural Aspects of Human Streptococcus suis Disease in Southeast Asian Countries
5.1. Thailand
5.2. Vietnam
5.3. Lao People’s Democratic Republic (Lao PDR)
5.4. Indonesia
5.5. Other Countries in Southeast Asia
6. Risk Assessment of Food Safety and Consumption of Raw Pork Dishes
7. Policy Implications
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—An update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Aragon, V.; Brockmeier, S.L.; Gebhart, C.; Greeff, A.; Kerdsin, A.; O’Dea, M.A.; Okura, M.; Saléry, M.; Schultsz, C.; et al. Update on Streptococcus suis Research and Prevention in the Era of Antimicrobial Restriction: 4th International Workshop on S. suis. Pathogens 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Nghia, H.D.; Hoa, N.T.; Linh, L.; Campbell, J.; Diep, T.S.; Chau, N.V.; Mai, N.T.; Hien, T.T.; Spratt, B.; Farrar, J.; et al. Human case of Streptococcus suis serotype 16 infection. Emerg. Infect. Dis. 2008, 14, 155–157. [Google Scholar] [CrossRef] [Green Version]
- Kerdsin, A.; Oishi, K.; Sripakdee, S.; Boonkerd, N.; Polwichai, P.; Nakamura, S.; Uchida, R.; Sawanpanyalert, P.; Dejsirilert, S. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J. Med. Microbiol. 2009, 58, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Kerdsin, A.; Dejsirilert, S.; Sawanpanyalert, P.; Boonnark, A.; Noithachang, W.; Sriyakum, D.; Simkum, S.; Chokngam, S.; Gottschalk, M.; Akeda, Y.; et al. Sepsis and spontaneous bacterial peritonitis in Thailand. Lancet 2011, 378, 960. [Google Scholar] [CrossRef]
- Kerdsin, A.; Hatrongjit, R.; Gottschalk, M.; Takeuchi, D.; Hamada, S.; Akeda, Y.; Oishi, K. Emergence of Streptococcus suis serotype 9 infection in humans. J. Microbiol. Immunol. Infect. 2017, 50, 545–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerdsin, A.; Akeda, Y.; Takeuchi, D.; Dejsirilert, S.; Gottschalk, M.; Oishi, K. Genotypic diversity of Streptococcus suis strains isolated from humans in Thailand. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 917–925. [Google Scholar] [CrossRef]
- Callejo, R.; Prieto, M.; Salamone, F.; Auger, J.P.; Goyette-Desjardins, G.; Gottschalk, M. Atypical Streptococcus suis in man, Argentina, 2013. Emerg. Infect. Dis. 2014, 20, 500–502. [Google Scholar] [CrossRef]
- Hatrongjit, R.; Kerdsin, A.; Gottschalk, M.; Takeuchi, D.; Hamada, S.; Oishi, K.; Akeda, Y. First human case report of sepsis due to infection with Streptococcus suis serotype 31 in Thailand. BMC Infect. Dis. 2015, 15, 392. [Google Scholar] [CrossRef]
- Liang, P.; Wang, M.; Gottschalk, M.; Vela, A.I.; Estrada, A.A.; Wang, J.; Du, P.; Luo, M.; Zheng, H.; Wu, Z. Genomic and pathogenic investigations of Streptococcus suis serotype 7 population derived from a human patient and pigs. Emerg. Microbes Infect. 2021, 10, 1960–1974. [Google Scholar] [CrossRef]
- Gottschalk, M.; Xu, J.; Calzas, C.; Segura, M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010, 5, 371–391. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Sroka, J.; Zając, V.; Wasiński, B.; Cisak, E.; Sawczyn, A.; Kloc, A.; Wójcik-Fatla, A. Streptococcus suis: A re-emerging pathogen associated with occupational exposure to pigs or pork products. Part I—Epidemiology. Ann. Agric. Environ. Med. 2017, 24, 683–695. [Google Scholar] [CrossRef]
- Kerdsin, A.; Dejsirilert, S.; Puangpatra, P.; Sripakdee, S.; Chumla, K.; Boonkerd, N.; Polwichai, P.; Tanimura, S.; Takeuchi, D.; Nakayama, T.; et al. Genotypic profile of Streptococcus suis serotype 2 and clinical features of infection in humans, Thailand. Emerg. Infect. Dis. 2011, 17, 835–842. [Google Scholar] [CrossRef]
- Takeuchi, D.; Kerdsin, A.; Pienpringam, A.; Loetthong, P.; Samerchea, S.; Luangsuk, P.; Khamisara, K.; Wongwan, N.; Areeratana, P.; Chiranairadul, P.; et al. Population-based study of Streptococcus suis infection in humans in Phayao Province in northern Thailand. PLoS ONE 2012, 7, e31265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huong, V.T.; Hoa, N.T.; Horby, P.; Bryant, J.E.; Van Kinh, N.; Toan, T.K.; Wertheim, H.F. Raw pig blood consumption and potential risk for Streptococcus suis infection, Vietnam. Emerg. Infect. Dis. 2014, 20, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, D.; Wongsawan, K.; Osaki, M.; Nishino, H.; Ishiji, T.; Tharavichitkul, P.; Khantawa, B.; Fongcom, A.; Takai, S.; Sekizaki, T. Streptococcus suis in humans, Thailand. Emerg. Infect. Dis. 2008, 14, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.T.; Hoa, N.T.; Nga, T.V.; Linh, L.; Chau, T.T.; Sinh, D.X.; Phu, N.H.; Chuong, L.V.; Diep, T.S.; Campbell, J.; et al. Streptococcus suis meningitis in adults in Vietnam. Clin. Infect. Dis. 2008, 46, 659–667. [Google Scholar] [PubMed] [Green Version]
- Susilawathi, N.M.; Tarini, N.; Fatmawati, N.; Mayura, P.; Suryapraba, A.; Subrata, M.; Sudewi, A.; Mahardika, G.N. Streptococcus suis-associated meningitis, Bali, Indonesia, 2014–2017. Emerg. Infect. Dis. 2019, 25, 2235–2242. [Google Scholar] [CrossRef] [Green Version]
- Wongjittraporn, S.; Teerasukjinda, O.; Yee, M.; Chung, H.H. Streptococcus suis meningoencephalitis with seizure from raw pork ingestion: A case report. Hawaii J. Med. Public Health 2014, 73, 13–14. [Google Scholar]
- Lee, G.T.; Chiu, C.Y.; Haller, B.L.; Denn, P.M.; Hall, C.S.; Gerberding, J.L. Streptococcus suis meningitis, United States. Emerg. Infect. Dis. 2008, 14, 183–185. [Google Scholar] [CrossRef]
- Rajahram, G.S.; Hameed, A.A.; Menon, J.; William, T.; Tambyah, P.A.; Yeo, T.W. Case report: Two human Streptococcus suis infections in Borneo, Sabah, Malaysia. BMC Infect. Dis. 2017, 17, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlieghe, E. The Microbiological Spectrum of Invasive Bacterial Infections in Cambodian Adults and Implications for Standard Treatment Guidelines. Master’s Thesis, University of Leuven, Leuven, Belgium, 2014. [Google Scholar]
- Tambyah, P.A.; Kumarasinghe, G.; Chan, H.L.; Lee, K.O. Streptococcus suis infection complicated by purpura fulminans and rhabdomyolysis: Case report and review. Clin. Infect. Dis. 1997, 24, 710–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, Y.C.; Wilder-Smith, A.; Ong, B.K.; Kumarasinghe, G.; Wilder-Smith, E. Adult community acquired bacterial meningitis in a Singaporean teaching hospital. A seven-year overview (1993–2000). Singap. Med. J. 2002, 43, 632–636. [Google Scholar] [PubMed]
- Tan, J.H.; Yeh, B.I.; Seet, C.S. Deafness due to haemorrhagic labyrinthitis and a review of relapses in Streptococcus suis meningitis. Singap. Med. J. 2010, 51, e30–e33. [Google Scholar]
- Rayanakorn, A.; Ademi, Z.; Liew, D.; Lee, L.H. Burden of disease and productivity impact of Streptococcus suis infection in Thailand. PLoS Negl. Trop. Dis. 2021, 15, e0008985. [Google Scholar] [CrossRef]
- Thongsawad, S. Burden and Epidemiological Characterisations of Streptococcus suis in Chiang Mai, Thailand. Ph.D. Thesis, The University of Edinburgh, Edinburgh, UK, 2016. [Google Scholar]
- Huong, V.; Turner, H.C.; Kinh, N.V.; Thai, P.Q.; Hoa, N.T.; Horby, P.; van Doorn, H.R.; Wertheim, H. Burden of disease and economic impact of human Streptococcus suis infection in Viet Nam. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Huong, V.; Long, H.B.; Kinh, N.V.; Ngan, T.; Dung, V.; Nadjm, B.; van Doorn, H.R.; Hoa, N.T.; Horby, P.; Wertheim, H. Long-term outcomes of patients with Streptococcus suis infection in Viet Nam: A case-control study. J. Infect. 2018, 76, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Wongsawat, S. Streptococcus suis serotype 2 outbreak at Chomthong district, Chiang Mai province, June–July, 2008. Lanna Public Health J. 2010, 6, 322–336. [Google Scholar]
- Burniston, S.; Okello, A.L.; Khamlome, B.; Inthavong, P.; Gilbert, J.; Blacksell, S.D.; Allen, J.; Welburn, S.C. Cultural drivers and health-seeking behaviours that impact on the transmission of pig-associated zoonoses in Lao People’s Democratic Republic. Infect. Dis. Poverty 2015, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Meekhanon, N.; Kaewmongkol, S.; Phimpraphai, W.; Okura, M.; Osaki, M.; Sekizaki, T.; Takamatsu, D. Potentially hazardous Streptococcus suis strains latent in asymptomatic pigs in a major swine production area of Thailand. J. Med. Microbiol. 2017, 66, 662–669. [Google Scholar] [CrossRef]
- Ngo, T.H.; Tran, T.B.; Tran, T.T.; Nguyen, V.D.; Campbell, J.; Pham, H.A.; Huynh, H.T.; Nguyen, V.V.; Bryant, J.E.; Tran, T.H.; et al. Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PLoS ONE 2011, 6, e17943. [Google Scholar]
- Nutravong, T.; Angkititrakul, S.; Jiwakanon, N.; Wongchanthong, W.; Dejsirilerts, S.; Nawa, Y. Identification of major Streptococcus suis serotypes 2, 7, 8 and 9 isolated from pigs and humans in upper northeastern Thailand. Southeast Asian J. Trop. Med. Public Health 2014, 45, 1173–1181. [Google Scholar]
- Thongkamkoon, P.; Kiatyingangsulee, T.; Gottschalk, M. Serotypes of Streptococcus suis isolated from healthy pigs in Phayao Province, Thailand. BMC Res. Notes 2017, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Kerdsin, A.; Takeuchi, D.; Nuangmek, A.; Akeda, Y.; Gottschalk, M.; Oishi, K. Genotypic comparison between Streptococcus suis isolated from pigs and humans in Thailand. Pathogens 2020, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Prasertsang, T.; Cheveerach, P. Risk factors on contamination of Streptococcus suis in slaughterhouse in Maha Sarakham province. KKU Vet. J. 2019, 29, 61–69. [Google Scholar]
- Holt, H.R.; Inthavong, P.; Khamlome, B.; Blaszak, K.; Keokamphe, C.; Somoulay, V.; Phongmany, A.; Durr, P.A.; Graham, K.; Allen, J.; et al. Endemicity of zoonotic diseases in pigs and humans in Lowland and Upland Lao PDR: Identification of socio-cultural risk factors. PLoS Negl. Trop. Dis. 2016, 10, e0003913. [Google Scholar] [CrossRef] [Green Version]
- Wongnak, P.; Wiratsudakul, A.; Nuanualsuwan, S. A risk assessment of pathogenic Streptococcus suis in pork supply chains and markets in Thailand. Food Control 2020, 118, 107432. [Google Scholar] [CrossRef]
- Boonyong, N.; Kaewmongkol, S.; Khunbutsri, D.; Satchasataporn, K.; Meekhanon, N. Contamination of Streptococcus suis in pork and edible pig organs in central Thailand. Vet. World 2019, 12, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Noppon, B.; Khaeng, S.; Sopa, A.; Phuaram, P.; Wongsan, R.; Laohasinnurak, T. Streptococcus suis serotype 2 in uncooked pork meat products in Khon Kaen, northeastern Thailand, and their antimicrobial profiles. Int. J. Sci. Eng. Res. 2014, 5, 1130–1133. [Google Scholar]
- Praphasiri, P.; Owusu, J.T.; Thammathitiwat, S.; Ditsungnoen, D.; Boonmongkon, P.; Sangwichian, O.; Prasert, K.; Srihapanya, S.; Sornwong, K.; Kerdsin, A.; et al. Streptococcus suis infection in hospitalized patients, Nakhon Phanom Province, Thailand. Emerg. Infect. Dis. 2015, 21, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Huong, V.T.; Thanh, L.V.; Phu, V.D.; Trinh, D.T.; Inui, K.; Tung, N.; Oanh, N.T.; Trung, N.V.; Hoa, N.T.; Bryant, J.E.; et al. Temporal and spatial association of Streptococcus suis infection in humans and porcine reproductive and respiratory syndrome outbreaks in pigs in northern Vietnam. Epidemiol. Infect. 2016, 144, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Nilubol, D.; Tripipat, T.; Hoonsuwan, T.; Kortheerakul, K. Porcine reproductive and respiratory syndrome virus, Thailand, 2010–2011. Emerg. Infect. Dis. 2012, 18, 2039–2043. [Google Scholar] [CrossRef] [Green Version]
- Khadthasrima, N.; Hannwong, T.; Thammawitjaya, P.; Pingsusean, D.; Akkanij, B.; Jaikhar, A.; Paungmali, P.; Yudee, P.; Wongyai, S.; Samerchea, S.; et al. Human Streptococcus suis outbreak in Phayao Province, Thailand, 2007. OSIR 2008, 1, 4–7. [Google Scholar]
- Juntasiriyarkorn, S.; Henpraserttae, N.; Iamsirithaworn, S.; Thepsittha, K.; Saengrueng, S. Outbreak verification summary-17th Week 25 April–1 May 2010. WESR 2010, 41, 265–266. Available online: https://wesr-doe.moph.go.th/wesr_new/index.php (accessed on 6 December 2021).
- Fongcom, A.; Pruksakorn, S.; Netsirisawan, P.; Pongprasert, R.; Onsibud, P. Streptococcus suis infection: A prospective study in northern Thailand. Southeast Asian J. Trop. Med. Public Health 2009, 40, 511–517. [Google Scholar]
- Navacharoen, N.; Chantharochavong, V.; Hanprasertpong, C.; Kangsanarak, J.; Lekagul, S. Hearing and vestibular loss in Streptococcus suis infection from swine and traditional raw pork exposure in northern Thailand. J. Laryngol. Otol. 2009, 123, 857–862. [Google Scholar] [CrossRef]
- Kaewmoon, P. Knowledge and Practice in Prevention of Streptococcus suis Infection among People in Tha-It Subdistrict, Mueang District, Uttaradit Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2009. (In Thai). [Google Scholar]
- Yana, A. Knowledge and Practice in Prevention of Streptococcus suis Infection among People in Mae-Narua Sub-District, Mueang District, Phayao Province. Master’s Thesis, Chiang Mai University, Chiang Mai, Thailand, 2008. (In Thai). [Google Scholar]
- Sutthaluang, N.; Korwanich, K.; Korwanich, N. Perception and preventive behaviors for Streptococcus suis infection of people in Pua sub-district, Pua district, Nan province. Dis. Control J. 2021, 47, 445–455. [Google Scholar]
- Wertheim, H.F.; Nguyen, H.N.; Taylor, W.; Lien, T.T.; Ngo, H.T.; Nguyen, T.Q.; Nguyen, B.N.; Nguyen, H.H.; Nguyen, H.M.; Nguyen, C.T.; et al. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS ONE 2009, 4, e5973. [Google Scholar] [CrossRef]
- Nghia, H.D.; Tu, L.; Wolbers, M.; Thai, C.Q.; Hoang, N.V.; Nga, T.V.; Thao, L.; Phu, N.H.; Chau, T.T.; Sinh, D.X.; et al. Risk factors of Streptococcus suis infection in Vietnam. A case-control study. PLoS ONE 2011, 6, e17604. [Google Scholar]
- Yang, L.; Darasavath, C.; Chang, K.; Vilay, V.; Sengduangphachanh, A.; Adsamouth, A.; Vongsouvath, M.; Keolouangkhot, V.; Robinson, M.T. Cluster of angiostrongyliasis cases following consumption of raw monitor lizard in the Lao People’s Democratic Republic and review of the literature. Trop. Med. Infect. Dis. 2021, 6, 107. [Google Scholar] [CrossRef]
- Tran, D.S.; Odermatt, P.; Le, T.O.; Huc, P.; Druet-Cabanac, M.; Barennes, H.; Strobel, M.; Preux, P.M. Prevalence of epilepsy in a rural district of central Lao PDR. Neuroepidemiology 2006, 26, 199–206. [Google Scholar] [CrossRef]
- Xayaseng, V.; Phongluxa, K.; van Eeuwijk, P.; Akkhavong, K.; Odermatt, P. Raw fish consumption in liver fluke endemic areas in rural southern Laos. Acta Trop. 2013, 127, 105–111. [Google Scholar] [CrossRef]
- Besung, I.; Suarjana, I.; Agustina, K.K.; Winaya, I.; Soeharsono, H.; Suwiti, N.K.; Mahardika, G.N. Isolation and identification of Streptococcus suis from sick pigs in Bali, Indonesia. BMC Res. Notes 2019, 12, 795. [Google Scholar] [CrossRef] [Green Version]
- Aryasa, I.A.; Widiasari, N.P.A.; Susilawathi, N.M.; Fatmawati, N.N.D.; Adnyana, I.M.O.; Sudewi, A.A.R.; Adi Tarini, N.M. Streptococcus suis meningitis related to processing and consuming raw pork during Balinese tradition, Mebat. Med. J. Indones 2020, 29, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Rozo, M.; Schully, K.L.; Philipson, C.; Fitkariwala, A.; Nhim, D.; Som, T.; Sieng, D.; Huot, B.; Dul, S.; Gregory, M.J.; et al. An observational study of sepsis in Takeo Province Cambodia: An in-depth examination of pathogens causing severe infections. PLoS Negl. Trop. Dis. 2020, 14, e0008381. [Google Scholar] [CrossRef]
- Rayanakorn, A.; Goh, B.H.; Lee, L.H.; Khan, T.M.; Saokaew, S. Risk factors for Streptococcus suis infection: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 13358. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, D.; Kerdsin, A.; Akeda, Y.; Chiranairadul, P.; Loetthong, P.; Tanburawong, N.; Areeratana, P.; Puangmali, P.; Khamisara, K.; Pinyo, W.; et al. Impact of a food safety campaign on Streptococcus suis infection in humans in Thailand. Am. J. Trop. Med. Hyg. 2017, 96, 1370–1377. [Google Scholar] [CrossRef]
- Horby, P.; Wertheim, H.; Ha, N.H.; Trung, N.V.; Trinh, D.T.; Taylor, W.; Ha, N.M.; Lien, T.T.; Farrar, J.; Van Kinh, N. Stimulating the development of national Streptococcus suis guidelines in Viet Nam through a strategic research partnership. Bull. World Health Organ. 2010, 88, 458–461. [Google Scholar] [CrossRef]
- Soontornpipit, P.; Viwatwongkasem, C.; Taratep, C.; Teerawat, W.; Vanitchatchavan, P. Development of the Electronic Surveillance Monitoring System on Web Applications. Procedia Comput. Sci. 2016, 86, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Sathawornwiwat, A.; Kengkanpanich, M.; Saengrat, N.; Sangian, J. Factor predicting raw meat consumption behavior among people Chiang Klang District, Nan Province. Thail. J. Health Educ. 2020, 43, 25–37. [Google Scholar]
- Nga, T.V.; Nghia, H.D.; Tu, L.; Diep, T.S.; Mai, N.T.; Chau, T.T.; Sinh, D.X.; Phu, N.H.; Nga, T.T.; Chau, N.V.; et al. Real-time PCR for detection of Streptococcus suis serotype 2 in cerebrospinal fluid of human patients with meningitis. Diagn. Microbiol. Infect. Dis. 2011, 70, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, T.; Zhao, J.; Takeuchi, D.; Kerdsin, A.; Chiranairadul, P.; Areeratana, P.; Loetthong, P.; Pienpringam, A.; Akeda, Y.; Oishi, K. Colloidal gold-based immunochromatographic strip test compromising optimised combinations of anti-S. suis capsular polysaccharide polyclonal antibodies for detection of Streptococcus suis. Biosens. Bioelectron. 2014, 60, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Z.; Wang, K. Diagnosis of Streptococcus suis meningoencephalitis with metagenomic next-generation sequencing of the cerebrospinal fluid: A case report with literature review. BMC Infect. Dis. 2020, 20, 884. [Google Scholar] [CrossRef] [PubMed]
- Thu, I.; Tragoolpua, K.; Intorasoot, S.; Anukool, U.; Khamnoi, P.; Kerdsin, A.; Tharinjaroen, C.S. Direct detection of Streptococcus suis from cerebrospinal fluid, positive hemoculture, and simultaneous differentiation of serotypes 1, 1/2, 2, and 14 within single reaction. Pathogens 2021, 10, 996. [Google Scholar] [CrossRef]
- Thayawiwat, C.; Wichaikam, O.; Painpringam, A. Streptococcus suis infection in the patients in Chiang Kham hospital Chiang Kham district, Phayao province 2009–2011: Screening test for Streptococcus suis infection. J. Prevent. Med. Assoc. Thail. 2012, 2, 119–134. [Google Scholar]
| Country | Serotype | Clonal Complex | Sequence Type | Reference |
|---|---|---|---|---|
| Thailand | 2 | 1 | 1, 11, 105, 126, 144, 298, 337 | [4,5,6,7,9,16], https://pubmlst.org/organisms/streptococcus-suis (accessed on 3 January 2022) |
| 25 | 25, 102, 103, 380, 381, 395, 515, 516 | |||
| 28 | 28, 382 | |||
| 104 | 101, 104, 391, 392, 393, 512, 513, 514 | |||
| 233/379 | 233, 379, 1656 | |||
| 1687/1688 | 1687, 1688 | |||
| Singleton | 236 | |||
| 4 | 94 | 94 | ||
| 5 | 221/234 | 221 | ||
| Singleton | 181, 235 | |||
| 9 | 16 | 16 | ||
| 14 | 1 | 11, 105, 127 | ||
| 24 | 221/234 | 221, 234 | ||
| 31 | 221/234 | 221 | ||
| Vietnam | 2 | 1 | 1, 105, 107, 144, 159, 160, 161, 325, 326, 869, 951 | [3,17], https://pubmlst.org/organisms/streptococcus-suis (accessed on 3 January 2022) |
| 14 | 1 | 1, 105 | ||
| 16 | 106 | |||
| Indonesia | 2 or 1/2 | ND | ND | [18] |
| The Philippines | ND | ND | ND | [19,20] |
| Malaysia | ND | ND | ND | [21] |
| Cambodia | 2 | ND | ND | [22] |
| Singapore | ND | ND | ND | [23,24,25] |
| Laos | ND | ND | ND | [1] |
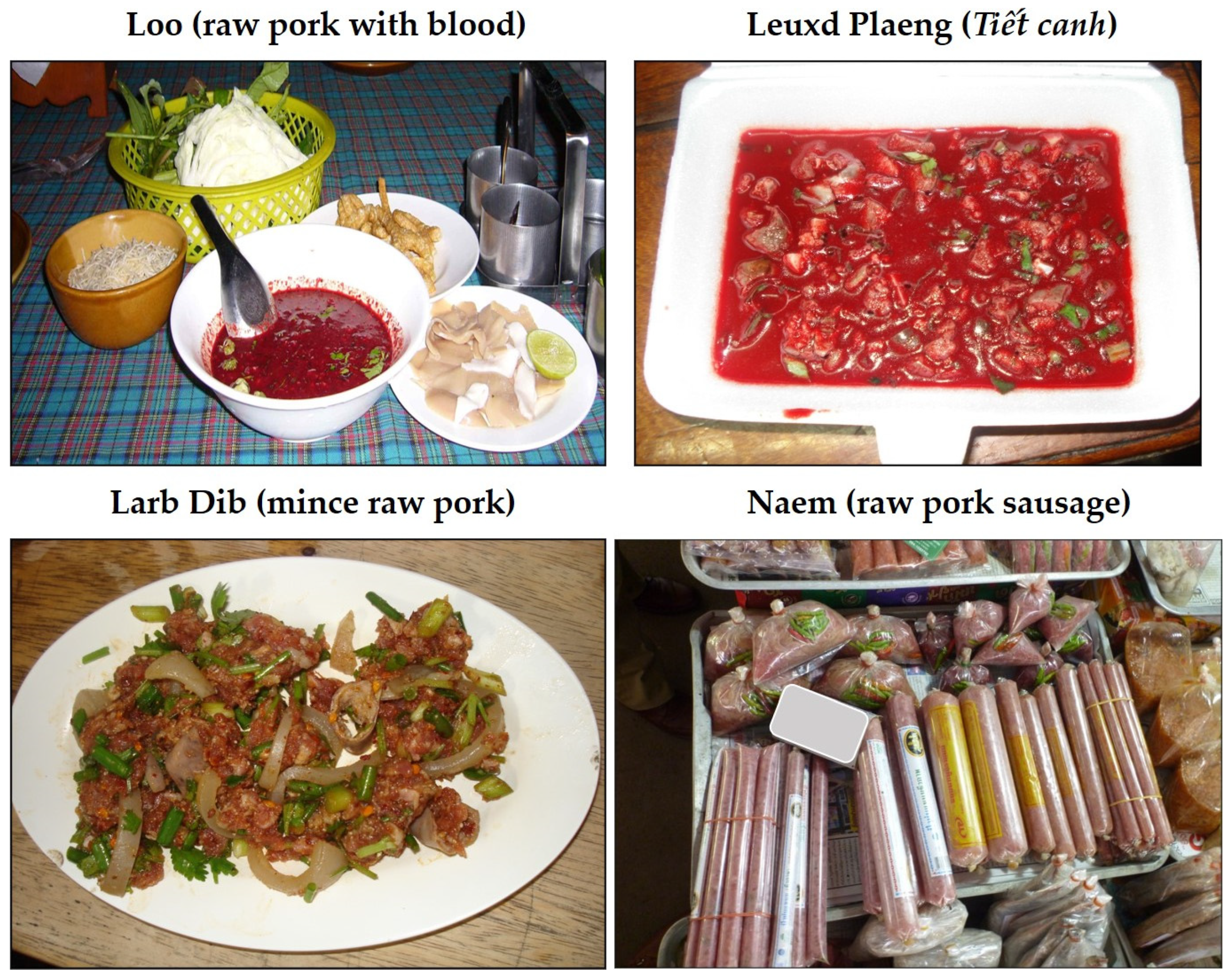
| Country | High-Risk Dishes | Description | Related to Human S. suis Infections |
|---|---|---|---|
| Thailand | Loo | Raw pig’s blood soup combined with a spice mixture and served with raw pig’s kidney, raw pork, crispy deep-fried noodles, and kaffir lime leaf. | Yes |
| Larb Dib | Minced raw pork mixed with seasonings, roasted rice, and vegetables. Sometimes, blood may be mixed with the minced raw pork. | Yes | |
| Leuxd Plaeng or Tiết Canh | Raw blood pudding mixed with cooked pork and offal products, together with crushed peanuts and chopped herbs | Yes | |
| Naem (synonym: som moo, naem maw, chin som) | A red-colored, semi-dry, fermented minced raw pork and pork skin sausage. | No report yet | |
| Vietnam | Tiết Canh | Raw blood pudding mixed with cooked meat such as pork and offal products, together with crushed peanuts and chopped herbs. | Yes |
| Lao PDR | Larb Dib | Minced raw pork mixed with seasonings, roasted rice, and vegetables. | No report yet |
| Indonesia | Lawar | Minced pork mixed with raw pig’s blood and vegetables. | Yes |
| Komoh | Fresh pig’s blood and Balinese herbs soup. | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerdsin, A.; Segura, M.; Fittipaldi, N.; Gottschalk, M. Sociocultural Factors Influencing Human Streptococcus suis Disease in Southeast Asia. Foods 2022, 11, 1190. https://doi.org/10.3390/foods11091190
Kerdsin A, Segura M, Fittipaldi N, Gottschalk M. Sociocultural Factors Influencing Human Streptococcus suis Disease in Southeast Asia. Foods. 2022; 11(9):1190. https://doi.org/10.3390/foods11091190
Chicago/Turabian StyleKerdsin, Anusak, Mariela Segura, Nahuel Fittipaldi, and Marcelo Gottschalk. 2022. "Sociocultural Factors Influencing Human Streptococcus suis Disease in Southeast Asia" Foods 11, no. 9: 1190. https://doi.org/10.3390/foods11091190
APA StyleKerdsin, A., Segura, M., Fittipaldi, N., & Gottschalk, M. (2022). Sociocultural Factors Influencing Human Streptococcus suis Disease in Southeast Asia. Foods, 11(9), 1190. https://doi.org/10.3390/foods11091190








