The Effect of Ozone Treatment on Metabolite Profile of Germinating Barley
Abstract
:1. Introduction
2. Materials and Methods
2.1. Barley Seeds
2.2. Ozone Generation
2.3. Germination Test
2.4. Liquid absorption VOC Collection
2.5. HS-SPME VOC Collection
2.6. GC-MS Conditions
2.7. Data Analysis
3. Results and Discussions
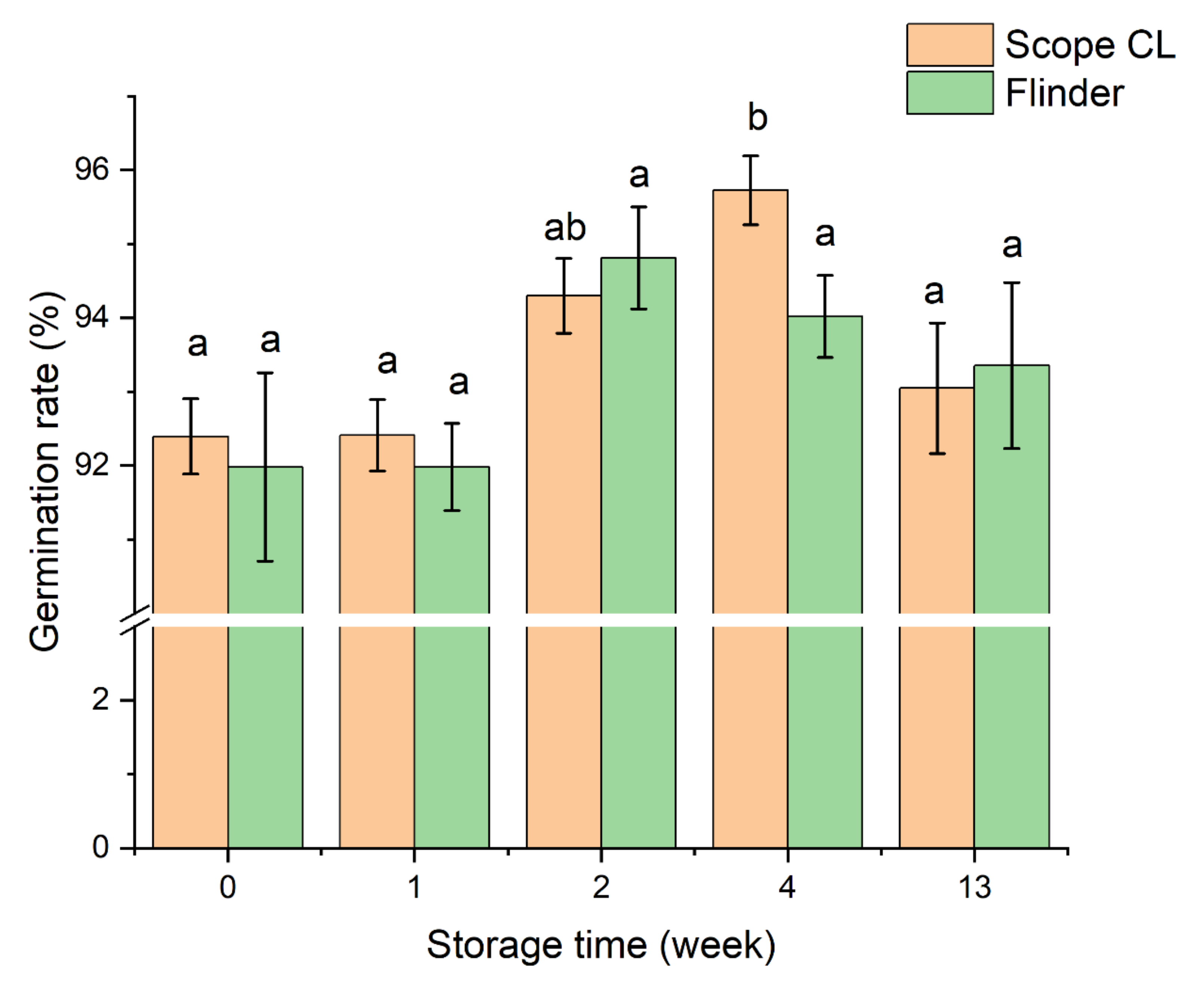
3.1. The Effect of Storage Time on Barley Germination
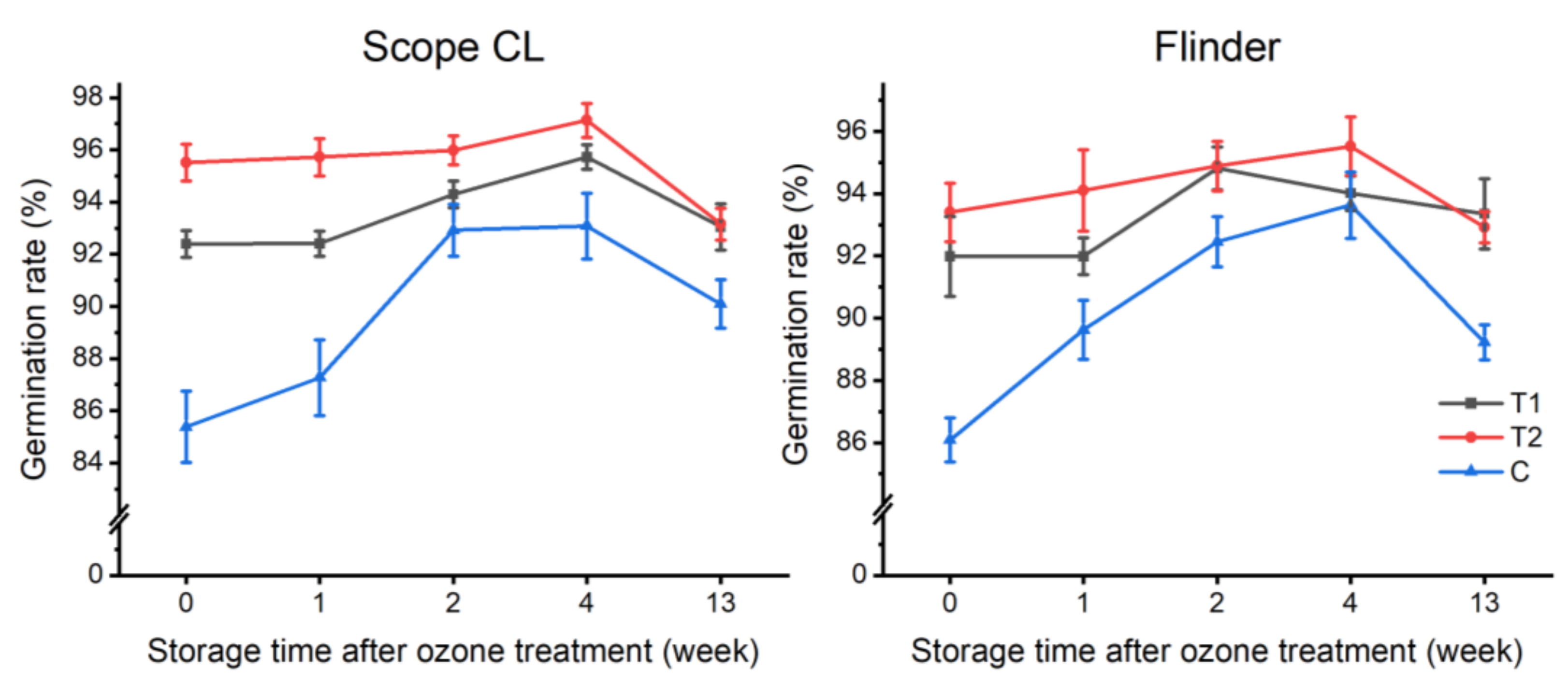
3.2. Influence of Ozone in Barley Seed Dormancy Alleviation
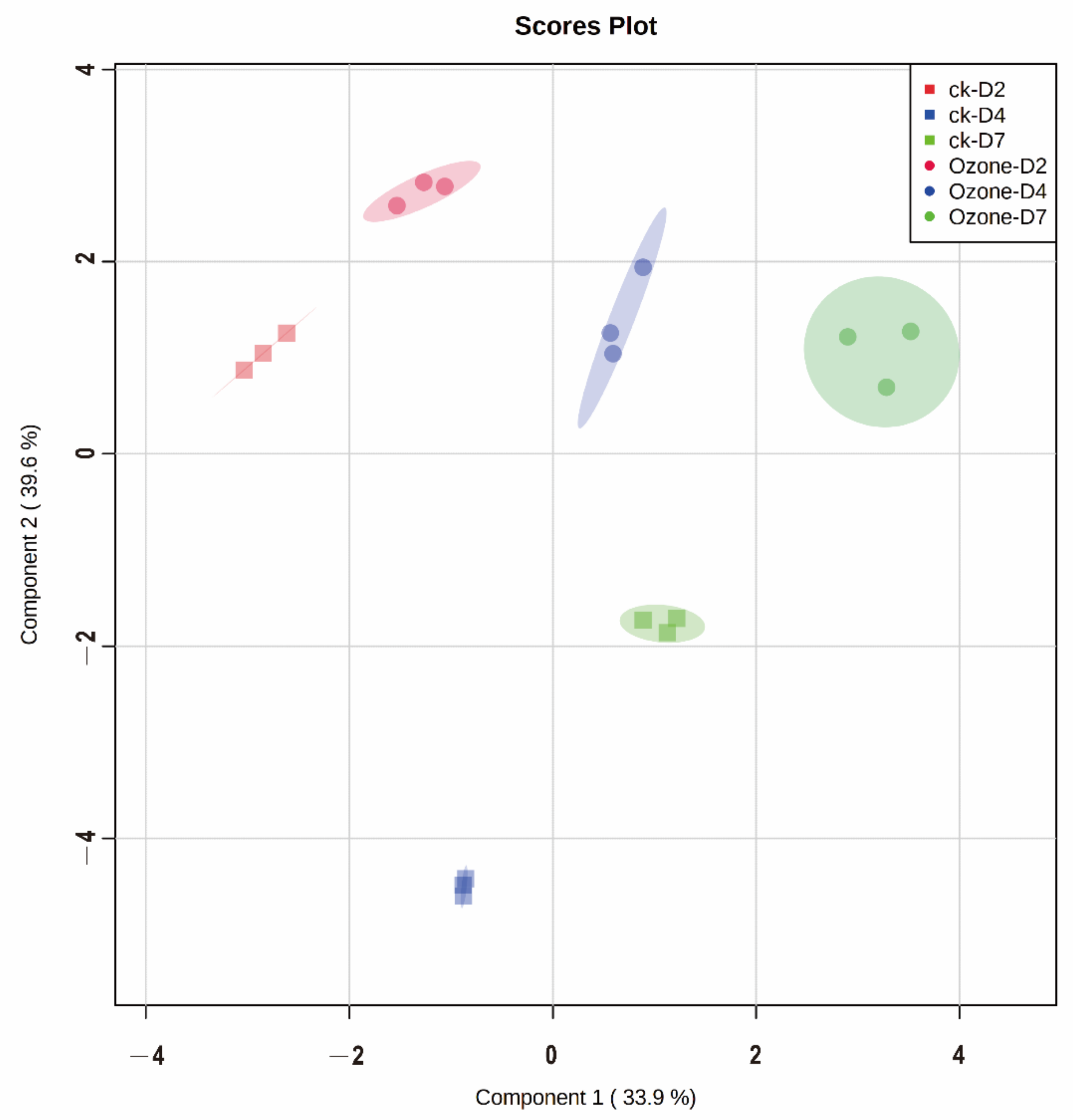
3.3. The Changes in Metabolite Profiles during Germination and Their Response to Ozone Treatment
3.4. Dynamic Changes of Metabolites in Barley during Germination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrestha, R.K.; Lindsey, L.E. Agronomic management of malting barley and research needs to meet demand by the craft brew industry. Agron. J. 2019, 111, 1570–1580. [Google Scholar] [CrossRef] [Green Version]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Biddulph, T.B.; Plummer, J.A.; Setter, T.L.; Mares, D.J. Influence of high temperature and terminal moisture stress on dormancy in wheat (Triticum aestivum L.). Field Crop Res. 2007, 103, 139–153. [Google Scholar] [CrossRef]
- Khan, M.; Weber, D. Dormancy, germination and viability of Salsola imbricata seeds in relation to light, temperature and salinity. Seed Sci. Technol. 2007, 35, 595–606. [Google Scholar]
- Nakamura, S. Grain dormancy genes responsible for preventing pre-harvest sprouting in barley and wheat. Breeding Sci. 2018, 68, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, M.W.; Young, C.A.; Sheffield, J.B. Germination and seedling growth of Desmanthus illinoensis and Desmodium canadense in response to mechanical scarification. HortScience 2010, 45, 1554–1558. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, I.; Sadanov, A. The Biological Method of Increasing Seed Germination and Productivity of Grain Crops. In Plant Growth Promoting Rhizobacteria (PGPR): Prospects for Sustainable Agriculture; Sayyed, R.Z., Reddy, M.S., Antonius, S., Eds.; Springer: Signapore, 2019; pp. 47–61. [Google Scholar]
- de Casas, R.R.; Kovach, K.; Dittmar, E.; Barua, D.; Barco, B.; Donohue, K. Seed after-ripening and dormancy determine adult life history independently of germination timing. New Phytol. 2012, 194, 868–879. [Google Scholar] [CrossRef]
- Liu, A.; Gao, F.; Kanno, Y.; Jordan, M.C.; Kamiya, Y.; Seo, M.; Ayele, B.T. Regulation of wheat seed dormancy by after-ripening is mediated by specific transcriptional switches that induce changes in seed hormone metabolism and signaling. PLoS ONE 2013, 8, e56570. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Jordan, M.C.; Ayele, B.T. Transcriptional programs regulating seed dormancy and its release by after-ripening in common wheat (Triticum aestivum L.). Plant Biotechnol. J. 2012, 10, 465–476. [Google Scholar] [CrossRef]
- Romagosa, I.; Prada, D.; Moralejo, M.; Sopena, A.; Muñoz, P.; Casas, A.; Swanston, J.; Molina-Cano, J.L. Dormancy, ABA content and sensitivity of a barley mutant to ABA application during seed development and after ripening. J. Exp. Bot. 2001, 52, 1499–1506. [Google Scholar] [CrossRef] [Green Version]
- Basbouss-Serhal, I.; Leymarie, J.; Bailly, C. Fluctuation of Arabidopsis seed dormancy with relative humidity and temperature during dry storage. J. Exp. Bot. 2016, 67, 119–130. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H. Seeds: Physiology of Development, Germination and Dormancy; Springer: New York, NY, USA, 2012. [Google Scholar]
- Tuttle, K.M.; Martinez, S.A.; Schramm, E.C.; Takebayashi, Y.; Seo, M.; Steber, C.M. Grain dormancy loss is associated with changes in ABA and GA sensitivity and hormone accumulation in bread wheat, Triticum aestivum (L.). Seed Sci. Res. 2015, 25, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, N.; Nagendra-Prasad, D.; Mohan, N.; Hill, B.; Gunasekaran, M.; Murugesan, K. Assessing influence of ozone in tomato seed dormancy alleviation. Am. J. Plant Sci. 2011, 2, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Monroy Vazquez, M.E.; Peña-Valdivia, C.B.; García, J.R.; Solano, E.; Campos, H.; García, E. Chemical scarification and ozone in seed dormancy alleviation of wild and domesticated Opuntia, Cactaceae. Ozone Sci. Eng. 2017, 39, 104–114. [Google Scholar] [CrossRef]
- Dong, X.; Sun, L.; Maker, G.; Ren, Y.; Yu, X. Ozone Treatment Increases the Release of VOC from Barley, Which Modifies Seed Germination. J. Agric. Food Chem. 2022, 70, 3127–3135. [Google Scholar] [CrossRef]
- Fincher, G.B. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu. Rev. Plant Biol. 1989, 40, 305–346. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Usadel, B.; Winter, A.; Radchuk, V.; Scholz, U.; Stein, N.; Weschke, W.; Strickert, M.; Close, T.J.; Stitt, M. Barley grain maturation and germination: Metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol. 2008, 146, 1738–1758. [Google Scholar] [CrossRef] [Green Version]
- Graham, I.A. Seed storage oil mobilization. Annu. Rev. Plant Biol. 2008, 59, 115–142. [Google Scholar] [CrossRef]
- Frankel, E. Volatile lipid oxidation products. Prog. Lipid Res. 1983, 22, 1–33. [Google Scholar] [CrossRef]
- Wanasundara, P.; Wanasundara, U.; Shahidi, F. Changes in flax (Linum usitatissimum L.) seed lipids during germination. J. Am. Oil Chem. Soc. 1999, 76, 41–48. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, J.; Hu, S.; Dong, J.; Yu, J.; Fang, L.; Huang, S.; Wang, L. Comparative proteomic analysis of different barley cultivars during seed germination. J. Cereal Sci. 2021, 102, 103357. [Google Scholar] [CrossRef]
- Leymarie, J.; Bruneaux, E.; Gibot-Leclerc, S.; Corbineau, F. Identification of transcripts potentially involved in barley seed germination and dormancy using cDNA-AFLP. J. Exp. Bot. 2007, 58, 425–437. [Google Scholar] [CrossRef] [Green Version]
- Frank, T.; Scholz, B.; Peter, S.; Engel, K.-H. Metabolite profiling of barley: Influence of the malting process. Food Chem. 2011, 124, 948–957. [Google Scholar] [CrossRef]
- Gupta, S.; Rupasinghe, T.; Callahan, D.L.; Natera, S.H.; Smith, P.; Hill, C.B.; Roessner, U.; Boughton, B.A. Spatio-temporal metabolite and elemental profiling of salt stressed barley seeds during initial stages of germination by MALDI-MSI and µ-XRF spectrometry. Front Plant Sci. 2019, 10, 1139. [Google Scholar] [CrossRef] [Green Version]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Basserdorf, Switzerland, 2006. [Google Scholar]
- Dong, X.; Agarwal, M.; Xiao, Y.; Ren, Y.; Maker, G.; Yu, X. Ozone Efficiency on Two Coleopteran Insect Pests and Its Effect on Quality and Germination of Barley. Insects 2022, 13, 318. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Pearce, D.W.; Poole, A.T.; Pharis, R.P.; Mander, L.N. Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol. Plant. 2002, 115, 428–441. [Google Scholar] [CrossRef]
- Favier, J. A model for germination rate during dormancy loss in Hordeum vulgare. Ann. Bot. 1995, 76, 631–638. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.; Baskin, J. A graphical method for identifying the six types of non-deep physiological dormancy in seeds. Plant Biol. 2017, 19, 673–682. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.-Q.; Luan, S.; Li, J.; He, Z.-H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [Green Version]
- Ketring, D.; Pattee, H. Ethylene and lipoxygenase in relation to afterripening of dormant NC-13 peanut seeds. Peanut Sci. 1985, 12, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, V.O.; Penido, A.C.; Pereira, D.d.S.; Oliveira, A.M.; Mendes, A.E.S.; Oliveira, J.A. Sanitary and Physiological Quality of Soybean Seeds Treated with Ozone. J. Agric. Sci. 2019, 11, 183–196. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, L.; Liu, J.; Dong, J.; Yin, H.; Yu, J.; Huang, S.; Hu, S.; Lin, H. Effect of hydrogen peroxide and ozone treatment on improving the malting quality. J. Cereal Sci. 2020, 91, 102882. [Google Scholar] [CrossRef]
- Michalak, M.; Plitta-Michalak, B.P.; Nadarajan, J.; Colville, L. Volatile signature indicates viability of dormant orthodox seeds. Physiol. Plant. 2021, 173, 788–804. [Google Scholar] [CrossRef]
- Kim, S.-C.; Lee, J.-H.; Kim, M.-H.; Lee, J.-A.; Kim, Y.B.; Jung, E.; Kim, Y.-S.; Lee, J.; Park, D. Hordenine, a single compound produced during barley germination, inhibits melanogenesis in human melanocytes. Food Chem. 2013, 141, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Mohammad, T.; Shamsi, A.; Queen, A.; Parveen, S.; Luqman, S.; Hasan, G.M.; Alamry, K.A.; Azum, N.; Asiri, A.M. Discovery of Hordenine as a potential inhibitor of pyruvate dehydrogenase kinase 3: Implication in lung Cancer therapy. Biomedicines 2020, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Borek, S.; Ratajczak, W.; Ratajczak, L. Regulation of storage lipid metabolism in developing and germinating lupin (Lupinus spp.) seeds. Acta Physiol. Plant. 2015, 37, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jin, E.S.; Sherry, A.D.; Malloy, C.R. Metabolism of glycerol, glucose, and lactate in the citric acid cycle prior to incorporation into hepatic acylglycerols. J. Biol. Chem. 2013, 288, 14488–14496. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [Green Version]
- Chiou, R.Y.-Y.; Ku, K.-L.; Chen, W.-L. Compositional characterization of peanut kernels after subjection to various germination times. J. Agric. Food Chem. 1997, 45, 3060–3064. [Google Scholar] [CrossRef]
- Li, P.; Zhu, Y.; Li, S.; Zhang, A.; Zhao, W.; Zhang, J.; Chen, Q.; Ren, S.; Liu, J.; Wang, H. Variation patterns of the volatiles during germination of the foxtail millet (setaria italic): The relationship between the volatiles and fatty acids in model experiments. Molecules 2020, 25, 1238. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.B. Acetone production during the germination of fatty seeds. Physiol. Plant. 1985, 63, 231–234. [Google Scholar] [CrossRef]
- Filipowska, W.; Jaskula-Goiris, B.; Ditrych, M.; Bustillo Trueba, P.; De Rouck, G.; Aerts, G.; Powell, C.; Cook, D.; De Cooman, L. On the contribution of malt quality and the malting process to the formation of beer staling aldehydes: A review. J. Inst. Brew. 2021, 127, 107–126. [Google Scholar] [CrossRef]
- Hambraeus, G.; Nyberg, N. Enzymatic hydrogenation of trans-2-nonenal in barley. J. Agric. Food Chem. 2005, 53, 8714–8721. [Google Scholar] [CrossRef]
- Janeš, D.; Kantar, D.; Kreft, S.; Prosen, H. Identification of buckwheat (Fagopyrum esculentum Moench) aroma compounds with GC–MS. Food Chem. 2009, 112, 120–124. [Google Scholar] [CrossRef]
- Darabi, H.R.; Mohandessi, S.; Balavar, Y.; Aghapoor, K. A structure-activity relationship study on a natural germination inhibitor, 2-methoxy-4-vinylphenol (MVP), in wheat seeds to evaluate its mode of action. Z. Fur Nat. 2007, 62, 694–700. [Google Scholar] [CrossRef]




| Chemical Groups | Compounds | RT a | RI (lib) b | RI (cal) c | Peak Area (log 10) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (Ozone (0 min)) | Ozone (15 min) | |||||||||
| D2 | D4 | D7 | D2 | D4 | D7 | |||||
| Alcohol | 2-Propanol, 1-methoxy- | 4.11 | 661 | n.d. d | n.d. | n.d. | 5.44 ± 0.40 e | n.d. | n.d. | |
| 1-Butanol, 3-methyl- | 5.45 | 736 | 732 | n.d. | 4.32 ± 0.21 | 4.74 ± 0.04 | n.d. | n.d. | 4.53 ± 0.06 | |
| 1-Butanol, 2-methyl- | 5.54 | 739 | 736 | n.d. | 4.18 ± 0.24 | 4.19 ± 0.03 | n.d. | 4.01 ± 0.19 | 4.37 ± 0.07 | |
| 2-Pentanol,4-methyl- | 6.67 | 758 | 778 | n.d. | n.d. | 4.67 ± 0.31 | n.d. | 4.63 ± 0.24 | 4.90 ± 0.86 | |
| 2,3-Butanediol | 7.01 | 788 | 791 | n.d. | n.d. | 4.80 ± 0.09 | 4.55 ± 0.44 | 5.12 ± 0.36 | 4.94 ± 0.09 | |
| 3-Hexen-1-ol, (Z) | 9.02 | 839 | 856 | n.d. | 4.93 ± 0.39 | 5.21 ± 0.20 | n.d. | n.d. | 4.91 ± 0.01 | |
| 1-Hexanol | 9.42 | 868 | 868 | n.d. | n.d. | 4.51 ± 0.04 | n.d. | n.d. | n.d. | |
| 1-Hexanol, 2-ethyl- | 14.67 | 1030 | 1029 | n.d. | 4.68 ± 0.15 | n.d. | n.d. | n.d. | 4.36 ± 0.17 | |
| 2-Nonen-1-ol, (E)- | 19.09 | 1169 | 1171 | 4.23 ± 0.14 | 4.58 ± 0.34 | 4.80 ± 0.14 | 4.63 ± 0.28 | 4.82 ± 0.06 | n.d. | |
| Aldehyde | Acetaldehyde | 2.75 | n.d. | 5.20 ± 0.09 | n.d. | n.d. | 5.07 ± 0.26 | n.d. | ||
| Hexanal | 7.26 | 801 | 801 | 4.24 ± 0.16 | 4.96 ± 0.06 | 4.60 ± 0.22 | 4.65 ± 0.24 | 4.37 ± 0.04 | 4.61 ± 0.23 | |
| Octanal | 13.85 | 1003 | 1003 | n.d. | n.d. | 4.53 ± 0.18 | n.d. | n.d. | n.d. | |
| 2-Nonenal, (E)- | 18.82 | 1162 | 1162 | 3.70 ± 0.14 | 4.90 ± 0.15 | 5.46 ± 0.17 | n.d. | 4.58 ± 0.48 | n.d. | |
| Heterocyclic | Furan, 2-pentyl- | 13.48 | 993 | 995 | 3.90 ± 0.16 | 4.53 ± 0.20 | 4.28 ± 0.08 | 4.09 ± 0.25 | 3.81 ± 0.21 | 4.34 ± 0.10 |
| Hydrocarbon | 7-Tetradecene | 24.33 | 1369 | 1356 | n.d. | 4.49 ± 0.14 | 4.59 ± 0.21 | n.d. | 3.71 ± 0.05 | 4.55 ± 0.09 |
| (-)-Aristolene | 26.39 | 1453 | 1435 | 4.30 ± 0.14 | 4.62 ± 0.27 | 4.35 ± 0.08 | 4.35 ± 0.09 | 4.05 ± 0.07 | 4.66 ± 0.02 | |
| Aromadendrene | 26.58 | 1461 | 1443 | 4.41 ± 0.38 | 4.84 ± 0.30 | n.d. | 4.13 ± 0.19 | 3.65 ± 0.10 | n.d. | |
| β-Guaiene | 26.87 | 1490 | 1454 | 4.70 ± 0.12 | 4.58 ± 0.04 | 4.28 ± 0.07 | 4.53 ± 0.12 | 4.26 ± 0.14 | 4.57 ± 0.11 | |
| Ketone | Acetone | 2.02 | n.d. | 5.62 ± 0.07 | 6.31 ± 0.12 | n.d. | 5.48 ± 0.10 | 6.30 ± 0.13 | ||
| Acetoin | 4.91 | 713 | 712 | n.d. | 5.82 ± 0.24 | 6.12 ± 0.14 | n.d. | 5.99 ± 0.39 | 5.72 ± 0.09 | |
| 5-Hepten-2-one, 6-methyl- | 13.34 | 986 | 991 | n.d. | 4.42 ± 0.25 | n.d. | 4.34 ± 0.07 | n.d. | n.d. | |
| 4-Acetyl-1-methylcyclohexene | 17.43 | 1110 | 1116 | 4.36 ± 0.14 | 4.76 ± 0.06 | n.d. | n.d. | n.d. | n.d. | |
| Phenol | Phenol | 13.07 | 981 | 982 | 4.08 ± 0.28 | n.d. | n.d. | 4.39 ± 0.19 | n.d. | n.d. |
| 2,4-Di-tert-butylphenol | 28.39 | 1519 | 1515 | n.d. | 4.51 ± 0.26 | n.d. | n.d. | n.d. | n.d. | |
| Chemical Groups | Compounds | RT a | RI (lib) b | RI (cal) c | Control (Ozone (0 min)) | Ozone (15 min) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| D2 | D4 | D7 | D2 | D4 | D7 | |||||
| Aldehyde | Benzeneacetaldehyde | 17.06 | 1045 | 1047 | n.d. d | 6.09 ± 0.04 e | 5.47 ± 0.02 | 5.45 ± 0.29 | 5.57 ± 0.17 | 5.30 ± 0.26 |
| 2-Decenal, (Z)- | 23.70 | 1252 | 1263 | 5.25 ± 0.06 | 5.65 ± 0.10 | 5.43 ± 0.01 | 5.11 ± 0.01 | 5.18 ± 0.07 | 5.16 ± 0.03 | |
| 2-Undecenal | 26.52 | 1367 | 1365 | 5.20 ± 0.05 | n.d. | n.d. | 4.95 ± 0.04 | 4.74 ± 0.01 | 5.01 ± 0.13 | |
| Alkaloid | Hordenine | 29.21 | 1495 | 1479 | 6.15 ± 0.01 | 6.02 ± 0.03 | 6.63 ± 0.16 | 6.13 ± 0.06 | 6.38 ± 0.12 | 6.55 ± 0.08 |
| Fatty acid | Acetic acid | 5.18 | 610 | 4.94 ± 0.09 | 7.19 ± 0.03 | 7.04 ± 0.05 | n.d. | 6.74 ± 0.05 | 7.01 ± 0.05 | |
| Palmitic acid | 36.87 | 1968 | 1962 | 5.43 ± 0.01 | 6.85 ± 0.02 | 6.91 ± 0.06 | 6.12 ± 0.21 | 6.62 ± 0.08 | n.d. | |
| Oleic acid | 38.23 | 2133 | 2141 | 5.20 ± 0.07 | 6.55 ± 0.08 | 6.73 ± 0.01 | 5.71 ± 0.12 | 6.44 ± 0.13 | 6.88 ± 0.01 | |
| stearic acid | 38.37 | 2172 | 2161 | 4.36 ± 0.17 | 5.55 ± 0.02 | 5.53 ± 0.05 | n.d. | n.d. | 6.73 ± 0.09 | |
| Heterocyclic | 2-Furanmethanol | 10.88 | 860 | 869 | n.d. | 6.22 ± 0.08 | 5.67 ± 0.18 | n.d. | 5.66 ± 0.13 | 5.52 ± 0.44 |
| 2-furancarboxaldehyde, 5-methyl | 14.30 | 964 | 965 | n.d. | 5.82 ± 0.12 | 5.33 ± 0.04 | n.d. | n.d. | 5.44 ± 0.12 | |
| 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | 14.98 | 989 | 984 | 4.61 ± 0.23 | 6.29 ± 0.13 | 5.76 ± 0.19 | n.d. | 5.18 ± 0.25 | 5.73 ± 0.40 | |
| Furaneol | 17.61 | 1070 | 1063 | n.d. | 6.37 ± 0.01 | 5.90 ± 0.02 | n.d. | 5.48 ± 0.05 | 5.79 ± 0.23 | |
| Maltol | 19.30 | 1110 | 1116 | 5.19 ± 0.02 | 6.24 ± 0.03 | 5.12 ± 0.03 | n.d. | n.d. | 5.50 ± 0.06 | |
| 2(3H)-Furanone, dihydro-4-hydroxy- | 20.68 | 1185 | 1161 | 4.34 ± 0.10 | 6.49 ± 0.18 | 5.62 ± 0.07 | n.d. | n.d. | 5.68 ± 0.19 | |
| 5-Hydroxymethylfurfural | 22.72 | 1232 | 1229 | 4.55 ± 0.09 | 6.94 ± 0.08 | 5.76 ± 0.17 | n.d. | n.d. | 5.61 ± 0.19 | |
| Ketone | 2-Propanone,1-hydroxy | 5.87 | 666 | 4.66 ± 0.02 | 6.76 ± 0.01 | 6.74 ± 0.09 | n.d. | 6.51 ± 0.06 | 6.71 ± 0.02 | |
| 4-Cyclopentene-1,3-dione | 11.51 | 883 | 886 | n.d. | 6.16 ± 0.07 | 5.67 ± 0.01 | n.d. | 5.49 ± 0.15 | 5.68 ± 0.20 | |
| 2-Cylopenten-1-one, 2-hydroxy- | 13.13 | 926 | 932 | 5.06 ± 0.15 | 6.44 ± 0.10 | 5.99 ± 0.05 | 5.38 ± 0.17 | 5.72 ± 0.11 | 5.93 ± 0.21 | |
| 2-Hydroxy-gamma-butyrolactone | 15.36 | 1011 | 995 | 6.30 ± 0.13 | 6.42 ± 0.01 | 6.18 ± 0.02 | 5.16 ± 0.35 | 5.78 ± 0.05 | 6.16 ± 0.07 | |
| 1,2-Cyclopentanedione,3-methyl- | 16.54 | 1028 | 1031 | 5.72 ± 0.09 | n.d. | n.d. | n.d. | n.d. | 5.25 ± 0.14 | |
| 2-Pentanone, 5-(acetyloxy)- | 17.32 | 1053 | 1055 | n.d. | n.d. | n.d. | n.d. | 4.94 ± 0.19 | 5.53 ± 0.05 | |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 20.27 | 1150 | 1147 | n.d. | 7.00 ± 0.07 | 6.49 ± 0.13 | n.d. | 5.79 ± 0.06 | 6.58 ± 0.20 | |
| 4H-Pyran-4-one, 3,5-dihydroxy-2-methyl- | 21.53 | 1196 | 1188 | n.d. | 5.73 ± 0.10 | n.d. | n.d. | n.d. | 5.36 ± 0.08 | |
| 2-Heptadecanone | 36.36 | 1904 | 1903 | 5.18 ± 0.01 | 5.18 ± 0.01 | n.d. | 4.97 ± 0.09 | 4.70 ± 0.02 | n.d. | |
| Phenol | 4-Vinylphenol | 22.38 | 1223 | 1217 | n.d. | 5.54 ± 0.01 | 5.27 ± 0.12 | n.d. | n.d. | 5.32 ± 0.20 |
| 2-Methoxy-4-vinylphenol | 25.25 | 1316 | 1318 | n.d. | 5.44 ± 0.07 | 5.39 ± 0.18 | 4.76 ± 0.13 | 5.01 ± 0.08 | 5.58 ± 0.17 | |
| Phenol, 4-ethenyl-2,6-dimethoxy- | 31.68 | 1567 | 1584 | n.d. | 5.52 ± 0.05 | 5.78 ± 0.13 | n.d. | 5.23 ± 0.04 | 5.92 ± 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Sun, L.; Agarwal, M.; Maker, G.; Han, Y.; Yu, X.; Ren, Y. The Effect of Ozone Treatment on Metabolite Profile of Germinating Barley. Foods 2022, 11, 1211. https://doi.org/10.3390/foods11091211
Dong X, Sun L, Agarwal M, Maker G, Han Y, Yu X, Ren Y. The Effect of Ozone Treatment on Metabolite Profile of Germinating Barley. Foods. 2022; 11(9):1211. https://doi.org/10.3390/foods11091211
Chicago/Turabian StyleDong, Xue, Litao Sun, Manjree Agarwal, Garth Maker, Yitao Han, Xiangyang Yu, and Yonglin Ren. 2022. "The Effect of Ozone Treatment on Metabolite Profile of Germinating Barley" Foods 11, no. 9: 1211. https://doi.org/10.3390/foods11091211






