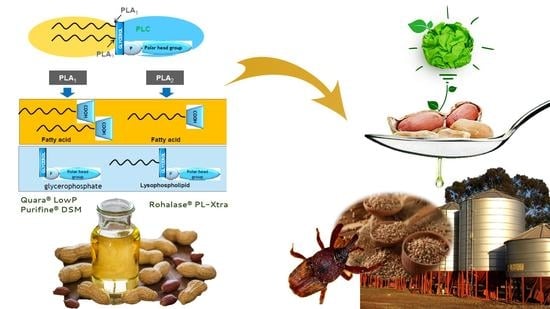
Chemical vs. Enzymatic Refining to Produce Peanut Oil for Edible Use or to Obtain a Sustainable and Cost-Effective Protector for Stored Grains against Sitophilus zeamais (Coleoptera: Curculionidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Refining Process
2.3. Enzymes
- Quara® LowP, produced by Novozymes (Monza, Italy) and distributed in Italy by Univar Solutions;
- ROHALASE® PL-XTRA, produced by AB Enzymes and distributed in Italy by Barentz;
- Purifine® DSM, produced by DSM Food Specialties B.V. (Delft, The Netherlands).
- Quara® LowP is a PLA1, having hydrolytic activity on fatty acids in sn-1 position and partial activity also tested on fatty acids in sn-2 position;
- ROHALASE® PL-XTRA is a PLA2, having exclusive hydrolytic activity on the fatty acids in the sn-2 position;
- Purifine® DSM is a PLA1, a (lyso-) phospholipase that cuts the sn-1 position of a (lyso-) phospholipid.
- Enzyme dosage equal to 30 g/t of crude oil;
- Temperature of the enzymatic degumming phase equal to 60 °C;
- Water dosage in the enzymatic degumming phase equal to 2.5%;
- 50% citric acid dosage in the enzymatic degumming phase equal to 650 g/t oil (pH mixture with oil ≅ 4);
- Contact time of the enzymatic degumming phase equal to 30 min.
2.4. Samples
- RA—Conventional degumming SALOV;
- RD—Rectified with Purifine® DSM;
- RQ—Rectified with Quara® LowP;
- RR—Rectified with ROHALASE® PL-XTRA;
- S—Crude peanut oil.
2.5. Reagents
- Citric acid (C6H8O7) at 50% by Bionova;
- 50% NaOH by Subolab GmbH;
- Drinking tap water;
- Ethanol blend—diethyl ether by Applichem 1:1 v/v + 15 mg/L phenophthalein by Biopharm;
- NaOH 0.1 N by Sigma-Aldrich;
- 10° Bé solution of sodium sulphate (Na2SO4) by Sigma-Aldrich;
- 98% sulfuric acid (H2SO4) by Sigma-Aldrich;
- Citric acid (C6H8O7) monohydrate by Bionova;
- Bleaching earths (CC160 Carbonitalia srl).
2.6. Free Fatty Acidity
2.7. Color Measurement
2.8. Phospholipids
2.9. Sitophilus Zeamais Rearing Conditions
2.10. Toxicity of the Oils on Sitophilus Zeamais
2.11. Statistical Data Processing
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gargouri, B.; Zribi, A.; Bouaziz, M. Effect of containers on the quality of Chemlali olive oil during storage. J. Food Sci. Technol. 2015, 52, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Eftekhardadkhah, M.; Øye, G. Correlations between Crude Oil Composition and Produced Water Quality: A Multivariate Analysis Approach. Ind. Eng. Chem. Res. 2013, 52, 17315–17321. [Google Scholar] [CrossRef]
- Lercker, G.; Rodriguez-Estrada, M.T. Chromatographic analysis of unsaponifiable compounds of olive oils and fat-containing foods. J. Chromatogr. A 2000, 881, 105–129. [Google Scholar] [CrossRef]
- Erol, A.S.; Özcan, M.M.; Er, F. Composition and characteristics of some seed oils. Asian J. Chem. 2011, 23, 1851–1853. [Google Scholar]
- Dijkstra, A.J. Enzymatic degumming. Eur. J. Lipid Sci. Technol. 2010, 112, 1178–1189. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.H.; Yang, J.G. Optimization of enzymatic degumming process for rapeseed oil. J. Am. Oil Chem. Soc. 2006, 83, 653–658. [Google Scholar] [CrossRef]
- Sampaio, K.A.; Zyaykina, N.; Wozniak, B.; Tsukamoto, J.; De Greyt, W.; Stevens, C.V. Enzymatic degumming: Degumming efficiency versus yield increase. Eur. J. Lipid Sci. Technol. 2015, 117, 81–86. [Google Scholar] [CrossRef]
- Mei, L.; Wang, L.; Li, Q.; Yu, J.; Xu, X. Comparison of acid degumming and enzymatic degumming process for Silybum marianum seed oil. J. Sci. Food Agric. 2013, 93, 2822–2828. [Google Scholar] [CrossRef]
- Cerminati, S.; Paoletti, L.; Aguirre, A.; Peirú, S.; Menzella, H.G.; Castelli, M.E. Industrial uses of phospholipases: Current state and future applications. Appl. Microbiol. Biotechnol. 2019, 103, 2571–2582. [Google Scholar] [CrossRef]
- Meinert, H.; Yi, D.; Zirpel, B.; Schuiten, E.; Geißler, T.; Gross, E.; Brückner, S.I.; Hartmann, B.; Röttger, C.; Ley, J.P.; et al. Discovery of Novel Bacterial Chalcone Isomerases by a Sequence-Structure-Function-Evolution Strategy for Enzymatic Synthesis of (S)-Flavanones. Angew. Chem. 2021, 60, 16874–16879. [Google Scholar] [CrossRef] [PubMed]
- Madhaven, B.N. Final report on the safety assessment of peanut (Arachis hypogaea) oil, hydrogenated peanut oil, peanut acid, peanut glycerides, and peanut (Arachis hypogaea) flour. Int. J. Toxicol. 2001, 20 (Suppl. 2), 65–77. [Google Scholar] [CrossRef]
- Hill, J.; Schoonhoven, A.V. Effectiveness of vegetable oil fractions in controlling the Mexican bean weevil on stored beans. J. Econ. Entomol. 1981, 74, 478–479. [Google Scholar] [CrossRef]
- Ivbijaro, M.F. Groundnut oil as a protectant of maize from damage by the maize weevil Sitophilus zeamais Motsch. Protect. Ecol. 1984, 6, 267–270. [Google Scholar]
- Ivbijaro, M.F.; Ligan, C.; Youdeowei, A. Control of rice weevils, Sitophilus oryzae (L.), in stored maize with vegetable oils. Agric. Ecosyst. Environ. 1985, 14, 237–242. [Google Scholar] [CrossRef]
- Kellouche, A.; Soltani, N.; Kreiter, S.; Auger, J.; Arnold, I.; Kreiter, P. Biological activity of four vegetable oils on Callosobruchus maculatus (Fabricius) (Coleoptera Bruchidae). Redia 2004, 87, 39–47. [Google Scholar]
- Law-Ogbomo, K.; Enobakhare, D.A. Efficacy of rubber seed oil, palm oil and palm kernel oil as grain protectants against Sitophilus zeamais (Mots.) (Coleoptera: Curculionidae) in three maize varieties. J. Entomol. 2006, 3, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Gemechu, F.; Santiago, D.R.; Sori, W. Laboratory evaluation of cotton (Gossypium hirsutum) and Ethiopian mustard (Brassica carinata) seed oils as grain protectants against maize weevil, Sitophilus zeamais M. (Coleoptera: Curculionidae). Afr. J. Agric. Res. 2013, 8, 4374–4379. [Google Scholar] [CrossRef]
- Wale, M.; Assegie, H. Efficacy of castor bean oil (Ricinus communis L.) against maize weevils (Sitophilus zeamais Mots.) in northwestern Ethiopia. J. Stored Prod. Res. 2015, 63, 38–41. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 261, 916–919. [Google Scholar] [CrossRef] [Green Version]
- Singano, C.D.; Mvumi, B.M.; Stathers, T.E.; Machekano, H.; Nyamukondiwa, C. What does global warming mean for stored-grain protection? Options for Prostephanus truncatus (Horn) control at increased temperatures. J. Stored Prod. Res. 2020, 85, 101532. [Google Scholar] [CrossRef]
- Pierattini, E.C.; Bedini, S.; Venturi, F.; Ascrizzi, R.; Flamini, G.; Bocchino, R.; Girardi, J.; Giannotti, P.; Ferroni, G.; Conti, B. Sensory quality of essential oils and their synergistic effect with diatomaceous earth, for the control of stored grain insects. Insects 2019, 10, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi, H.; Toprak, U.; Emekci, M.; Bagci, F.; Ferizli, A.G. Persistence of diatomaceous earth, SilicoSec® against three stored grain beetles. J. Stored Prod. Res. 2020, 89, 101724. [Google Scholar] [CrossRef]
- Salim, M.; Gökçe, A.; Naqqash, N.M.; Ersoy, O. Insecticidal potential of native diatomaceous earth against Sitophilus granarius (Coleoptera: Curculionidae). Sarhad J. Agric. 2020, 36, 729–733. [Google Scholar] [CrossRef]
- Bertoli, A.; Conti, B.; Mazzoni, V.; Meini, L.; Pistelli, L. Volatile chemical composition and bioactivity of six essential oils against the stored food insect Sitophilus zeamais Motsch. (Coleoptera Dryophthoridae). Nat. Prod. Res. 2012, 26, 2063–2071. [Google Scholar] [CrossRef]
- Bougherra, H.H.; Bedini, S.; Flamini, G.; Cosci, F.; Belhamel, K.; Conti, B. Pistacia lentiscus essential oil has repellent effect against three major insect pests of pasta. Ind. Crops Prod. 2015, 63, 249–255. [Google Scholar] [CrossRef]
- Bedini, S.; Bougherra, H.H.; Flamini, G.; Cosci, F.; Belhamel, K.; Ascrizzi, R.; Conti, B. Repellency of anethole- and estragole-type fennel essential oils against stored grain pests: The different twins. Bull. Insectol. 2016, 69, 149–157. [Google Scholar]
- Romani, R.; Bedini, S.; Salerno, G.; Ascrizzi, R.; Flamini, G.; Echeverria, M.C.; Farina, P.; Conti, B. Andean flora as a source of new repellents against insect pests: Behavioral, morphological and electrophysiological studies on Sitophilus zeamais (Coleoptera: Curculionidae). Insects 2019, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Adarkwah, C.; Obeng-Ofori, D.; Opuni-Frimpong, E.; Ulrichs, C.; Schöller, M. Predator-parasitoid-host interaction: Biological control of Rhyzopertha dominica and Sitophilus oryzae by a combination of Xylocoris flavipes and Theocolax elegans in stored cereals. Entomol. Exp. Appl. 2019, 167, 118–128. [Google Scholar] [CrossRef]
- Kamboh, K.M.S.; Aqueel, M.A.; Raza, M.A. Evaluation of parasitic potential of Anisopteromalus calandrae (Howard) against Callosobruchus maculatus (F.), Rhyzopertha dominica (F.) and Sitophilus oryzae (L.) in grains treated with diatomaceous earths. Pak. J. Agric. Sci. 2021, 58, 1161–1167. [Google Scholar] [CrossRef]
- Tsaganou, F.K.; Vassilakos, T.N.; Athanassiou, C.G. Insecticidal effect of thiamethoxam against seven stored-product beetle species. J. Stored Prod. Res. 2021, 93, 101843. [Google Scholar] [CrossRef]
- Afful, E.; Elliott, B.; Nayak, M.K.; Phillips, T.W. Phosphine resistance in North American field populations of the lesser grain borer, Rhyzopertha dominica (Coleoptera: Bostrichidae). J. Econ. Entomol. 2018, 111, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Lampiri, E.; Agrafioti, P.; Athanassiou, C.G. Delayed mortality, resistance and the sweet spot, as the good, the bad and the ugly in phosphine use. Sci. Rep. 2021, 11, 3933. [Google Scholar] [CrossRef] [PubMed]
- Wakil, W.; Kavallieratos, N.G.; Usman, M.; Gulzar, S.; El-Shafie, H.A.F. Detection of phosphine resistance in field populations of four key stored-grain insect pests in Pakistan. Insects 2021, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, R.; Rosentrater, K.A.; Bern, C.J. Evaluation of maize weevils Sitophilus zeamais Motschulsky infestation on seven varieties of maize. J. Stored Prod. Res. 2015, 64, 97–102. [Google Scholar] [CrossRef]
- Ojo, J.A.; Omoloye, A.A. Development and life history of Sitophilus zeamais (Coleoptera: Curculionidae) on cereal crops. Adv. Agric. 2016, 2016, 7836379. [Google Scholar] [CrossRef] [Green Version]
- Flori, L.; Macaluso, M.; Taglieri, I.; Sanmartin, C.; Sgherri, C.; Leo, M.D.; Ciccone, V.; Donnini, S.; Venturi, F.; Pistelli, L. Development of Fortified Citrus Olive Oils: From Their Production to Their Nutraceutical Properties on the Cardiovascular System. Nutrients 2020, 12, 1557. [Google Scholar] [CrossRef]
- Szabó, R.T.; Mézes, M.; Szalai, T.; Zajácz, E.; Weber, M. Colour identification of honey and methodical development of its instrumental measuring. Columella J. Agric. Environ. Sci. 2016, 3, 29–36. [Google Scholar] [CrossRef]
- Bacle, A.; Kadri, L.; Khoury, S.; Ferru-Clément, R.; Faivre, J.-F.; Cognard, C.; Bescond, J.; Krzesiak, A.; Contzler, H.; Delpech, N.; et al. A comprehensive study of phospholipid fatty acid rearrangements in the metabolic syndrome: Correlations to organ dysfunction. Dis. Models Mech. 2020, 13, dmm043927. [Google Scholar] [CrossRef] [Green Version]
- Abbott, W.J. A method of computing effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 256–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, NY, USA, 1971; p. 333. [Google Scholar] [CrossRef]
- Bedini, S.; Muniz, E.R.; Tani, C.; Conti, B.; Ruiu, L. Insecticidal potential of Brevibacillus laterosporus against dipteran pest species in a wide ecological range. J. Invertebr. Pathol. 2020, 177, 107493. [Google Scholar] [CrossRef] [PubMed]
- Legge 13 febbraio 1957, n. 12. Conversione in legge del decreto-legge 20 dicembre 1956, n. 1380, pubblicato nella Gazzetta Ufficiale n. 321 del 21 dicembre 1956, che proroga le disposizioni di cui al decreto—legge 2 febbraio 1956, n. 28, convertito con modificazioni, nella legge 27 marzo 1956, n. 162, ed apporta modificazioni all’art. 30 del testo unico delle disposizioni concernenti la disciplina fiscale della lavorazione dei semi oleosi e degli oli da essi ottenuti, approvato con decreto del Presidente della Repubblica 22 dicembre 1954, n. 1217. Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:legge:1957-02-13;12@originale (accessed on 14 March 2022).
- Obeng-Ofori, D.; Amiteye, S. Efficacy of mixing vegetable oils with pirimiphos-methyl against the maize weevil, Sitophilus zeamais Motschulsky in stored maize. J. Stored Prod. Res. 2005, 41, 57–66. [Google Scholar] [CrossRef]
- Tembo, E.; Murfitt, R.F.A. Effect of combining vegetable oil with pirimiphos-methyl for protection of stored wheat against Sitophilus granarius (L.). J. Stored Prod. Res. 1995, 31, 77–81. [Google Scholar] [CrossRef]
- Aider, F.A.; Kellouche, A.; Fellag, H.; Debras, J.F. Evaluation of the bio-insecticidal effects of the main fatty acids of olive oil on Callosobruchus maculatus F. (Coleoptera-Bruchidae) in cowpea (Vigna unguiculata (L.)). J. Plant Dis. Prot. 2016, 123, 235–245. [Google Scholar] [CrossRef]

| Acidity Free (% Oleic Acid) | RA | RQ | RR | RD | F-Value | R2 |
|---|---|---|---|---|---|---|
| Starting acidity sample crude oil (%) | 1.63 a ± 0.05 | 1.63 a ± 0.06 | 1.63 a ± 0.06 | 1.36 b ± 0.05 | 19.07 | 0.87 |
| Oil acidity degummed (%) | 1.47 b ± 0.09 | 1.73 a ± 0.06 | 1.71 a ± 0.03 | 1.12 c ± 0.03 | 196.62 | 0.99 |
| Neutral oil acidity (%) | 0.34 b ± 0.05 | 0.23 c ± 0.13 | 0.27 c ± 0.19 | 0.47 a ± 0.14 | 5.79 | 0.69 |
| Olein acidity (%) | 65.44 c ± 0.83 | 70.54 bc ± 2.83 | 76.15 ab ± 7.35 | 96.82 a ± 10.35 | 16.25 | 0.86 |
| Physical Parameters | RA | RQ | RR | RD | F-Value | R2 |
|---|---|---|---|---|---|---|
| Sample starting weight crude (g) | 600.06 a ± 0.06 | 600.08 a ± 0.11 | 600.05 a ± 0.04 | 600.00 a ± 0.02 | 0.63 | 0.19 |
| Total weight of degummed oil obtained from the first phase of degumming (g) | 587.68 b ± 1.04 | 589.24 a ± 1.13 | 589.22 a ± 1.17 | 585.94 c ± 1.12 | 1.80 | 0.40 |
| Total weight of tires separated from the first degumming phase (g) | 8.27 b ± 1.32 | 7.08 c ± 1.86 | 7.67 c ± 1.23 | 12.37 a ± 1.34 | 49.96 | 0.95 |
| Total weight of degummed oil obtained from the second degumming phase (g) | 575.87 b ± 2.01 | 579.52 a ± 2.35 | 579.29 a ± 5.62 | 580.71 a ± 5.62 | 1.04 | 0.28 |
| Total weight of separate tires from the second phase of degumming (g) | 10.48 a ± 1.21 | 8.94 b ± 1.34 | 9.94 b ± 0.75 | 8.18 c ± 0.84 | 3.59 | 0.58 |
| Total volume of separate tires from the second phase of degumming (mL) | 10.63 a ± 1.43 | 8.00 b ± 1.50 | 8.05 b ± 1.61 | 5.02 c ± 1.54 | 3.60 | 0.57 |
| Total oil weight retained by the soap pastes (g) | 1.04 b ± 0.63 | 0.59 c ± 0.32 | 0.70 c ± 0.20 | 2.06 a ± 0.30 | 25.74 | 0.90 |
| Total weight of soap pastes degreased separated by the neutralization phase (g) | 18.04 b ± 0.44 | 21.73 a ± 0.68 | 21.21 a ± 1.13 | 12.85 c ± 1.22 | 28.21 | 0.91 |
| Olein weight obtained for 12 g of split soapy pastes (g) | 3.98 b ± 0.17 | 4.31 a ± 1.11 | 4.35 a ± 1.04 | 4.30 a ± 1.03 | 1.63 | 0.37 |
| Colorimetric Test (Lovibond) | RA | RQ | RR | RD | F-Value | R2 |
|---|---|---|---|---|---|---|
| R | 0.60 a ± 0.05 | 0.50 b ± 0.02 | 0.50 b ± 0.03 | 0.50 b ± 0.02 | 0.60 | 0.19 |
| Y | 4.20 a ± 0.30 | 4.17 a ± 0.07 | 4.07 a ± 0.35 | 4.12 a ± 0.30 | 0.16 | 0.06 |
| Phosphorus and Phospholipids | S | RA | RQ | RR | RD | F-Value | R2 |
|---|---|---|---|---|---|---|---|
| Content in P (ppm) | 127.11 a ± 5.22 | 69.12 b ± 0.13 | 60.22 d ± 0.11 | 52.17 e ± 0.14 | 63.52 c ± 0.12 | 497.02 | 0.99 |
| Phospholipid content (ppm) | 3289.17 a ± 16.22 | 1718.17 b ± 15.32 | 1503.66 c ± 11.13 | 1266.82 d ± 10.12 | 1613.17 b ± 12.32 | 11,135.08 | 0.99 |
| Phosphorus abatement compared to crude oil (%) | - | 45.62 | 52.62 | 58.95 | 50.02 | - | - |
| Theoretical Returns of Process Phases | RA | RQ | RR | RD |
|---|---|---|---|---|
| Yield of degumming phase (%) | 95.97 | 96.57 | 96.54 | 96.74 |
| Yield phase neutralization (%) | 96.51 | 96.57 | 96.62 | 94.95 |
| Washing phase yield (%) | 98.50 | 98.64 | 98.79 | 98.26 |
| Oil | LC50 (95% FL) | Intercept ± SE | p-Value |
|---|---|---|---|
| RA | 1.372 (1.025–1.858) a | −0.117 ± 0.055 | 0.03 |
| RD | 2.453 (1.796–3.429) b | −0.330 ± 0.060 | <0.001 |
| RQ | 4.076 (2.930–5.952) c | −0.518 ± 0.060 | <0.001 |
| RR | 3.153 (2.295–4.491) bc | −0.423 ± 0.060 | <0.001 |
| S | 1.836 (1.367–2.525) ab | −0.224 ± 0.055 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macaluso, M.; Farina, P.; Rossi, L.; Bianchi, A.; Venturi, F.; Chiriboga Ortega, R.D.; Bedini, S.; Conti, B.; Guidi, L.; Zinnai, A. Chemical vs. Enzymatic Refining to Produce Peanut Oil for Edible Use or to Obtain a Sustainable and Cost-Effective Protector for Stored Grains against Sitophilus zeamais (Coleoptera: Curculionidae). Foods 2022, 11, 1224. https://doi.org/10.3390/foods11091224
Macaluso M, Farina P, Rossi L, Bianchi A, Venturi F, Chiriboga Ortega RD, Bedini S, Conti B, Guidi L, Zinnai A. Chemical vs. Enzymatic Refining to Produce Peanut Oil for Edible Use or to Obtain a Sustainable and Cost-Effective Protector for Stored Grains against Sitophilus zeamais (Coleoptera: Curculionidae). Foods. 2022; 11(9):1224. https://doi.org/10.3390/foods11091224
Chicago/Turabian StyleMacaluso, Monica, Priscilla Farina, Linda Rossi, Alessandro Bianchi, Francesca Venturi, Rodrigo Daniel Chiriboga Ortega, Stefano Bedini, Barbara Conti, Luca Guidi, and Angela Zinnai. 2022. "Chemical vs. Enzymatic Refining to Produce Peanut Oil for Edible Use or to Obtain a Sustainable and Cost-Effective Protector for Stored Grains against Sitophilus zeamais (Coleoptera: Curculionidae)" Foods 11, no. 9: 1224. https://doi.org/10.3390/foods11091224
APA StyleMacaluso, M., Farina, P., Rossi, L., Bianchi, A., Venturi, F., Chiriboga Ortega, R. D., Bedini, S., Conti, B., Guidi, L., & Zinnai, A. (2022). Chemical vs. Enzymatic Refining to Produce Peanut Oil for Edible Use or to Obtain a Sustainable and Cost-Effective Protector for Stored Grains against Sitophilus zeamais (Coleoptera: Curculionidae). Foods, 11(9), 1224. https://doi.org/10.3390/foods11091224