Efficacy of Pectin-Based Coating Added with a Lemon Byproduct Extract on Quality Preservation of Fresh-Cut Carrots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Lemon Extract (LE)
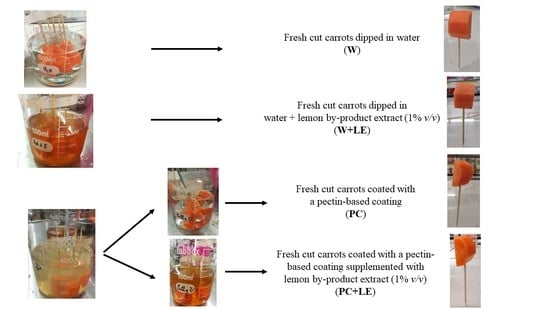
2.3. Preparation of the Dipping and Coating Solutions
2.4. Physicochemical Properties
- Colour
- Hardness
2.5. Microbiological Analysis
2.6. Respiratory Activity
2.7. Extraction and Determination of Total Phenolic and Content and Antioxidant Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Microbial Growth
3.3. Respiratory Activity
3.4. Bioactive Compounds Content and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Enhancing phenolic content in carrots by pulsed electric fields during post-treatment time: Effects on cell viability and quality attributes. Innov. Food Sci. Emerg. Technol. 2020, 59, 102252. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Miguel, M.G.C.; Faleiro, M.L.; Antunes, M.D.C. The influence of edible coatings enriched with citral and eugenol on the raspberry storage ability, nutritional and sensory quality. Food Packag. Shelf Life 2016, 9, 20–28. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Packaging strategies to prolong the shelf life of fresh carrots (Daucus carota L.). Innov. Food Sci. Emerg. Technol. 2012, 13, 215–220. [Google Scholar] [CrossRef]
- Piscopo, A.; Zappia, A.; Princi, M.P.; De Bruno, A.; Araniti, F.; Lupini, A.; Abenavoli, M.R.; Poiana, M. Quality of shredded carrots minimally processed by different dipping solutions. J. Food Sci. Technol. 2019, 56, 2584–2593. [Google Scholar] [CrossRef]
- Huber, K.C.; Embuscado, M. Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Bourtoom, T. Review article-edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioproc. Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Salmieri, S.; Lacroix, M. Physicochemical properties of alginate/polycaprolactone-based films containing essential oils. J. Agric. Food Chem. 2006, 54, 10205–10214. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and Uses of Pectin—A Review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, W.; Gao, W.; Tian, G.; Zhao, C.; Bao, Y.; Cui, J.; Lian, Y.; Zheng, J. Effect of mesoscopic structure of citrus pectin on its emulsifying properties: Compactness is more important than size. J. Colloid Interface Sci. 2020, 570, 80–88. [Google Scholar] [CrossRef]
- Baeva, M.; Panchev, I. Investigation of the retaining effect of a pectin-containing edible film upon the crumb ageing of dietetic sucrose-free sponge cake. Food Chem. 2005, 92, 343–348. [Google Scholar] [CrossRef]
- Corbo, M.R.; Campaniello, D.; Speranza, B.; Bevilacqua, A.; Sinigaglia, M. Non-conventional tools to preserve and prolong the quality of minimally-processed fruits and vegetables. Coatings 2015, 5, 931–961. [Google Scholar] [CrossRef] [Green Version]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Petkova, X.; Todorova, M.; Ivanov, I.; Deseva, I.; Mihaylova, D.; Ibrahim, S.A. Effects of pectin-based edible coatings containing a bacteriocin of Bacillus methylotrophicus BM47 on the quality and storage life of fresh blackberries. Ital. J. Food Sci. 2020, 32, 420–427. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Amodio, M.L.; Colelli, G. Potential use of microwave treatment on fresh-cut carrots: Physical, chemical and microbiological aspects. J. Sci. Food Agric. 2016, 96, 2063–2072. [Google Scholar] [CrossRef]
- Attar, R.F.; Sedaghat, N.; Pasban, A.; Yeganehzad, S.; Hesarinejad, M.A. Modeling the respiration rate of chitosan coated fresh in-hull pistachios (Pistacia vera L. cv. Badami) for modified atmosphere packaging design. J. Food Meas. Charact. 2022, 16, 1049–1061. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; Delŕio, M.A.; Pérez-Gago, M.B. Antimicrobial edible films and coatings for fresh and minimally processed fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 872–900. [Google Scholar] [CrossRef]
- Song, Z.; Li, F.; Guan, H.; Xu, Y.; Fu, Q.; Li, D. Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Porat, R.; Poverenov, E. Enhancement of agricultural produce quality and storability using citral-based edible coatings; the valuable effect of nano-emulsification in a solid-state delivery on fresh-cut melons model. Food Chem. 2019, 277, 205–212. [Google Scholar] [CrossRef]
- Rangel-Marrón, M.; Mani-López, E.; Palou, E.; López-Malo, A. Effects of alginate-glycerol-citric acid concentrations on selected physical, mechanical, and barrier properties of papaya puree-based edible films and coatings, as evaluated by response surface methodology. LWT 2019, 101, 83–91. [Google Scholar] [CrossRef]
- Nair, A.K.; Mukherjee, M.; Nag, S.; Pandimadevi, M. Antioxidant and antimicrobial activities of citrus lemon peels encapsulated in PVA. Carpathian J. Food Sci. Technol. 2019, 11, 111–126. [Google Scholar]
- Mathew, B.B.; Shajie, D.; Wadhwa, N.; Murthy, N.K.; Murthy, T.K.; Rashmi, M.V. Comparative antioxidant efficacy of Citrus limonum pulp and peel—An in vitro study. Drug Inven. Today 2013, 5, 296–301. [Google Scholar] [CrossRef]
- Al-Qassabi, J.S.A.; Weli, A.M.; Hossain, M.A. Comparison of total phenols content and antioxidant potential of peel extracts of local and imported lemons samples. Sustain. Chem. Pharm. 2018, 8, 71–75. [Google Scholar] [CrossRef]
- O’Shea, N.; Arendt, E.K.; Gallagher, E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. Technol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes—A review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [Green Version]
- Imeneo, V.; Romeo, R.; Gattuso, A.; De Bruno, A.; Piscopo, A. Functionalized Biscuits with Bioactive Ingredients Obtained by Citrus Lemon Pomace. Foods 2021, 10, 2460. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.; Torre, G.; Sarò, M.; Dugo, P.; Mondello, L. Underestimated sources of flavonoids, limonoids and dietary fibre: Availability in lemon’s by-products. J. Funct. Foods 2014, 9, 18–26. [Google Scholar] [CrossRef]
- Imeneo, V.; Romeo, R.; De Bruno, A.; Piscopo, A. Green-sustainable extraction techniques for the recovery of antioxidant compounds from “citrus Limon” by-products. J. Environ. Sci. Health B 2022, 1–13. [Google Scholar] [CrossRef]
- AOAC, Association of Official Analytical Chemists. Hydrogen-ion activity (pH) method. In Method 14.022, 13th ed.; Horwitz, W., Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1980; p. 213. [Google Scholar]
- AOAC, Association of Official Analytical Chemists. Method 942.15. Acidity of fruit products. In Official Methods of Analysis, 17th ed.; Horwitz, W., Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000; p. 213. [Google Scholar]
- AOAC, Association of Official Analytical Chemists. Determination of Water/Dry Matter (Moisture) in Animal Feed, Grain, and Forage (Plant Tissue); In Official Methods of Analysis, 17th ed.; Horwitz, W., Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000; p. 12. [Google Scholar]
- Thompson, B. Printing Materials Science and Technology, 2nd ed.; Pira International: Surrey, UK, 2004. [Google Scholar]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Enhancing hydroxycinnamic acids and flavan-3-ol contents by pulsed electric fields without affecting quality attributes of apple. Food Res. Int. 2019, 121, 433–440. [Google Scholar] [CrossRef]
- Fan, L.; Song, J. Microbial quality assessment methods for fresh-cut fruits and vegetables. Stewart Postharvest Rev. 2008, 4, 1–9. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed electric fields affect endogenous enzyme activities, respiration and biosynthesis of phenolic compounds in carrots. Postharvest Biol. Technol. 2020, 168, 111284. [Google Scholar] [CrossRef]
- Tappi, S.; Berardinelli, A.; Ragni, L.; Dalla Rosa, M.; Guarnieri, A.; Rocculi, P. Atmospheric gas plasma treatment of fresh-cut apples. Innov. Food Sci. Emerg. Technol. 2014, 21, 114–122. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- González-Casado, S.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. Enhancing the carotenoid content of tomato fruit with pulsed electric field treatments: Effects on respiratory activity and quality attributes. Postharvest Biol. Technol. 2018, 137, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Gross, J. Pigments in Vegetables: Chlorophylls and Carotenoids; Van Nostrand Reinhold: New York, NY, USA, 1991; 278p. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- de Oliveira, K.Á.R.; Fernandes, K.F.D.; de Souza, E.L. Current advances on the development and application of probiotic-loaded edible films and coatings for the bioprotection of fresh and minimally processed fruit and vegetables. Foods 2021, 10, 2207. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible coating to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria x Ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Fai, A.E.C.; Alves de Souza, M.R.; de Barros, S.T.; Bruno, N.V.; Ferreira, M.S.L.; Gonçalves, T.C.B.D.A.; de Andrade, É.C.B. Development and evaluation of biodegradable films and coatings obtained from fruit and vegetable residues applied to fresh-cut carrot (Daucus carota L.). Postharvest Biol. Technol. 2016, 112, 194–204. [Google Scholar] [CrossRef]
- Howard, L.R.; Griffin, L.E. Lignin Formation and Surface Discoloration of Minimally Processed Carrot Sticks. J. Food Sci. 1993, 58, 1065–1067. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R. Processing effects on carrot phytonutrients. HortScience 2006, 41, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Howard, L.R.; Griffin, L.E.; Lee, Y. Steam treatment of minimally processed carrot sticks to control surface discoloration. J. Food Sci. 1994, 59, 356–358. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, A.; Wojciechowska, R.; Stanisław, R. Phenolic metabolism in root slices of selected carrot cultivars. Acta Physiol. Plant. 1997, 19, 319–325. [Google Scholar] [CrossRef]
- Pace, B.; Capotorto, I.; Cefola, M.; Minasi, P.; Montemurro, N.; Carbone, V. Evaluation of quality, phenolic and carotenoid composition of fresh-cut purple Polignano carrots stored in modified atmosphere. J. Food Compost. Anal. 2020, 86, 103363. [Google Scholar] [CrossRef]
- Vargas, M.; Chiralt, A.; Albors, A.; González-Martínez, C. Effect of chitosan-based edible coatings applied by vacuum impregnation on quality preservation of fresh-cut carrot. Postharvest Biol. Technol. 2009, 51, 263–271. [Google Scholar] [CrossRef]
- Mei, Y.; Zhao, Y.; Furr, H.C. Using Edible Coating to Enhance Nutritional and Sensory Qualities of Baby Carrots. J. Food Sci. 2002, 67, 1964–1968. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Saltveit, M.E.; Krochta, J.M. Hygroscopic Coatings Control Surface White Discoloration of Peeled (Minimally Processed) Carrots during Storage. J. Food Sci. 1997, 62, 363–366. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Park, H.J.; Lee, C.Y.; Choi, W.Y. Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. Lebensm.-Wiss. Technol. 2003, 36, 323–329. [Google Scholar] [CrossRef]
- Olivas, G.I.; Mattinson, D.S.; Barbosa-Canovas, G.V. Alginate coatings for reservation of minimally processed ‘Gala’ apples. Postharvest Biol. Technol. 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Rojas-Grau, M.A.; Tapia, M.S.; Martín-Belloso, O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. Lebensm.-Wiss. Technol. 2008, 41, 139–147. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Sarantópoulos, C.I.G.L.; Carmello-Guerreiro, S.M.; Hubinger, M.D. Effect of Osmotic Dehydration and Pectin Edible Coatings on Quality and Shelf Life of Fresh-Cut Melon. Food Bioprocess Technol. 2013, 6, 80–91. [Google Scholar] [CrossRef]
- Shigematsu, E.; Dorta, C.; Santos, D.N.; Ferreira, K.A.; Góes-Favoni, S.P.; Oshiiwa, M.; Mauro, M.A. Edible coating with coconut water to preserve probiotic strains and sensory characteristics of minimally processed carrots. Int. Food Res. J. 2019, 26, 1285–1292. [Google Scholar]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanatidou, A.; Slump, R.A.; Gorris, L.G.M.; Smid, E.J. High oxygen and high carbon dioxide modified atmospheres for shelf-life extension of minimally processed carrots. J. Food Sci. 2000, 65, 61–66. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Maherani, B.; Manus, J.; Salmieri, S.; Lacroix, M. Physicochemical and microbiological characterization of pectin-based gelled emulsions coating applied on pre-cut carrots. Food Hydrocoll. 2020, 101, 105573. [Google Scholar] [CrossRef]
- Budiati, T.; Suryaningsih, W.; Yudistira, H.; Azhar, S.W. Antimicrobial activity of jengkol and petai peel extract to inhibit listeria monocytogenes. IOP Conf. Ser. Earth Environ. Sci. 2021, 672, 012046. [Google Scholar] [CrossRef]
- Ivasenko, S.; Orazbayeva, P.; Skalicka–wozniak, K.; Ludwiczuk, A.; Marchenko, A.; Ishmuratova, M.; Poleszak, E.; Korona-Glowniak, I.; Akhmetova, S.; Karilkhan, I.; et al. Antimicrobial activity of ultrasonic extracts of two chemotypes of thymus serpyllum l. Of central kazakhstan and their polyphenolic profiles. Maced. J. Med. Sci. 2021, 9, 61–67. [Google Scholar] [CrossRef]
- Leceta, I.; Molinaro, S.; Guerrero, P.; Kerry, J.P.; De la Caba, K. Quality attributes of map packaged ready-to-eat baby carrots by using chitosan-based coatings. Postharvest Biol. Technol. 2015, 100, 142–150. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Hernández-Jover, T.; Martín-Belloso, O. Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chem. 2009, 112, 258–266. [Google Scholar] [CrossRef]
- Ranjitha, K.; Sudhakar Rao, D.V.; Shivashankara, K.S.; Oberoi, H.S.; Roy, T.K.; Bharathamma, H. Shelf-life extension and quality retention in fresh-cut carrots coated with pectin. Innov. Food Sci. Emerg. Technol. 2017, 42, 91–100. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Izumi, H.; Watada, A.E. Calcium Treatments Affect Storage Quality of Shredded Carrots. J. Food Sci. 1994, 59, 106–109. [Google Scholar] [CrossRef]
- Muley, A.B.; Singhal, R.S. Extension of postharvest shelf life of strawberries (Fragaria ananassa) using a coating of chitosan-whey protein isolate conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef] [PubMed]







| Storage Time (Day) | |||||
|---|---|---|---|---|---|
| 1 | 3 | 7 | 10 | 14 | |
| Acidity | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| W | 0.10 ± 0.00 ab,A | 0.07 ± 0.01 C | 0.07 ± 0.01 b,BC | 0.08 ± 0.01 b,B | 0.10 ± 0.00 a,A |
| W + LE | 0.10 ± 0.01 ab,A | 0.07 ± 0.00 BC | 0.11 ± 0.00 a,A | 0.08 ± 0.00 b,AB | 0.05 ± 0.02 b,C |
| PC | 0.11 ± 0.00 a,A | 0.08 ± 0.01 B | 0.08 ± 0.00 b,B | 0.12 ± 0.03 a,A | 0.07 ±0.00 ab,B |
| PC + LE | 0.09 ± 0.01 b | 0.07 ± 0.01 | 0.09 ± 0.01 a,b | 0.07 ± 0.01 b | 0.09 ± 0.02 a |
| Significance | * | ns | ** | ** | ** |
| pH | |||||
| W | 6.34 ± 0.04 BC | 6.32 ± 0.03 C | 6.43 ± 0.01 AB | 6.6 ± 0.03 a,A | 6.49 ± 0.10 a,B |
| W + LE | 6.27 ± 0.09 | 6.46 ± 0.10 | 6.54 ± 0.08 | 6.51 ± 0.25 a | 6.43 ± 0.00 a |
| PC | 6.31 ± 0.05 A | 6.38 ± 0.01 A | 6.40 ± 0.06 A | 6.04 ± 0.26 b,B | 6.21 ± 0.00 b,AB |
| PC + LE | 6.31 ± 0.01 C | 6.29 ± 0.16 C | 6.52 ± 0.13 B | 6.73 ± 0.06 a,A | 6.24 ± 0.06 b,C |
| Significance | ns | ns | ns | ** | ** |
| Soluble solids content (°Brix) | |||||
| W | 3.6 ± 0.14 d | 3.25 ± 0.07 c | 3.60 ± 0.57 c | 3.70 ± 0.28 c | 3.90 ± 0.99 b |
| W + LE | 5.65 ± 0.21 b,A | 5.20 ± 0.42 b,AB | 5.00 ± 0.42 b,B | 5.65 ± 0.07 b,A | 5.60 ± 0.28 a,A |
| PC | 5.35 ± 0.07 c | 5.40 ± 0.14 b | 4.90 ± 0.42 b | 5.30 ± 0.28 b | 5.35 ± 1.34 a,b |
| PC + LE | 6.4 ± 0.14 a,A | 6.05 ± 0.07 a,C | 6.25 ± 0.07 a,ABC | 6.35 ± 0.21 a,AB | 6.10 ± 0.14 a,BC |
| Significance | ** | ** | ** | ** | ** |
| Dry matter | |||||
| W | 9.24 ± 0.18 b | 9.94 ± 0.94 a | 9.99 ± 1.15 | 9.60 ± 0.45 ab | 9.50 ± 0.07 |
| W + LE | 10.47 ± 0.78 a | 9.71 ± 0.21 a | 10.48 ± 1.76 | 10.11 ± 0.32 a | 9.19 ± 0.41 |
| PC | 10.96 ± 0.50 a,A | 9.73 ± 0.10 a,B | 9.18 ± 0.18 B | 9.41 ± 0.03 b,B | 9.31 ± 0.84 B |
| PC + LE | 9.20 ± 0.36 b | 8.68 ± 0.28 b | 8.73 ± 0.68 | 9.55 ± 0.50 ab | 9.22 ± 1.53 |
| Significance | ** | ** | ns | * | ns |
| ΔE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Storage Time (Day) | ||||||||||
| 1 | 3 | 7 | 10 | 14 | ||||||
| Cortical Tissue | Vascular Cylinder | Cortical Tissue | Vascular Cylinder | Cortical Tissue | Vascular Cylinder | Cortical Tissue | Vascular Cylinder | Cortical Tissue | Vascular Cylinder | |
| W | 3.63 ± 0.56 a | 7.09 ± 0.53 ab,B | 5.85 ± 1.24 a | 11.31 ± 1.48 a,A | 5.60 ± 1.27 a | 9.67 ± 1.42 a,A | 5.83 ± 2.04 a | 9.84 ± 1.52 A | 5.47 ± 0.94 a | 9.62 ± 1.72 a,A |
| W + LE | 3.01 ± 0.30 ab | 6.49 ± 1.35 a,B | 2.33 ± 0.93 b | 13.87 ± 2.00 a,A | 2.87 ± 0.04 b | 9.01 ± 1.88 a,AB | 3.53 ± 1.26 a,b | 7.89 ± 2.35 B | 2.33 ± 0.91 b | 9.57 ± 1.67 a,AB |
| PC | 2.20 ± 0.64 bc | 3.17 ± 0.56 ab | 2.07 ± 0.56 b | 6.21 ± 0.70 b | 1.42 ± 0.61 b | 4.41 ± 1.26 b | 1.66 ± 0.58 b | 5.93 ± 2.58 | 1.63 ± 0.69 b | 6.14 ± 2.06 a,b |
| PC + LE | 1.63 ± 0.19 c,B | 1.74 ± 0.66 b,B | 3.06 ± 0.26 b,A | 2.07 ± 0.71 c,AB | 1.00 ± 0.28 b,B | 3.02 ± 0.19 b,AB | 1.22 ± 0.07 b,B | 4.38 ± 1.78 A | 2.65 ± 0.36 b,A | 3.76 ± 0.28 b,AB |
| Sign. | ** | * | ** | ** | ** | ** | ** | ns | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imeneo, V.; Piscopo, A.; Martín-Belloso, O.; Soliva-Fortuny, R. Efficacy of Pectin-Based Coating Added with a Lemon Byproduct Extract on Quality Preservation of Fresh-Cut Carrots. Foods 2022, 11, 1314. https://doi.org/10.3390/foods11091314
Imeneo V, Piscopo A, Martín-Belloso O, Soliva-Fortuny R. Efficacy of Pectin-Based Coating Added with a Lemon Byproduct Extract on Quality Preservation of Fresh-Cut Carrots. Foods. 2022; 11(9):1314. https://doi.org/10.3390/foods11091314
Chicago/Turabian StyleImeneo, Valeria, Amalia Piscopo, Olga Martín-Belloso, and Robert Soliva-Fortuny. 2022. "Efficacy of Pectin-Based Coating Added with a Lemon Byproduct Extract on Quality Preservation of Fresh-Cut Carrots" Foods 11, no. 9: 1314. https://doi.org/10.3390/foods11091314
APA StyleImeneo, V., Piscopo, A., Martín-Belloso, O., & Soliva-Fortuny, R. (2022). Efficacy of Pectin-Based Coating Added with a Lemon Byproduct Extract on Quality Preservation of Fresh-Cut Carrots. Foods, 11(9), 1314. https://doi.org/10.3390/foods11091314