Profiling of Primary Metabolites and Volatiles in Apricot (Prunus armeniaca L.) Seed Kernels and Fruits in the Context of Its Different Cultivars and Soil Type as Analyzed Using Chemometric Tools
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, SPME and Chemicals
2.2. Sample Preparation
2.3. GC–MS Analysis of Silylated Primary Metabolites
2.4. SPME–GC–MS Volatile Analysis
2.5. Metabolites Identification and Multivariate Data Analyses
2.6. Statistical Analysis
3. Results and Discussion
3.1. Primary Metabolites Profiling of Apricot Fruits and Seed Kernels via GC-MS Analysis (Post-Silylation)
3.1.1. Sugars
3.1.2. Organic Acids
3.1.3. Amino Acids
3.1.4. Free Fatty Acids
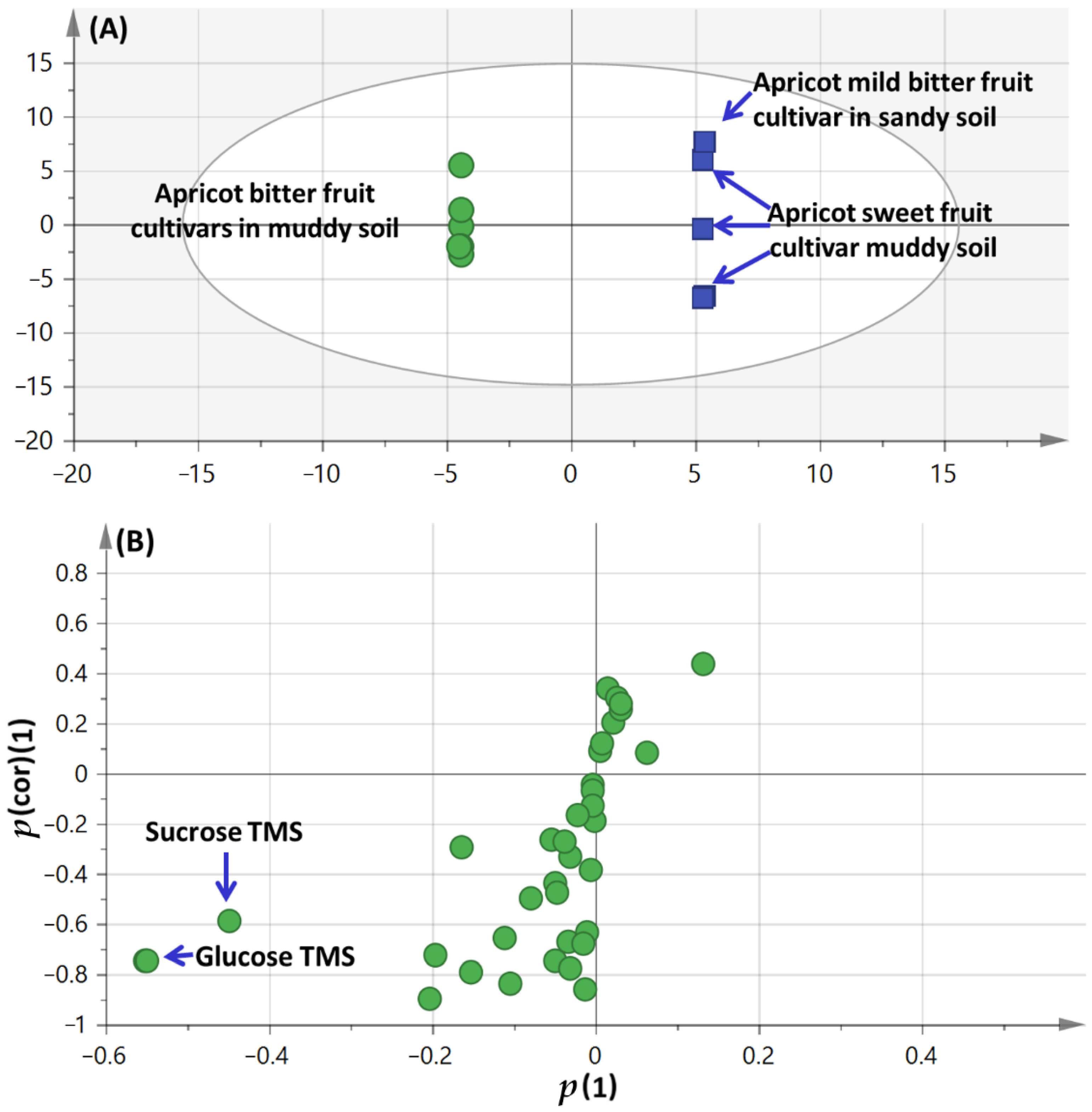
3.2. Multivariate Data Analyses of Primary Silylated Metabolites of the Different Apricot Cultivars Fruit and Seed Kernel Cultivated in Different Soil Types
3.2.1. Multivariate Data Analyses of the Primary Silylated Metabolites in Fruits and Seed Kernels Models
3.2.2. OPLS-DA Analysis of Apricot Fruit Cultivar Propagating in Sandy Versus Muddy Soil Primary Silylated Metabolites Dataset
3.3. Apricot Fruit Headspace Volatile Analysis via SPME–GC–MS
3.3.1. Lactones
3.3.2. Fatty Acids/Esters
3.3.3. Alcohols
3.3.4. Aldehydes
3.3.5. Ionones
3.3.6. Ethers/Oxides
3.3.7. Ketones
3.3.8. Esters
3.3.9. Aliphatic/Aromatic Hydrocarbons
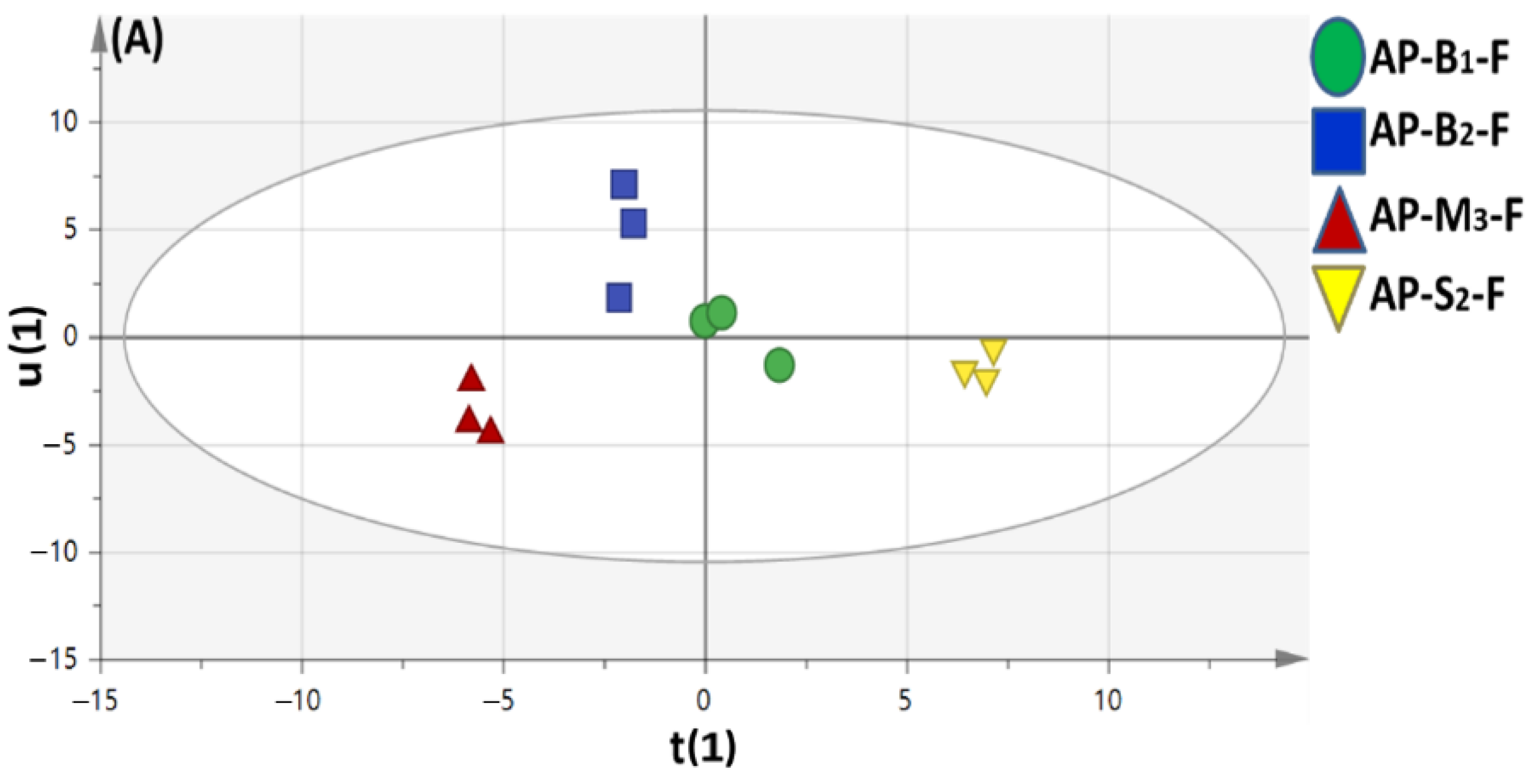
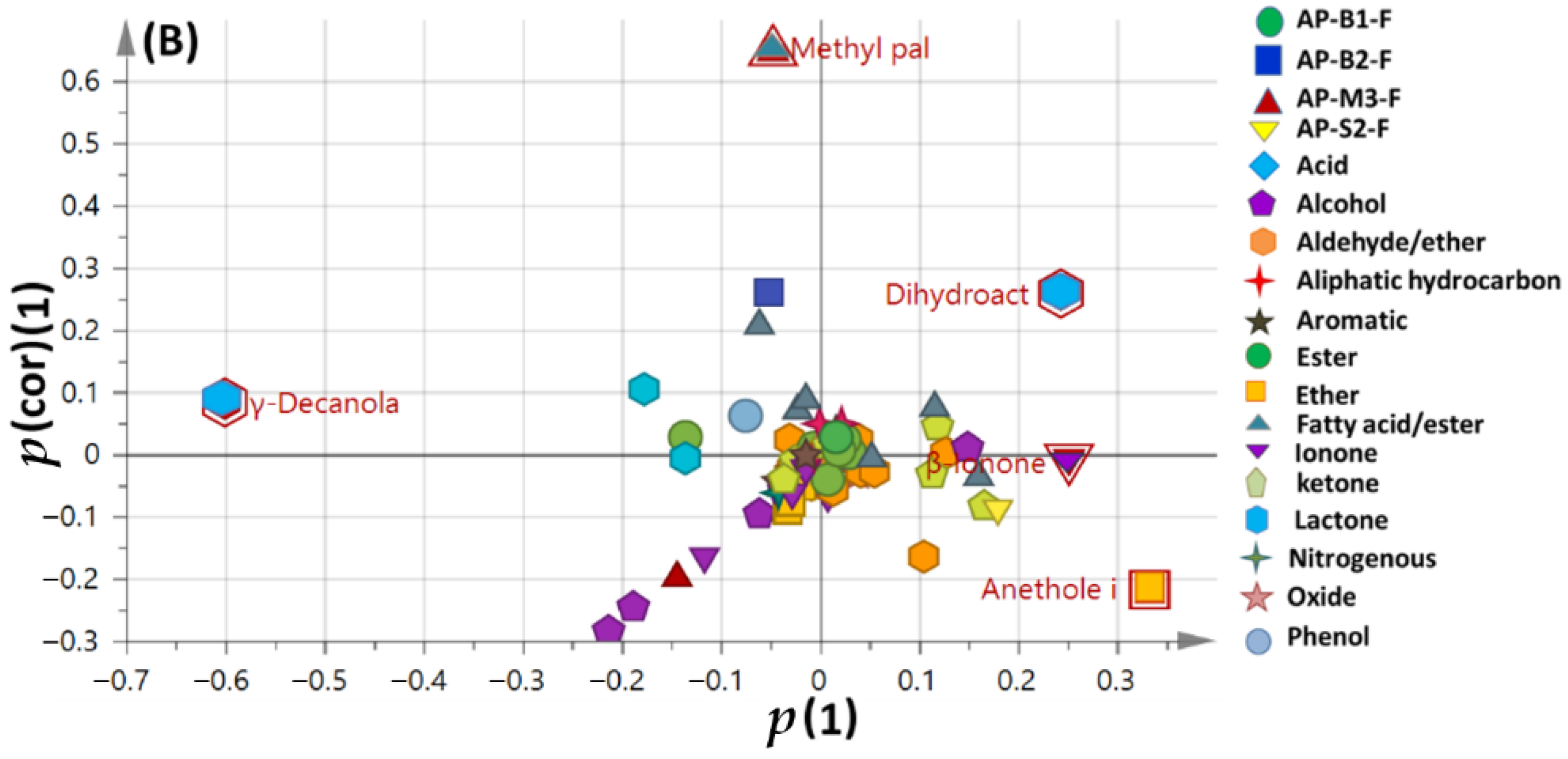
3.4. Multivariate Data Analyses of Sweet and Bitter Apricot Fruit Cultivars Aroma Profile Cultivated in Different Soil Types
OPLS-DA Analyses of Volatile Metabolites in Different Apricot Fruit Cultivars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farag, M.A.; El Senousy, A.S.; El-Ahmady, S.H.; Porzel, A.; Wessjohann, L.A. Comparative Metabolome-Based Classification of Senna Drugs: A Prospect for Phyto-Equivalency of Its Different Commercial Products. Metabolomics 2019, 15, 80. [Google Scholar] [CrossRef]
- Ramadan, N.S.; Wessjohann, L.A.; Mocan, A.; Vodnar, D.C.; ElSayed, N.H.; ElToumy, S.A.; Mohamed, D.A.; Aziz, Z.A.; Ehrlich, A.; Farag, M.A. Nutrient and Sensory Metabolites Profiling of Averrhoa carambola L. (Starfruit) in the Context of Its Origin and Ripening Stage by GC/MS and Chemometric Analysis. Molecules 2020, 25, 2423. [Google Scholar] [CrossRef]
- Mesarović, J.; Trifković, J.; Tosti, T.; Fotirić Akšić, M.; Milatović, D.; Ličina, V.; Milojković-Opsenica, D. Relationship between Ripening Time and Sugar Content of Apricot (Prunus armeniaca L.) Kernels. Acta Physiol. Plant. 2018, 40, 157. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, J.; Wang, S.; Yang, L.; Luo, Y.; Gao, S.; Zhang, M.; Wu, S.; Hu, S.; Sun, H.; et al. The Apricot (Prunus armeniaca L.) Genome Elucidates Rosaceae Evolution and Beta-Carotenoid Synthesis. Hortic. Res. 2019, 6, 128. [Google Scholar] [CrossRef] [Green Version]
- Kovacikova, E.; Kovacik, A.; Halenar, M.; Tokarova, K.; Chrastinova, L.; Ondruska, L.; Jurcik, R.; Kolesar, E.; Valuch, J.; Kolesarova, A. Potential Toxicity of Cyanogenic Glycoside Amygdalin and Bitter Apricot Seed in Rabbits—Health Status Evaluation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 695–703. [Google Scholar] [CrossRef]
- Yiǧit, D.; Yiǧit, N.; Mavi, A. Antioxidant and Antimicrobial Activities of Bitter and Sweet Apricot (Prunus armeniaca L.) Kernels. Braz. J. Med. Biol. Res. 2009, 42, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Wani, S.M.; Jan, N.; Wani, T.A.; Ahmad, M.; Masoodi, F.A.; Gani, A. Optimization of Antioxidant Activity and Total Polyphenols of Dried Apricot Fruit Extracts (Prunus armeniaca L.) Using Response Surface Methodology. J. Saudi Soc. Agric. Sci. 2017, 16, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Raafat, K.; El-Darra, N.; Saleh, F.A.; Rajha, H.N.; Maroun, R.G.; Louka, N. Infrared-Assisted Extraction and HPLC-Analysis of Prunus armeniaca L. Pomace and Detoxified-Kernel and Their Antidiabetic Effects. Phytochem. Anal. 2018, 29, 156–167. [Google Scholar] [CrossRef]
- Merzouki, A.; Ed-derfoufi, F.; Molero Mesa, J. Contribution to the Knowledge of Rifian Traditional Medicine. II: Folk Medicine in Ksar Lakbir District (NW Morocco). Fitoterapia 2000, 71, 278–307. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 28 November 2021).
- Awad, N.; Gabr, M.; Gawish, M. Morphological Evaluation and Genetic Identification of Some Local Apricot Lines. J. Plant Prod. 2019, 10, 843–848. [Google Scholar] [CrossRef]
- Zohry, A.E.H.; Ouda, S.; Hamd-Alla, W.; Shalaby, E.S. Evaluation of Different Crop Sequences for Wheat and Maize in Sandy Soil. Acta Agric. Slov. 2017, 109, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Katkat, A.V. Effects of Soil and Foliar Applications of Humic Substances on Dry Weight and Mineral Nutrients Uptake of Wheat under Calcareous Soil Conditions. Aust. J. Basic Appl. Sci. 2009, 3, 1266–1273. [Google Scholar]
- Usowicz, B.; Lipiec, J. Spatial Variability of Soil Properties and Cereal Yield in a Cultivated Field on Sandy Soil. Soil Tillage Res. 2017, 174, 241–250. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Zhang, Y.; Yu, X.; Sun, M.; Xu, M.; Zhang, Q.; Liu, S. Kernel-Using Apricot Resources and Its Utilization. Acta Hortic. 2012, 966, 189–192. [Google Scholar] [CrossRef]
- Ighbareyeh, J.M.H.; Carmona, E.C.; Mohammed, M.H.; Suliemieh, A.A.A. Study of Biology and Bioclimatology Applied of Apricot (Prunus armeniaca L.): To Increase the Economy and Maintaining Food Security in Palestine. Int. J. Res. Stud. Biosci. 2016, 4, 25–33. [Google Scholar] [CrossRef]
- Negri, P.; Bassi, D.; Magnanini, E.; Rizzo, M.; Bartolozzi, F. Bitterness Inheritance in Apricot (P. armeniaca L.) Seeds. Tree Genet. Genomes 2008, 4, 767–776. [Google Scholar] [CrossRef]
- Tousson, E.; Hafez, E.; Abo Gazia, M.M.; Salem, S.B.; Mutar, T.F. Hepatic Ameliorative Role of Vitamin B17 against Ehrlich Ascites Carcinoma–Induced Liver Toxicity. Environ. Sci. Pollut. Res. 2020, 27, 9236–9246. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Martínez-Nicolás, J.J.; Legua, P.; Martínez, R.; Hernández, F. Antioxidant Activity, Volatile Composition and Sensory Profile of Four New Very-Early Apricots (Prunus armeniaca L.). J. Sci. Food Agric. 2014, 94, 85–94. [Google Scholar] [CrossRef]
- Schmitzer, V.; Slatnar, A.; Mikulic-Petkovsek, M.; Veberic, R.; Krska, B.; Stampar, F. Comparative Study of Primary and Secondary Metabolites in Apricot (Prunus armeniaca L.) Cultivars. J. Sci. Food Agric. 2011, 91, 860–866. [Google Scholar] [CrossRef]
- Solis-Solis, H.M.; Calderon-Santoyo, M.; Gutierrez-Martinez, P.; Schorr-Galindo, S.; Ragazzo-Sanchez, J.A. Discrimination of Eight Varieties of Apricot (Prunus armeniaca) by Electronic Nose, LLE and SPME Using GC-MS and Multivariate Analysis. Sens. Actuators B Chem. 2007, 125, 415–421. [Google Scholar] [CrossRef]
- Stryjecka, M.; Kiełtyka-Dadasiewicz, A.; Michalak, M.; Rachoń, L.; Głowacka, A. Chemical Composition and Antioxidant Properties of Oils from the Seeds of Five Apricot (Prunus armeniaca L.) Cultivars. J. Oleo Sci. 2019, 68, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, W.; Fang, J.; Chen, S.; Liu, Y.; Wu, B.; Li, S. Volatile Profiles of Apricot Cultivars (Prunus armeniaca Lam.) Evaluated by Head Space Solid Phase Microextraction Gas Chromatography Mass Spectrometry. Anal. Lett. 2014, 47, 433–452. [Google Scholar] [CrossRef]
- Bhanger, M.I.; Anwar, F.; Memon, N.; Qadir, R. Cold Pressed Apricot (Prunus armeniaca L.) Kernel Oil. In Cold Pressed Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 725–730. [Google Scholar]
- Luber, F.; Demmel, A.; Hosken, A.; Busch, U.; Engel, K.H. Apricot DNA as an Indicator for Persipan: Detection and Quantitation in Marzipan Using Ligation-Dependent Probe Amplification. J. Agric. Food Chem. 2012, 60, 5853–5858. [Google Scholar] [CrossRef]
- Farag, M.A.; Khattab, A.R.; Shamma, S.; Afifi, S.M. Profiling of Primary Metabolites and Volatile Determinants in Mahlab Cherry (Prunus mahaleb L.) Seeds in the Context of Its Different Varieties and Roasting as Analyzed Using Chemometric Tools. Foods 2021, 10, 728. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, H.; Wang, X.; Cao, J.; Jiang, W. Sugar and Organic Acid Composition of Apricot and Their Contribution to Sensory Quality and Consumer Satisfaction. Sci. Hortic. 2017, 225, 553–560. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.K.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H.; Jun, J.H. Assessment of Organic Acid and Sugar Composition in Apricot, Plumcot, Plum, and Peach during Fruit Development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar]
- Caliskan, O.; Bayazit, S.; Sumbul, A. Fruit Quality and Phytochemical Attributes of Some Apricot (Prunus armeniaca L.) Cultivars as Affected by Genotypes and Seasons. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua Pod Metabolome in Context of Its Different Geographical Origin, Ripening Stage and Roasting Process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef]
- Naryal, A.; Acharya, S.; Bhardwaj, A.K.; Kant, A.; Chaurasia, O.P.; Stobdan, T. Altitudinal Effect on Sugar Contents and Sugar Profiles in Dried Apricot (Prunus armeniaca L.) Fruit. J. Food Compos. Anal. 2019, 76, 27–32. [Google Scholar] [CrossRef]
- Xi, W.; Zheng, H.; Zhang, Q.; Li, W. Profiling Taste and Aroma Compound Metabolism during Apricot Fruit Development and Ripening. Int. J. Mol. Sci. 2016, 17, 998. [Google Scholar] [CrossRef] [Green Version]
- Milošević, T.; Milošević, N.; Glišić, I.; Mladenović, J. Fruit Quality, Phenolics Content and Antioxidant Capacity of New Apricot Cultivars from Serbia. Acta Sci. Pol. Hortorum Cultus 2012, 11, 3–15. [Google Scholar]
- Gill, S.K.; Lever, E.; Emery, P.W.; Whelan, K. Nutrient, Fibre, Sorbitol and Chlorogenic Acid Content of Prunes (Prunus domestica): An Updated Analysis and Comparison of Different Countries of Origin and Database Values. Int. J. Food Sci. Nutr. 2019, 70, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Dine Tariq Bouhlali, E.; Derouich, M.; Meziani, R.; Bourkhis, B.; Filali-Zegzouti, Y.; Alem, C. Nutritional, Mineral and Organic Acid Composition of Syrups Produced from Six Moroccan Date Fruit (Phoenix dactylifera L.) Varieties. J. Food Compos. Anal. 2020, 93, 103591. [Google Scholar] [CrossRef]
- Choe, U.; Sun, J.; Bailoni, E.; Chen, P.; Li, Y.; Gao, B.; Wang, T.T.Y.; Rao, J.; Yu, L.L. Chemical Composition of Tomato Seed Flours, and Their Radical Scavenging, Anti-Inflammatory and Gut Microbiota Modulating Properties. Molecules 2021, 26, 1478. [Google Scholar] [CrossRef]
- Nazir, N.; Khalil, A.A.K.; Nisar, M.; Zahoor, M.; Ahmad, S. HPLC-UV Characterization, Anticholinesterase, and Free Radical-Scavenging Activities of Rosa moschata Herrm. Leaves and Fruits Methanolic Extracts. Braz. J. Bot. 2020, 43, 523–530. [Google Scholar] [CrossRef]
- Świątkowski, M.; Lanka, S.; Czylkowska, A.; Gas, K.; Sawicki, M. Structural, Spectroscopic, Thermal, and Magnetic Properties of a New Dinuclear Copper Coordination Compound with Tiglic Acid. Materials 2021, 14, 2148. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Ruiz, D.; Valero, D.; Rivera, D.; Obón, C.; Sánchez-Roca, C.; Gil, M.I. Health Benefits from Pomegranates and Stone Fruit, Including Plums, Peaches, Apricots and Cherries. Bioact. Fruit Health Benefits Funct. Foods 2013, 19, 125–167. [Google Scholar]
- Gurrieri, F.; Audergon, J.-M.; Albagnac, G.; Reich, M. Soluble Sugars and Carboxylic Acids in Ripe Apricot Fruit as Parameters for Distinguishing Different Cultivars. Euphytica 2001, 117, 183–189. [Google Scholar] [CrossRef]
- Ibrahim, N.; Taleb, M.; Heiss, A.G.; Kropf, M.; Farag, M.A. GC-MS Based Metabolites Profiling of Nutrients and Anti-Nutrients in 10 Lathyrus Seed Genotypes: A Prospect for Phyto-Equivalency and Chemotaxonomy. Food Biosci. 2021, 42, 101183. [Google Scholar] [CrossRef]
- Field, C.J.; Blewett, H.H.; Proctor, S.; Vine, D. Human Health Benefits of Vaccenic Acid. Appl. Physiol. Nutr. Metab. 2009, 34, 979–991. [Google Scholar] [CrossRef]
- Aumeistere, L.; Beluško, A.; Ciproviča, I.; Zavadska, D. Trans Fatty Acids in Human Milk in Latvia: Association with Dietary Habits during the Lactation Period. Nutrients 2021, 13, 2967. [Google Scholar] [CrossRef]
- Hrichi, S.; Rigano, F.; Chaabane-Banaoues, R.; Oulad El Majdoub, Y.; Mangraviti, D.; Di Marco, D.; Babba, H.; Dugo, P.; Mondello, L.; Mighri, Z. Identification of Fatty Acid, Lipid and Polyphenol Compounds from Prunus armeniaca L. Kernel Extracts. Foods 2020, 9, 896. [Google Scholar] [CrossRef]
- Farag, M.A.; Afifi, S.M.; Rasheed, D.M.; Khattab, A.R. Revealing Compositional Attributes of Glossostemon Bruguieri Desf. Root Geographic Origin and Roasting Impact via Chemometric Modeling of SPME-GC-MS and NMR Metabolite Profiles. J. Food Compos. Anal. 2021, 102, 104073. [Google Scholar] [CrossRef]
- Azodanlou, R.; Darbellay, C.; Luisier, J.-L.; Villettaz, J.-C.; Amadò, R. Development of a Model for Quality Assessment of Tomatoes and Apricots. LWT-Food Sci. Technol. 2003, 36, 223–233. [Google Scholar] [CrossRef]
- Lo Bianco, R.; Farina, V.; Indelicato, S.G.; Filizzola, F.; Agozzino, P. Fruit Physical, Chemical and Aromatic Attributes of Early, Intermediate and Late Apricot Cultivars. J. Sci. Food Agric. 2010, 90, 1008–1019. [Google Scholar] [CrossRef]
- Pintea, A.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Pop, E.A.; Opriță, V.-A.; Giuffrida, D.; Cacciola, F.; Bartolomeo, G.; Mondello, L. Carotenoids, Fatty Acids, and Volatile Compounds in Apricot Cultivars from Romania—A Chemometric Approach. Antioxidants 2020, 9, 562. [Google Scholar] [CrossRef]
- Guillot, S.; Peytavi, L.; Bureau, S.; Boulanger, R.; Lepoutre, J.P.; Crouzet, J.; Schorr-Galindo, S. Aroma Characterization of Various Apricot Varieties Using Headspace-Solid Phase Microextraction Combined with Gas Chromatography-Mass Spectrometry and Gas Chromatography-Olfactometry. Food Chem. 2006, 96, 147–155. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal Family as Source for Industrial Uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.N.A.; Fekry, M.I.; Farag, M.A. Metabolome Based Volatiles Profiling in 13 Date Palm Fruit Varieties from Egypt via SPME GC–MS and Chemometrics. Food Chem. 2017, 217, 171–181. [Google Scholar] [CrossRef]
- Göğüş, F.; Özel, M.Z.; Lewis, A.C. The Effect of Various Drying Techniques on Apricot Volatiles Analysed Using Direct Thermal Desorption-GC–TOF/MS. Talanta 2007, 73, 321–325. [Google Scholar] [CrossRef]
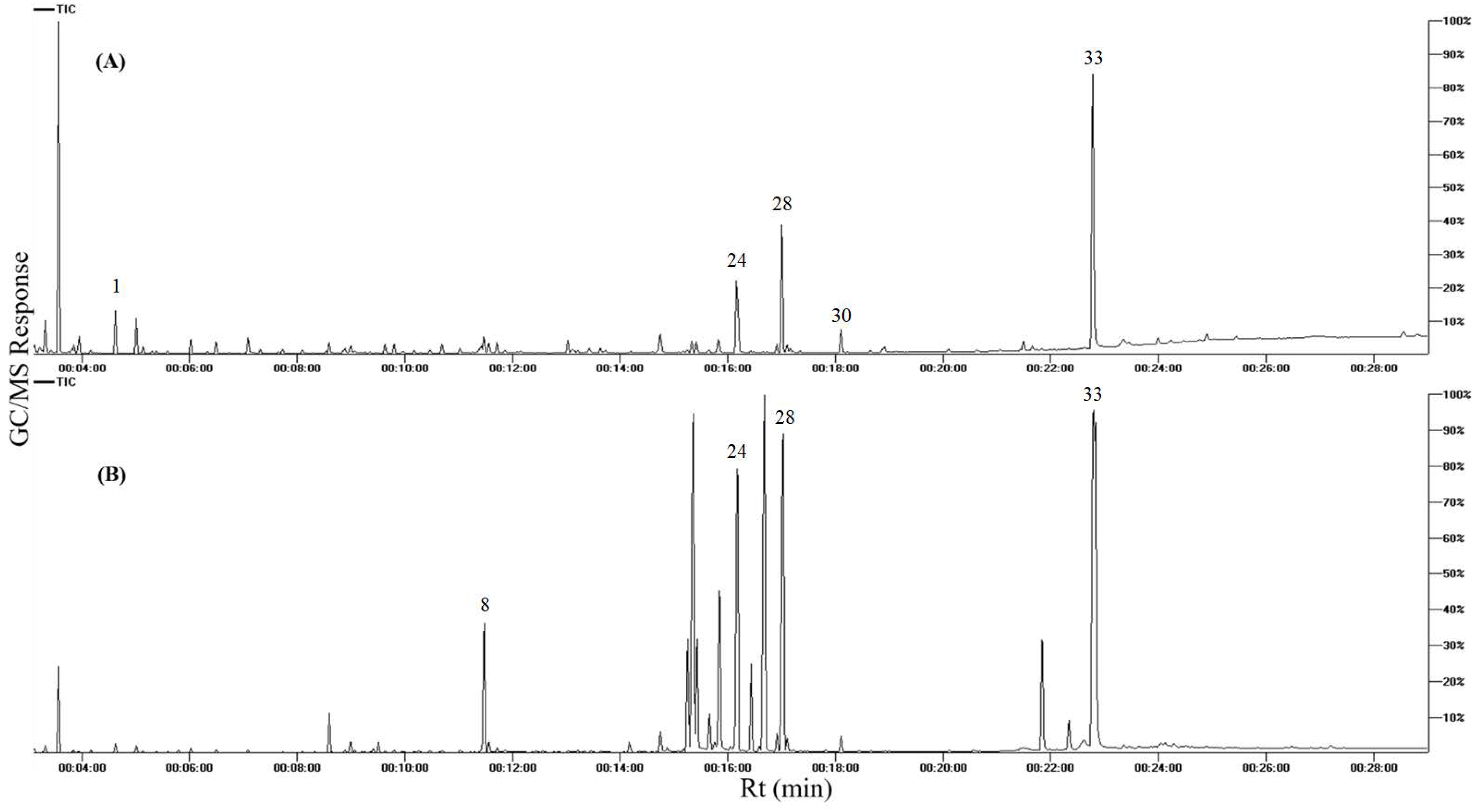


| Fruit Codes | Seed Codes | Collection Location/Soil Nature | Type |
|---|---|---|---|
| AP-B1-F | AP-B1-S | Manshiet Al-Ammar apricots (Muddy soil) | Bitter |
| AP-B2-F | AP-B2-S | Al-Amar Al-Kubra apricots (Muddy soil) | Bitter |
| AP-M3-F | AP-M3-S | Nubaria mountain apricots (Sandy soil) | Bitter |
| AP-S2-F | AP-S2-S | Al-Amar Al-Kubra apricots (Muddy soil) | Sweet |
| Rt (min) | KI | Name | AP-B1-F | AP-B1-S | AP-B2-F | AP-B2-S | AP-S2-F | AP-S2-S | AP-M3 F | AP-M3-S | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Organic Acids (mg/g) | |||||||||||
| 1 | 4.614 | 1029 | Tiglic acid (TMS) | 3.76 ± 0.91 | 2.65 ± 0.26 | 2.80 ± 0.39 | 3.60 ± 1.10 | 2.84 ± 0.47 | 2.82 ± 0.63 | 2.71 ± 0.61 | 3.17 ± 0.95 |
| 2 | 5.378 | 1077 | Lactic Acid (2TMS) | 0.41 ± 0.05 b | 0.24 ± 0.15 abc | 0.45 ± 0.14 c | 0.15 ± 0.05 a | 0.25 ± 0.08 abc | 0.15 ± 0.04 c | 0.21 ± 0.08 abc | 0.17 ± 0.03 bc |
| 3 | 5.584 | 1089 | Glycolic acid (2TMS) | 0.34 ± 0.07 | 0.21 ± 0.00 | 0.23 ± 0.02 | 0.26 ± 0.09 | 0.22 ± 0.02 | 0.21 ± 0.06 | 0.20 ± 0.05 | 0.23 ± 0.06 |
| 4 | 5.784 | 1102 | Pyruvic acid (2TMS) | 2.61 ± 0.99 a | 0.05 ± 0.02 c | 1.48 ± 0.20 c | 0.06 ± 0.02 b | 1.46 ± 0.46 b | 0.07 ± 0.03 c | 0.88 ± 0.17 bc | 0.08 ± 0.00 c |
| 5 | 9.08 | 1322 | Succinic acid (TMS) | 0.01 ± 0.01 bc | 0.02 ± 0.00 a | 0.01 ± 0.00 bc | 0.01 ± 0.01 bc | 0.00 ± 0.00 c | 0.01 ± 0.01 bc | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| 6 | 9.498 | 1353 | Fumaric acid (TMS) | 3.14 ± 0.77 bc | 0.02 ± 0.01 b | 4.00 ± 0.92 b | 0.02 ± 0.01 bc | 4.95 ± 3.19 bc | 0.11 ± 0.04 b | 6.78 ± 4.04 a | 0.10 ± 0.02 b |
| 7 | 9.621 | 1362 | Methylmaleic acid (2TMS) ester | 0.36 ± 0.04 ab | 0.21 ± 0.05 b | 0.35 ± 0.07 ab | 0.29 ± 0.10 ab | 0.35 ± 0.03 ab | 0.37 ± 0.08 ab | 0.43 ± 0.22 ab | 0.55 ± 0.15 a |
| 8 | 11.453 | 1505 | Malic acid (TMS) | 29.81 ± 9.94 a | 0.15 ± 0.07 b | 24.89 ± 5.99 b | 0.56 ± 0.18 a | 21.90 ± 9.27 a | 2.40 ± 0.97 b | 22.64 ± 12.51 a | 1.62 ± 0.41 b |
| 9 | 15.361 | 1850 | Isocitric acid (TMS) | 1.45 ± 0.09 a | 0.09 ± 0.02 b | 1.50 ± 0.11 b | 0.08 ± 0.07 a | 1.51 ± 0.27 a | 0.01 ± 0.01 b | 1.12 ± 0.23 a | 0.02 ± 0.00 b |
| 10 | 15.816 | 1894 | Quininic acid (5TMS) | 2.52 ±1.14 | 1.77 ± 2.54 | 2.36 ± 1.10 | 1.14 ± 0.92 | 0.63 ± 0.08 | 1.24 ± 0.58 | 1.40 ± 0.27 | 0.70 ± 0.16 |
| Total organic acids | 44.42 | 5.41 | 38.08 | 6.16 | 34.13 | 7.40 | 36.37 | 6.64 | |||
| Amino acids (mg/g) | |||||||||||
| 11 | 6.011 | 1116 | L-Alanine (2TMS) | 3.40 ± 1.86 a | 0.61 ± 0.11 b | 3.22 ± 0.19 ab | 1.13 ± 0.50 ab | 2.82 ± 0.79 ab | 1.87 ± 0.72 ab | 2.47 ± 1.48 ab | 1.47 ± 0.38 ab |
| 12 | 7.72 | 1226 | L-Valine, N-(TMS)-, trimethylsilyl ester | 0.39 ± 0.06 bcd | 0.24 ± 0.11 bcd | 0.20 ± 0.06 bcd | 0.34 ± 0.22 cd | 0.06 ± 0.03 d | 0.89 ± 0.39 ab | 0.85 ± 0.30 abc | 1.51 ± 0.40 a |
| 13 | 8.529 | 1284 | L-Leucine, N-(TMS)-, trimethylsilyl ester | 0.06 ± 0.02 c | 0.33 ± 0.13 bc | 0.05 ± 0.02 bc | 0.37 ± 0.28 c | 0.03 ± 0.01 c | 0.62 ± 0.33 ab | 0.16 ± 0.04 bc | 0.86 ± 0.21 a |
| 14 | 8.843 | 1306 | L-Isoleucine, N-(TMS)-, trimethylsilyl ester | 0.20 ± 0.03 bcd | 0.17 ± 0.08 bcd | 0.12 ± 0.02 bcd | 0.25 ± 0.15 cd | 0.06 ± 0.02 d | 0.59 ± 0.27 ab | 0.54 ± 0.21 abc | 0.87 ± 0.26 a |
| 15 | 8.878 | 1309 | L-Proline (2TMS) | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.01 | 0.00 ± 0.00 |
| 16 | 9.792 | 1374 | L-Serine (2TMS)-, trimethylsilyl ester | 0.99 ± 0.24 ab | 0.07 ± 0.03 d | 0.75 ± 0.32 cd | 0.17 ± 0.09 abc | 0.34 ± 0.14 bcd | 0.89 ± 0.25 ab | 0.82± 0.40 abc | 1.37 ± 0.21 a |
| 17 | 11.848 | 1537 | L-Aspartic acid, N-(TMS) (2TMS) ester | 0.96 ± 0.36 | 0.80 ± 1.21 | 0.60 ± 0.08 | 0.57 ± 0.33 | 1.24 ± 0.58 | 1.37 ± 0.57 | 0.76 ± 0.37 | 0.38 ± 0.10 |
| 18 | 13.019 | 1635 | Glutamic acid (TMS) | 0.79 ± 0.18 b | 0.13 ± 0.03 b | 0.45 ± 0.13 ab | 1.24 ± 0.77 b | 0.79 ± 0.39 b | 1.17 ± 0.55 ab | 0.94 ± 0.58 ab | 2.24 ± 0.74 a |
| Total amino acids | 6.82 | 2.36 | 5.40 | 4.08 | 5.33 | 7.39 | 6.54 | 8.71 | |||
| Sugars (mg/g) | |||||||||||
| 19 | 14.739 | 1789 | D-Xylose, tetrakis (TMS)- | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 20 | 15.25 | 1839 | D-Fructose, 1,3,4,5,6-pentakis-O-(TMS)- | 0.18 ± 0.03 | 0.04 ± 0.00 | 0.20 ± 0.05 | 0.03 ± 0.03 | 0.16 ± 0.07 | 0.01 ± 0.00 | 0.20 ± 0.26 | 0.02 ± 0.00 |
| 21 | 15.406 | 1855 | D-Fructose, 1,3,4,5,6-pentakis-O-(TMS)- | 14.62 ± 2.21 | 1.76 ± 0.39 | 17.55 ± 3.25 | 1.70 ± 1.01 | 12.49 ± 4.50 | 0.16 ± 0.07 | 27.23 ± 40.44 | 0.41 ± 0.11 |
| 22 | 15.639 | 1878 | D-Galactofuranose, 1,2,3,5,6-pentakis-O-(TMS)- | 6.16 ± 1.05 a | 0.45 ± 0.40 d | 4.38 ± 1.19 d | 0.24 ± 0.18 ab | 3.15 ± 1.46 bc | 0.02 ± 0.01 d | 1.27 ± 0.78 cd | 0.14 ± 0.01 d |
| 23 | 16.064 | 1920 | Galactonic acid, γ-lactone, 4TMS | 0.10 ± 0.06 a | 0.01 ± 0.01 b | 0.06 ± 0.01 ab | 0.03 ± 0.03 ab | 0.03 ± 0.00 b | 0.02 ± 0.01 b | 0.02 ± 0.02 b | 0.01 ± 0.01 b |
| 24 | 16.151 | 1928 | D-Glucose, 2,3,4,5,6-pentakis-O-(TMS)- | 62.22 ± 3.44 a | 13.41 ± 3.58 cd | 60.29 ± 4.87 cd | 16.69 ± 13.33 a | 47.46 ± 10.31 ab | 0.18 ± 0.11 d | 30.18 ± 14.00 bc | 8.67 ± 4.21 cd |
| 25 | 16.568 | 1970 | D-Mannitol, 1,2,3,4,5,6-hexakis-O-(TMS)- | 0.58 ± 0.42 | 0.09 ± 0.02 | 0.68 ± 0.28 | 0.06 ± 0.02 | 0.26 ± 0.14 | 0.07 ± 0.04 | 0.70 ± 0.93 | 0.07 ± 0.01 |
| 26 | 16.638 | 1977 | D-Glucitol, 6TMS | 0.13 ± 0.05 | 0.01 ± 0.00 | 0.16 ± 0.13 | 0.01 ± 0.00 | 0.12 ± 0.01 | 0.01 ± 0.00 | 0.17 ± 0.19 | 0.01 ± 0.00 |
| 27 | 16.9 | 2003 | D-Allofuranose, pentakis (TMS) | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 |
| 28 | 16.997 | 2014 | D-Glucose, 2,3,4,5,6-pentakis-O-(TMS)- | 70.11 ± 1.79 a | 16.73 ± 4.36 cd | 66.43 ± 5.69 cd | 19.03 ± 15.56 a | 54.74 ± 9.77 ab | 0.32 ± 0.20 d | 36.94 ± 13.56 bc | 9.10 ± 4.15 d |
| 29 | 17.084 | 2023 | Myo-Inositol, 1,2,3,4,5,6-hexakis-O-(TMS)- | 1.28 ± 0.23 a | 0.09 ± 0.02 bc | 1.23 ± 0.30 c | 0.03 ± 0.01 a | 0.60 ± 0.34 b | 0.14 ± 0.03 bc | 0.46 ± 0.20 bc | 0.24 ± 0.10 bc |
| 30 | 18.088 | 2132 | Inositol, 1,2,3,4,5,6-hexakis-O-(TMS)-, scyllo- | 1.60 ± 0.23 ab | 0.59 ± 0.08 abc | 1.50 ± 0.34 bc | 0.50 ± 0.32 ab | 0.93 ± 0.57 abc | 0.22 ± 0.02 c | 1.71 ± 0.73 a | 0.90 ± 0.40 abc |
| 31 | 21.478 | 2538 | Unknown sugar | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.05 ± 0.05 |
| 32 | 21.917 | 2593 | Sucrose, 8TMS | 0.03 ± 0.01 a | 0.01 ± 0.00 b | 0.01 ± 0.01 b | 0.00 ± 0.00 b | 0.01 ± 0.00 b | 0.00 ± 0.00 b | 0.01 ± 0.01 b | 0.01 ± 0.00 b |
| 33 | 22.768 | 2710 | Sucrose, 8TMS | 111.27± 4.13 a | 5.54 ± 3.86 b | 96.90 ± 18.21 b | 19.78 ± 6.04 a | 85.45 ± 13.76 a | 10.78 ± 4.95 b | 91.07 ± 19.55 a | 11.99 ± 7.27 b |
| Total sugars | 268.32 | 38.74 | 249.43 | 58.12 | 205.44 | 11.94 | 189.98 | 31.62 | |||
| Free fatty acids (mg/g) | |||||||||||
| 34 | 17.33 | 2050 | Palmitic acid, trimethylsilyl ester | 1.02 ± 0.16 a | 0.41 ± 0.09 b | 0.59 ± 0.17 b | 0.31 ± 0.10 ab | 0.69 ± 0.31 ab | 0.42 ± 0.13 b | 0.49 ± 0.25 b | 0.32 ± 0.06 b |
| 35 | 18.706 | 2200 | 9,12-Octadecadienoic acid (Z,Z)-, trimethylsilyl ester Linoleic acid | 3.31 ± 1.42 a | 0.58 ± 0.08 c | 3.07 ± 0.80 bc | 1.11 ± 1.15 ab | 0.32 ± 0.04 c | 1.22 ± 0.16 bc | 1.40 ±0.23 abc | 0.72 ± 0.32 c |
| 36 | 18.909 | 2224 | 11-cis-Octadecenoic acid, trimethylsilyl ester Vaccenic acid | 0.71 ± 0.15 a | 0.06 ± 0.05 b | 0.41 ± 0.05 b | 0.03 ± 0.00 ab | 0.79 ± 0.45 a | 0.08 ± 0.02 b | 0.30 ± 0.16 ab | 0.03 ± 0.01 b |
| Total free fatty acids | 5.03 | 1.05 | 4.06 | 1.45 | 1.80 | 1.72 | 2.19 | 1.06 | |||
| No. | Rt. (min.) | KI | Name | AP-B1-F | AP-B2-F | AP-M3-F | AP-S2-F |
|---|---|---|---|---|---|---|---|
| Alcohols | |||||||
| 1 | 5.217 | 774 | (Z)-Hex-3-en-1-ol | - | 0.07 ± 0.10 | - | 0.03 ± 0.02 |
| 2 | 8.27 | 1106 | Linalool | 0.10 ± 0.08 | 0.67 ± 0.63 | 8.86 ± 12.69 | 0.72 ±0.67 |
| 3 | 8.454 | 1123 | Phenylethyl Alcohol | 0.12 ±0.02 | 0.19 ± 0.10 | 0.84 ± 0.78 | 0.20 ± 0.15 |
| 4 | 9.162 | 1189 | Terpinen-4-ol | 0.08 ± 0.06 | 0.22 ± 0.18 | 0.16 ± 0.08 | 0.16 ± 0.13 |
| 5 | 9.21 | 1193 | p-Cymen-8-ol | 0.36 ± 0.20 b | 0.55 ± 0.40 a | 0.49 ± 0.19 ab | 0.90 ± 0.69 ab |
| 6 | 9.274 | 1199 | α-Terpineol | 9.25± 2.16 b | 7.09 ± 2.39 ab | 13.27 ± 9.59 ab | 6.09 ± 4.51 a |
| 7 | 9.841 | 1259 | Geraniol | 0.03 ± 0.01 b | 0.03 ± 0.01 a | 0.05 ± 0.06 ab | 0.03 ± 0.02 a |
| 8 | 10.114 | 1288 | 1,7-Nonadien-4-ol, 4,8-dimethyl- | 1.66 ± 0.79 b | 1.57 ±1.20 a | 0.46 ± 0.15 a | 2.91 ± 2.39 a |
| 9 | 10.32 | 1309 | p-Menth-1-en-9-ol | - | - | 0.32 ± 0.51 | 0.03 ± 0.03 |
| Total alcohols | 11.6 | 10.39 | 24.45 | 11.07 | |||
| Ketones | |||||||
| 10 | 7.805 | 1068 | Acetophenone | 0.061 ± 0.05 | 0.17 ± 0.12 | 0.09 ± 0.02 | 0.26 ± 0.24 |
| 11 | 7.87 | 1070 | Isophorone | 0.20 ± 0.16 | 1.71 ± 1.45 | 0.19 ± 0.19 | 2.46 ± 2.13 |
| 12 | 9.788 | 1254.48 | Pulegone | 0.01 ± 0.00 | 0.05 ± 0.04 | 0.10 ± 0.07 | 0.02 ± 0.02 |
| 13 | 11.124 | 1395 | Anisketone | 1.57 ± 0.79 | 0.30 ± 0.32 | 0.37 ± 0.23 | 3.76 ± 6.11 |
| 14 | 11.272 | 1410 | (Z)-Jasmone | 0.02 ± 0.00 | 0.05 ± 0.02 | 0.02 ± 0.00 | 0.04 ± 0.03 |
| 15 | 11.683 | 1450 | Geranyl acetone | 2.68 ± 0.68 a | 1.99 ± 1.23 b | 1.84 ± 0.55 ab | 3.61 ± 2.95 b |
| 16 | 14.06 | 1692 | Turmerone | 0.24 ± 0.01 a | 0.17 ± 0.11 b | 0.32 ± 0.22 ab | 0.12 ± 0.05 b |
| Total ketones | 4.78 | 4.44 | 2.93 | 10.27 | |||
| Aldehydes | |||||||
| 17 | 8.034 | 1085 | 5-formylfurfural | 0.33 ± 0.13 | 0.31 ± 0.50 | 0.38 ± 0.36 | 0.14 ± 0.06 |
| 18 | 9.121 | 1185 | Benzaldehyde, 2,5-dimethyl- | 0.16 ± 0.05 b | 0.22 ± 0.16 a | 0.21 ± 0.13 ab | 0.25 ± 0.21 a |
| 19 | 9.365 | 1209 | n-Decanal | 0.12 ± 0.03 | 0.11 ± 0.00 | 0.24 ± 0.20 | 0.08 ± 0.06 |
| 20 | 9.4 | 1212 | Safranal | 0.05 ± 0.03 | 0.05 ± 0.04 | 0.21 ± 0.03 | 0.14 ± 0.12 |
| 21 | 9.566 | 1229 | 5-Hydroxymethylfurfural | 2.71 ± 1.23 b | 0.87 ± 1.49 ab | 1.71 ± 1.47 ab | 0.82 ± 0.42 a |
| 22 | 9.613 | 1235 | β-Cyclocitral | 1.71 ± 1.30 b | 1.45 ± 1.19 a | 0.46 ± 0.28 a | 2.39 ± 2.06 a |
| 23 | 9.93 | 1268 | p-Anisaldehyde | 1.53 ± 0.48 | 1.76 ± 0.59 | 4.66 ± 4.56 | 7.08 ± 8.85 |
| 24 | 10.026 | 1279 | α-Citral | 0.17 ± 0.03 b | 0.18 ± 0.08 a | 0.30 ± 0.17 ab | 0.34 ± 0.04 a |
| 25 | 10.09 | 1285 | (E)-Cinnamaldehyde | 1.00 ± 0.46 | 0.35 ± 0.51 | 0.50 ± 0.40 | 0.79 ± 1.15 |
| 26 | 10.707 | 1351 | Piperonal | 0.10 ± 0.05 | 0.08 ± 0.07 | 0.07 ± 0.06 | 0.05 ± 0.09 |
| 27 | 11.263 | 1409 | Methyleugenol | 0.26 ± 0.09 b | 0.18 ± 0.10 a | 0.05 ± 0.03 a | 0.24 ± 0.19 a |
| 28 | 13.008 | 1579 | trans-4-Methoxycinnamaldehyde | 0.09 ± 0.05 | 0.02 ± 0.03 | 0.02 | 0.41 |
| Total Aldehyde | 8.23 | 5.58 | 8.81 | 12.73 | |||
| Aromatics | |||||||
| 29 | 8.2 | 1100 | p-Cymenene | 0.03 ± 0.02 | 0.07 ± 0.03 | 0.05 ± 0.06 | 0.10 ± 0.09 |
| 30 | 8.309 | 1110 | Unknown | 0.12 ± 0.05 | 0.94 ± 0.76 | 0.31 ± 0.23 | 0.66 ± 0.51 |
| 31 | 10.372 | 1315 | α-Methylnaphthalene | 0.15 ± 0.02 | 0.18 ± 0.03 | 0.38 ± 0.27 | 0.12 ± 0.04 |
| 32 | 10.541 | 1333 | β-Methylnaphthalene | 1.92 ± 3.00 | 0.02 ± 0.02 | 0.03 ± 0.02 | 0.22 ± 0.19 |
| 33 | 11.33 | 1415 | Diphenyl ether | 0.20 ± 0.07 | 0.13 ± 0.19 | 0.20 ± 0.15 | 0.10 ± 0.15 |
| 34 | 11.531 | 1435 | Dimethylnaphthalene | 0.14 ± 0.04 | 0.06 ± 0.03 | 0.03 ± 0.02 | 0.13 |
| 35 | 11.945 | 1475 | Unknown | 0.22 ± 0.11 | 0.10 ± 0.17 | 0.05 ± 0.06 | 0.02 ± 0.05 |
| Total aromatics | 2.78 | 1.5 | 1.05 | 1.35 | |||
| Esters | |||||||
| 36 | 8.26 | 1105 | Methyl benzoate | 2.02 ± 1.37 | 1.58 ± 0.69 | 2.22 ± 0.81 | 0.23 |
| 37 | 8.971 | 1171 | Benzyl acetate | 0.21 ± 0.16 | 0.13 ± 0.06 | 0.07 ± 0.08 | 0.19 |
| 38 | 9.341 | 1205 | Methyl salicylate | 0.11 ± 0.02 | 0.09 ± 0.03 | 0.09 ± 0.06 | 0.07 |
| 39 | 10.804 | 1361 | α-Terpinyl acetate | 0.08 ± 0.04 a | 0.01 ± 0.01 b | 0.11 ± 0.08 ab | 0.11 ab |
| 40 | 14.901 | 1785 | Benzyl Benzoate | 0.25 ± 0.11 | 0.40 ± 0.29 | 0.19 ± 0.12 | 0.35 |
| Total esters | 2.67 | 2.21 | 2.68 | 0.95 | |||
| Ethers/Oxides | |||||||
| 41 | 9.349 | 1207 | Estragole | 0.24 ± 0.12 | 0.17 ± 0.10 | 0.61 ± 0.46 | 0.28 ± 0.16 |
| 42 | 9.889 | 1264 | Anethole | 0.02 ± 0.00 | 0.09 ± 0.06 | 0.10 ± 0.05 | 0.13 ± 0.14 |
| 43 | 10.215 | 1299 | Anethole isomer | 6.02 ± 2.57 | 2.57 ± 3.02 | 4.53 ± 3.30 | 19.85 ± 32.11 |
| 44 | 10.266 | 1304 | Safrole | 0.07 ± 0.02 | 0.02 ± 0.00 | 0.16 ± 0.12 | 0.06 ± 0.04 |
| 45 | 10.882 | 1370 | Eugenol | 0.11 ± 0.02 | 0.02 ± 0.01 | 0.37 ± 0.02 | 0.15 ± 0.11 |
| 46 | 8.864 | 1161 | Nerol oxide | 0.77 ± 0.11 | 0.01 ± 0.00 | 0.26 ± 0.42 | 0.43 ± 0.28 |
| Total ethers/Oxides | 7.23 | 2.88 | 6.03 | 20.9 | |||
| Ionones | |||||||
| 47 | 9.951 | 1271 | α-Ionene | 0.047 ± 0.03 | 0.03 ± 0.02 | 0.42 ± 0.27 | 0.41 ± 0.38 |
| 48 | 10.913 | 1373 | Dehydro-ar-ionene | 0.65 ± 0.18 | 0.19 ± 0.07 | 2.35 ± 1.52 | 0.31 ± 0.27 |
| 49 | 10.942 | 1376 | Megastigma-4,6(Z),8(E)-triene | 0.03 ± 0.02 | 0.04 ±0.03 | 0.09 ± 0.05 | 0.05 ± 0.04 |
| 50 | 11.633 | 1445 | Dihydro-β-ionone | 0.02 ± 0.00 | - | 0.26 ± 0.17 | 0.08 ± 0.05 |
| 51 | 12.096 | 1490.73 | β-Ionone | 13.75 ± 3.37 a | 6.09 ± 0.29 b | 4.68 ± 3.40 b | 11.52 ± 8.80 b |
| Total ionones | 14.49 | 6.35 | 7.80 | 12.37 | |||
| Acids | |||||||
| 52 | 10.8 | 1361 | n-Decanoic acid | 0.06 ± 0.01 | 0.48 ± 0.64 | 0.07 ± 0.04 | 0.37 ± 0.31 |
| Total acids | 0.06 | 0.48 | 0.07 | 0.37 | |||
| Nitrogenous Compounds | |||||||
| 53 | 11.379 | 1420.76 | Methyl methanthranilate | 0.11 ± 0.02 | 0.05 ± 0.00 | 0.31 ± 0.10 | 0.08 ± 0.05 |
| Total Nitrogenous Compounds | 0.11 | 0.05 | 0.31 | 0.08 | |||
| Phenols | |||||||
| 54 | 11.737 | 1455.71 | Thymoquinol | 0.67 | 1.00 | 0.76 | 0.27 |
| Total phenols | 0.67 | 1.00 | 0.76 | 0.27 | |||
| Aliphatic hydrocarbon | |||||||
| 55 | 11.17 | 1400 | Tetradecane | 0.32 ± 0.21 b | 0.33 ± 0.24 a | 0.22 ± 0.20 ab | 0.19 ± 0.15 a |
| 56 | 13.219 | 1599 | Hexadecane | 0.06 ± 0.02 | 0.06 ± 0.04 | 0.02 ± 0.02 | 0.07 ± 0.03 |
| 57 | 14.244 | 1712 | Heptadecane | 0.10 ± 0.02 | 0.23 ± 0.18 | 0.04 ± 0.06 | 0.21± 0.14 |
| 58 | 15.195 | 1816 | Octadecane | 8.33 ± 14.41 | 0.14 ± 0.09 | 0.06 ± 0.04 | 0.11 ± 0.07 |
| 59 | 15.904 | 1891 | Nonadecane | 0.14 ± 0.04 | 0.32 ± 0.07 | 0.11 ± 0.03 | 0.25 ± 0.09 |
| Total aliphatic hydrocarbon | 8.95 | 1.08 | 0.45 | 0.83 | |||
| Lactones | |||||||
| 60 | 11.88 | 1469 | γ-Decanolactone | 17.14 ± 4.60 | 26.34 ± 6.66 | 31.19 ± 12.99 | 5.00 ± 4.37 |
| 61 | 12.179 | 1498 | δ-Decalactone | 2.16 ± 0.55 | 2.73 ± 0.16 | 2.51 ± 1.32 | 0.15 ± 0.13 |
| 62 | 12.698 | 1549 | Dihydroactinidiolide | 5.54 ± 1.16 a | 7.76 ± 2.45 ab | 1.46 ± 0.68 b | 9.13 ± 6.31 ab |
| 63 | 14.156 | 1703 | γ-Dodecalactone | 0.61 ± 0.13 | 1.48 ± 0.32 | 1.98 ± 0.72 | 0.54 ± 0.369 |
| Total lactones | 24.45 | 38.31 | 37.14 | 14.82 | |||
| Fatty acids/esters | |||||||
| 64 | 12.345 | 1515 | Methyl laurate | 0.75 ± 0.32 | 0.70 ± 0.34 | 0.23 ± 0.11 | 0.14 ± 0.12 |
| 65 | 14.439 | 1734 | Methyl myristate | 1.20 ± 0.47 | 2.13 ± 0.97 | 0.68 ± 0.23 | 0.34 ± 0.26 |
| 66 | 14.676 | 1761 | Myristic acid | 0.05 ± 0.01 | 0.11 ± 0.08 | 0.05 ± 0.03 | 0.32 ± 0.20 |
| 67 | 15.956 | 1896 | Methyl palmitoleate | 0.10 ± 0.04 a | 0.34 ± 0.27 b | 0.08 ± 0.03 ab | 0.08 ± 0.06 ab |
| 68 | 16.164 | 1918 | Methyl palmitate | 9.42 ± 3.29 a | 20.32 ± 8.86 b | 5.80 ± 1.90 b | 8.10 ± 6.70 b |
| 69 | 16.471 | 1950 | Palmitic acid | 0.31 ± 0.04 a | 0.68 ± 0.93 ab | 0.13 ± 0.07 b | 2.77 ± 2.04 b |
| 70 | 18.234 | 2134 | 8,11-Octadecadienoic acid, methyl ester | 0.76 ± 0.32 | 1.11 ± 0.33 | 0.22 ± 0.06 | 1.88 ± 1.90 |
| Total fatty acids/ester | 12.59 | 25.39 | 7.19 | 13.63 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.A.; Ramadan, N.S.; Shorbagi, M.; Farag, N.; Gad, H.A. Profiling of Primary Metabolites and Volatiles in Apricot (Prunus armeniaca L.) Seed Kernels and Fruits in the Context of Its Different Cultivars and Soil Type as Analyzed Using Chemometric Tools. Foods 2022, 11, 1339. https://doi.org/10.3390/foods11091339
Farag MA, Ramadan NS, Shorbagi M, Farag N, Gad HA. Profiling of Primary Metabolites and Volatiles in Apricot (Prunus armeniaca L.) Seed Kernels and Fruits in the Context of Its Different Cultivars and Soil Type as Analyzed Using Chemometric Tools. Foods. 2022; 11(9):1339. https://doi.org/10.3390/foods11091339
Chicago/Turabian StyleFarag, Mohamed A., Nehal S. Ramadan, Mohamed Shorbagi, Nermeen Farag, and Haidy A. Gad. 2022. "Profiling of Primary Metabolites and Volatiles in Apricot (Prunus armeniaca L.) Seed Kernels and Fruits in the Context of Its Different Cultivars and Soil Type as Analyzed Using Chemometric Tools" Foods 11, no. 9: 1339. https://doi.org/10.3390/foods11091339
APA StyleFarag, M. A., Ramadan, N. S., Shorbagi, M., Farag, N., & Gad, H. A. (2022). Profiling of Primary Metabolites and Volatiles in Apricot (Prunus armeniaca L.) Seed Kernels and Fruits in the Context of Its Different Cultivars and Soil Type as Analyzed Using Chemometric Tools. Foods, 11(9), 1339. https://doi.org/10.3390/foods11091339







