Comprehensive Evaluation of Probiotic Property, Hypoglycemic Ability and Antioxidant Activity of Lactic Acid Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Materials
2.3. Cell Free Supernatant (CFS), Intact Cell (IC) and Intracellular Cell Free Extraction (CFE) Preparation
2.4. Probiotic Property
2.4.1. Tolerance to Artificial Gastric Juice
2.4.2. Tolerance to Artificial Bile Salt
2.4.3. Cell Adhesion Activity
2.5. Hypoglycemic Ability
2.5.1. Inhibition of α-Amylase
2.5.2. Inhibition of α-Glucosidase
2.5.3. Inhibition of DPP4
2.6. Antioxidant Activity
2.6.1. DPPH Radical Scavenging Activity
2.6.2. Hydroxyl Radical Scavenging Activity
2.6.3. Superoxide Dismutase Activity
2.6.4. Ferric-Reducing Antioxidant Power (FRAP)
2.7. Antibiotic Susceptibility
2.8. Statistics Analysis
3. Result and Discussion
3.1. Tolerance to Artificial Gastric Juice and Bile Salt
3.1.1. Tolerance to Artificial Gastric Juice
3.1.2. Tolerance to Artificial Bile Salt
3.2. Cell Adhesion Ability
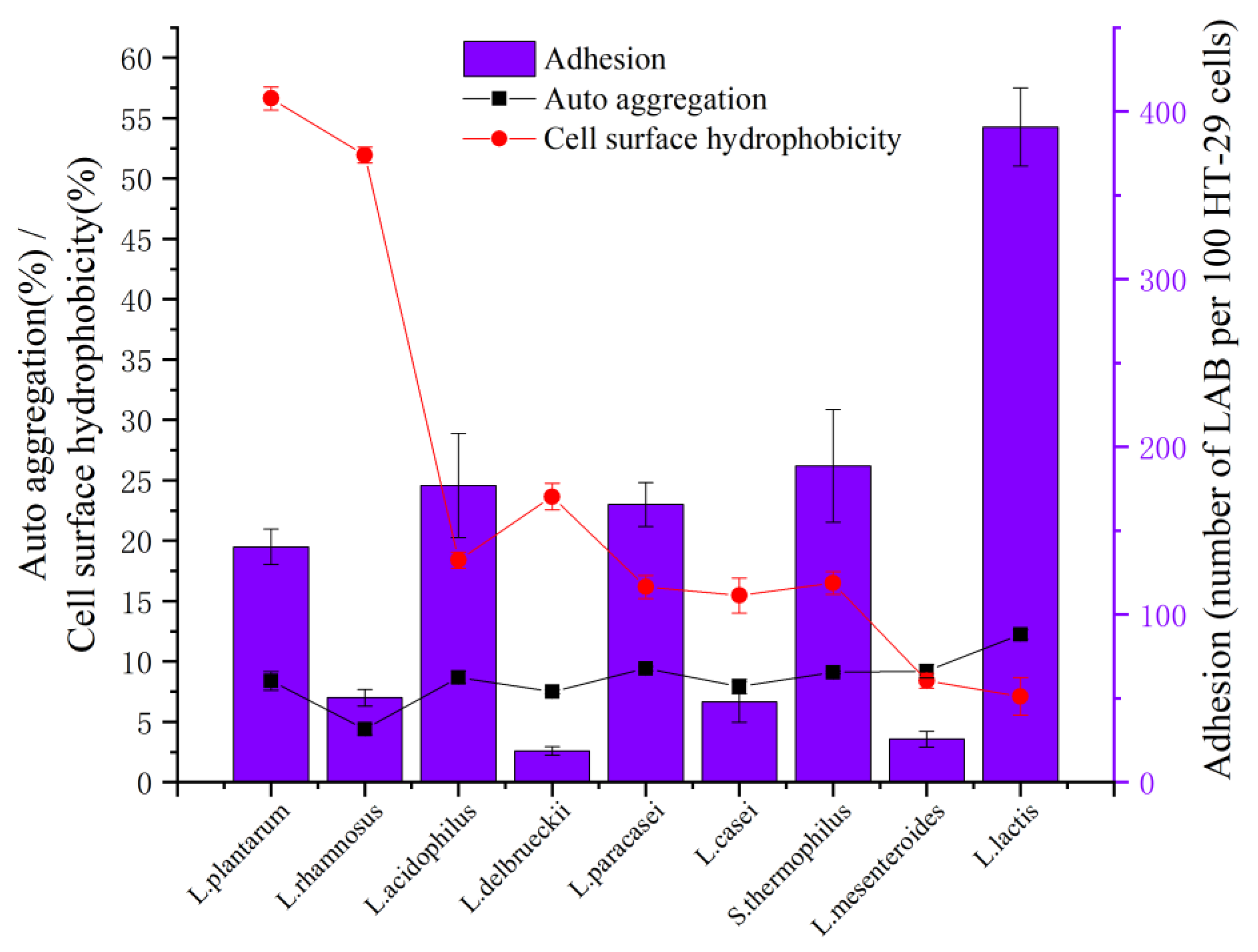
3.2.1. Auto Aggregation and Cell Surface Hydrophobicity
3.2.2. HT-29 Cell Adhesion Activity
3.3. Hypoglycemic Ability
3.3.1. Inhibition of α-Amylase
3.3.2. Inhibition of α-Glucosidase
3.3.3. Inhibition of Dipeptidyl Peptidase 4
3.4. Antioxidant Activity
3.4.1. DPPH Radical Scavenging Activity
3.4.2. Total Antioxidant Activity (FRAP)
3.4.3. Hydroxyl Radical Scavenging Activity
3.4.4. SOD Activity
3.5. Antibiotics Susceptibility
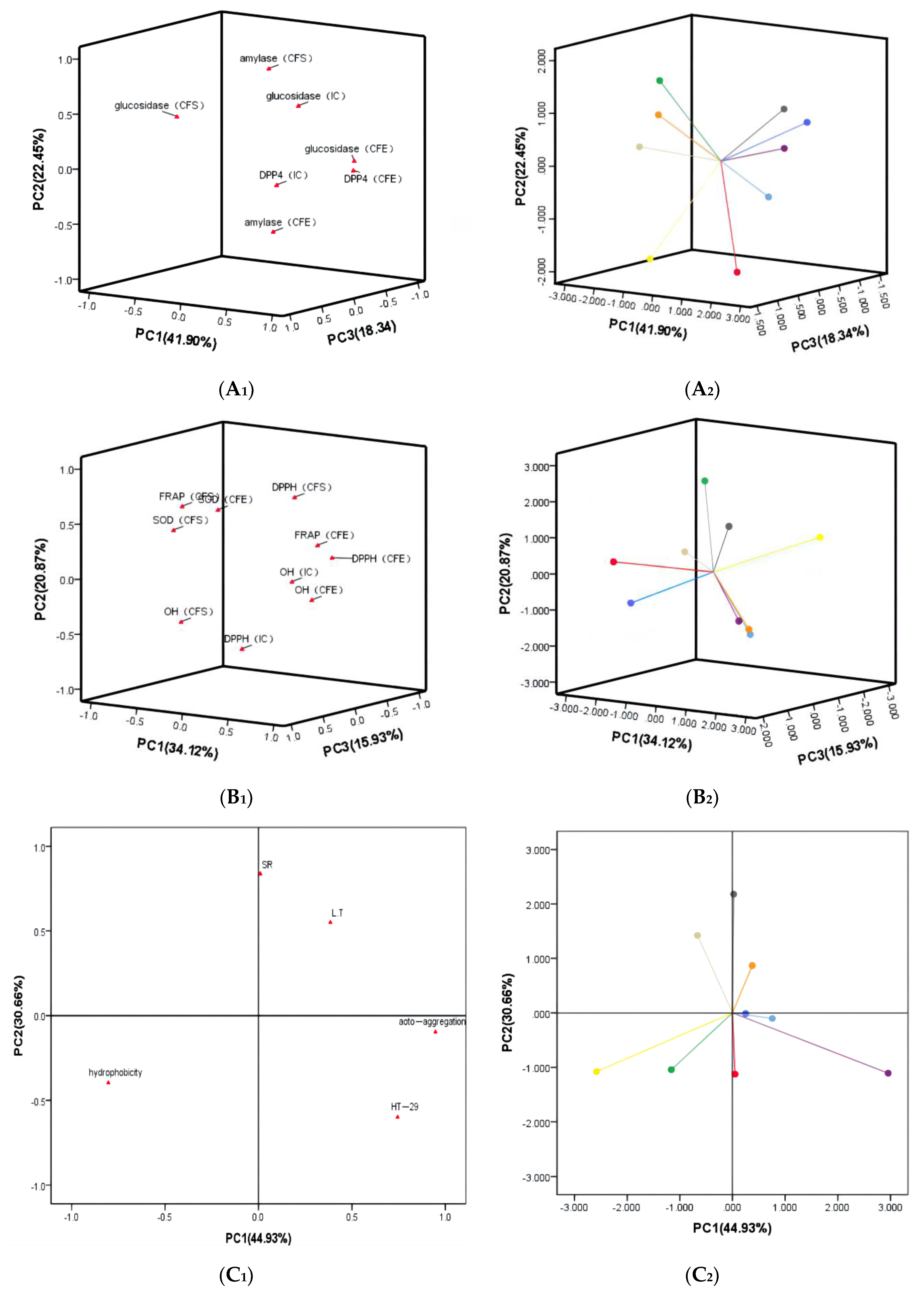
3.6. Principal Component Analysis (PCA) and Analytic Hierarchy Process (AHP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogurtsova, K.; Da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.; Park, J.B. Clinical implication of fasting glucose and systolic/diastolic blood pressure on the prevalence of periodontitis in non-diabetic and non-hypertensive adults using nationally representative data. Exp. Ther. Med. 2018, 16, 671–678. [Google Scholar] [CrossRef]
- Islam, M.S. Animal Models of Diabetic Neuropathy: Progress Since 1960s. J. Diabetes Res. 2013, 2013, 149452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, L. Effect of local insulin injection on wound vascularization in patients with diabetic foot ulcer. Exp. Ther. Med. 2015, 11, 397–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broichhagen, J.; Schönberger, M.; Cork, S.; Frank, J.A.; Marchetti, P.; Bugliani, M.; Shapiro, A.M.J.; Trapp, S.; Rutter, G.; Hodson, D.J.; et al. Optical control of insulin release using a photoswitchable sulfonylurea. Nat. Commun. 2014, 5, 5116. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of gallic acid on α-amylase and α-glucosidase inhibitory properties of acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Phoem, A.N.; Mayiding, A.; Saedeh, F.; Permpoonpattana, P. Evaluation of Lactobacillus plantarum encapsulated with Eleutherine americana oligosaccharide extract as food additive in yoghurt. Braz. J. Microbiol. 2019, 50, 237–246. [Google Scholar] [CrossRef]
- Niibo, M.; Shirouchi, B.; Umegatani, M.; Morita, Y.; Ogawa, A.; Sakai, F.; Kadooka, Y.; Sato, M. Probiotic Lactobacillus gasseri SBT2055 improves insulin secretion in a diabetic rat model. J. Dairy Sci. 2019, 102, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Toejing, P.; Khat-Udomkiri, N.; Intakhad, J.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Putative Mechanisms Responsible for the Antihyperglycemic Action of Lactobacillus paracasei HII01 in Experimental Type 2 Diabetic Rats. Nutrients 2020, 12, 3015. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S. Ameliorative Effects of Probiotic Lactobacillus paracasei NL41 on Insulin Sensitivity, Oxidative Stress, and Beta-Cell Function in a Type 2 Diabetes Mellitus Rat Model. Mol. Nutr. Food Res. 2019, 63, e1900457. [Google Scholar] [CrossRef]
- Obaroakpo, J.U.; Liu, L.; Zhang, S.; Lu, J.; Pang, X.; Lv, J. α-Glucosidase and ACE dual inhibitory protein hydrolysates and peptide fractions of sprouted quinoa yoghurt beverages inoculated with Lactobacillus casei. Food Chem. 2019, 299, 124985. [Google Scholar] [CrossRef]
- Ragul, K.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Evaluation of functional properties of potential probiotic isolates from fermented brine pickle. Food Chem. 2020, 311, 126057. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, N.; Yue, Y.; Wang, C.; Zhao, L.; Evivie, S.; Li, B.; Huo, G. Screening for Potential Novel Probiotics With Dipeptidyl Peptidase IV-Inhibiting Activity for Type 2 Diabetes Attenuation in vitro and in vivo. Front. Microbiol. 2020, 10, 2855. [Google Scholar] [CrossRef]
- Zeng, Z.; Luo, J.; Zuo, F.; Zhang, Y.; Ma, H.; Chen, S. Screening for potential novel probiotic Lactobacillus strains based on high dipeptidyl peptidase IV and α-glucosidase inhibitory activity. J. Funct. Foods 2016, 20, 486–495. [Google Scholar] [CrossRef]
- Walton, E.L. Oxidative stress and diabetes: Glucose response in the cROSsfire. Biomed. J. 2017, 40, 241–244. [Google Scholar] [CrossRef]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef] [Green Version]
- Son, S.-H.; Jeon, H.-L.; Jeon, E.B.; Lee, N.-K.; Park, Y.-S.; Kang, D.-K.; Paik, H.-D. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT 2017, 85, 181–186. [Google Scholar] [CrossRef]
- Liu, W.; Chen, M.; Duo, L.; Wang, J.; Guo, S.; Sun, H.; Menghe, B.; Zhang, H. Characterization of potentially probiotic lactic acid bacteria and bifidobacteria isolated from human colostrum. J. Dairy Sci. 2020, 103, 4013–4025. [Google Scholar] [CrossRef] [PubMed]
- Vasiee, A.; Falah, F.; Behbahani, B.A.; Tabatabaee-Yazdi, F. Probiotic characterization of Pediococcus strains isolated from Iranian cereal-dairy fermented product: Interaction with pathogenic bacteria and the enteric cell line Caco-2. J. Biosci. Bioeng. 2020, 130, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Panda, S.; Kumar, S.; Ray, P. In vitro evaluation of adherence and anti-infective property of probiotic Lactobacillus plantarum DM 69 against Salmonella enterica. Microb. Pathog. 2019, 126, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Manan, H.; Sallehhuddin, M.; Musa, N.; Ikhwanuddin, M. Screening of Lactic Acid Bacteria isolated from giant freshwater prawn (Macrobrachium rosenbergii) as potential probiotics. Aquac. Rep. 2020, 18, 100523. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M. Leuconostoc mesenteroides MKSR isolated from kimchi possesses α-glucosidase inhibitory activity, antioxidant activity, and cholesterol-lowering effects. LWT 2019, 116, 108570. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Li, L. The relationship between charge intensity and bioactivities/processing characteristics of exopolysaccharides from lactic acid bacteria. LWT 2022, 153, 112345. [Google Scholar] [CrossRef]
- Jung, J.; Jang, H.J.; Eom, S.J.; Choi, N.S.; Lee, N.-K.; Paik, H.-D. Fermentation of red ginseng extract by the probiotic Lactobacillus plantarum KCCM 11613P: Ginsenoside conversion and antioxidant effects. J. Ginseng Res. 2017, 43, 20–26. [Google Scholar] [CrossRef]
- Banothu, V.; Neelagiri, C.; Adepally, U.; Lingam, J.; Bommareddy, K. Phytochemical screening and evaluation of in vitro antioxidant and antimicrobial activities of the indigenous medicinal plant Albizia odoratissima. Pharm. Biol. 2017, 55, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Kwun, S.Y.; Bae, Y.W.; Yoon, J.A.; Park, E.H.; Kim, M.D. Isolation of acid tolerant lactic acid bacteria and evaluation of α-glucosidase inhibitory activity. Food Sci. Biotechnol. 2020, 29, 1125–1130. [Google Scholar] [CrossRef]
- Chen, T.; Wang, L.; Li, Q.; Long, Y.; Lin, Y.; Yin, J.; Zeng, Y.; Huang, L.; Yao, T.; Abbasi, M.N.; et al. Functional probiotics of lactic acid bacteria from Hu sheep milk. BMC Microbiol. 2020, 20, 228. [Google Scholar] [CrossRef]
- Fadare, O.S.; Singh, V.; Enabulele, O.I.; Shittu, O.H.; Pradhan, D. In vitro evaluation of the synbiotic effect of probiotic Lactobacillus strains and garlic extract against Salmonella species. LWT 2021, 153, 112439. [Google Scholar] [CrossRef]
- Rajab, S.; Tabandeh, F.; Shahraky, M.K.; Alahyaribeik, S. The effect of Lactobacillus cell size on its probiotic characteristics. Anaerobe 2020, 62, 102103. [Google Scholar] [CrossRef] [PubMed]
- Kebouchi, M.; Galia, W.; Genay, M.; Soligot, C.; LeComte, X.; Awussi, A.A.; Perrin, C.; Roux, E.; Dary-Mourot, A.; Le Roux, Y. Implication of sortase-dependent proteins of Streptococcus thermophilus in adhesion to human intestinal epithelial cell lines and bile salt tolerance. Appl. Microbiol. Biotechnol. 2016, 100, 3667–3679. [Google Scholar] [CrossRef]
- Cai, T.; Wu, H.; Qin, J.; Qiao, J.; Yang, Y.; Wu, Y.; Qiao, D.; Xu, H.; Cao, Y. In vitro evaluation by PCA and AHP of potential antidiabetic properties of lactic acid bacteria isolated from traditional fermented food. LWT 2019, 115, 108455. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. Screening for potential new probiotic based on probiotic properties and α-glucosidase inhibitory activity. Food Control 2014, 35, 65–72. [Google Scholar] [CrossRef]
- Han, Q.; Kong, B.; Chen, Q.; Sun, F.; Zhang, H. In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J. Funct. Foods 2017, 32, 391–400. [Google Scholar] [CrossRef]
- Jara, J.; Pérez-Ramos, A.; del Solar, G.; Rodríguez, J.M.; Fernández, L.; Orgaz, B. Role of Lactobacillus biofilms in Listeria monocytogenes adhesion to glass surfaces. Int. J. Food Microbiol. 2020, 334, 108804. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, W.; Sun, C.; Gu, L.; Liu, Z.; Yu, W.; Chen, L.; Jiang, Z.; Hou, J. Effects of environmental stresses on the physiological characteristics, adhesion ability and pathogen adhesion inhibition of Lactobacillus plantarum KLDS 1.0328. Process Biochem. 2020, 92, 426–436. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control 2019, 111, 107057. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, M.; Wang, M.; Chen, W.; Li, J.; Yao, Y. The correlation between colonization and the biological properties of Lactobacillus sp. Food Biosci. 2020, 36, 100613. [Google Scholar] [CrossRef]
- Abushelaibi, A.; Al-Mahadin, S.; El-Tarabily, K.; Shah, N.P.; Ayyash, M. Characterization of potential probiotic lactic acid bacteria isolated from camel milk. LWT 2017, 79, 316–325. [Google Scholar] [CrossRef]
- Rocha-Mendoza, D.; Kosmerl, E.; Miyagusuku-Cruzado, G.; Giusti, M.M.; Jiménez-Flores, R.; García-Cano, I. Growth of lactic acid bacteria in milk phospholipids enhances their adhesion to Caco-2 cells. J. Dairy Sci. 2020, 103, 7707–7718. [Google Scholar] [CrossRef] [PubMed]
- Kalita, D.; Holm, D.G.; LaBarbera, D.V.; Petrash, J.M.; Jayanty, S.S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS ONE 2018, 13, e0191025. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Abdullah; Zhang, Y.; Zhao, M.; Zhang, J.; Zhang, H.; Xi, Y.; Cai, H.; Feng, F. Screening of novel potential antidiabetic Lactobacillus plantarum strains based on in vitro and in vivo investigations. LWT 2020, 139, 110526. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Production and identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from discarded Sardine pilchardus protein. Food Chem. 2020, 328, 127096. [Google Scholar] [CrossRef] [PubMed]
- Kaprasob, R.; Sarkar, D.; Kerdchoechuen, O.; Laohakunjit, N.; Khanongnuch, C.; Shetty, K. Beneficial lactic acid bacteria based bioprocessing of cashew apple juice for targeting antioxidant nutraceutical inhibitors as relevant antidotes to type 2 diabetes. Process Biochem. 2019, 82, 40–50. [Google Scholar] [CrossRef]
- Tang, W.; Xing, Z.; Li, C.; Wang, J.; Wang, Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, X.; Hu, Y.-S.; Wu, Y.; Wang, Q.-Z.; Li, N.-N.; Guo, Q.-C.; Dong, X.-C. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009, 115, 32–36. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, X.; Tuo, Y.; Zhang, H.; Li, Y.; Xu, C.; Mu, G.; Jiang, S. Reducing antigenicity of β-lactoglobulin, probiotic properties and safety evaluation of Lactobacillus plantarum AHQ-14 and Lactobacillus bulgaricus BD0390. Food Biosci. 2021, 42, 101137. [Google Scholar] [CrossRef]

| Hypoglycemic Ability | Strain | L. plantarum | L. rhamnosus | L. acidophilus | L. delbrueckii | L. paracasei | L. casei | S. thermophilus | L. mesenteroides | L. lactis | Acarbose |
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-amylase inhibitory activities(%) | CFS | 83.36 ± 0.77 a | 62.28 ± 0.67 e | 72.51 ± 1.73 d | 82.83 ± 0.55 ab | 82.21 ± 1.12 abc | 79.27 ± 1.45 abc | 71.09 ± 1.81 d | 78.47 ± 0.88 c | 78.83 ± 0.77 bc | 78.65 ± 1.78 |
| IC | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| CFE | ND | ND | 7.77 ± 0.21 | ND | ND | ND | ND | ND | ND | ||
| α-glucosidase inhibitory activities (%) | CFS | 85.16 ± 0.32 a | 82.55 ± 1.76 a | 29.04 ± 2.79 e | 56.90 ± 1.64 c | 44.53 ± 2.49 d | 69.66 ± 1.21 b | 52.86 ± 2.67 c | 83.20 ± 1.15 a | 51.95 ± 3.68 cd | 91.90 ± 2.94 |
| IC | 83.81 ± 1.78 a | 73.81 ± 5.26 c | 82.86 ± 2.33 ab | 78.10 ± 3.37 bc | 84.29 ± 1.17 a | 81.90 ± 3.56 a | 78.10 ± 1.78 abc | 81.43 ± 1.17 abc | 78.57 ± 2.33 abc | ||
| CFE | 5.21 ± 1.44 d | 9.38 ± 1.69 cd | 17.84 ± 1.61 b | 4.56 ± 2.08 d | 26.17 ± 1.94 a | 28.78 ± 1.81 a | 16.15 ± 2.26 bc | 8.07 ± 1.61 d | 29.17 ± 3.81 a | ||
| DPP4 inhibitory activities (%) | CFS | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| IC | 14.91 ± 1.84 d | 7.22 ± 2.01 e | 27.30 ± 1.58 b | ND | 42.51 ± 1.25 a | 25.55 ± 1.61 bc | 36.69 ± 1.37 a | 6.13 ± 2.04 e | 20.61 ± 1.72 cd | ||
| CFE | 11.31 ± 5.43 b | 10.56 ± 5.48 b | 23.44 ± 4.69 ab | 12.83 ± 5.34 b | 32.66 ± 4.12 a | ND | ND | ND | ND |
| Antioxidant activities | Strain | L. plantarum | L. rhamnosus | L. acidophilus | L. delbrueckii | L. paracasei | L. casei | S. thermophilus | L. mesenteroides | L. lactis |
|---|---|---|---|---|---|---|---|---|---|---|
| Scavenging rate of DPPH radical (%) | CFS | 71.37 ± 0.53 a | 73.02 ± 0.24 a | 60.08 ± 0.81 e | 66.01 ± 0.45 bc | 62.56 ± 0.30 d | 64.64 ± 0.40 c | 57.43 ± 0.29 f | 67.28 ± 0.11 b | 59.04 ± 0.67 ef |
| IC | 8.15 ± 1.71 de | 6.06 ± 0.31 ef | 11.81 ± 0.66 c | 7.13 ± 0.56 e | 9.77 ± 0.42 cd | 4.50 ± 0.25 f | 9.77 ± 0.59 cd | 19.66 ± 0.08 a | 15.41 ± 0.37 b | |
| CFE | 18.46 ± 2.33 b | 26.37 ± 1.42 a | 10.65 ± 1.66 c | 11.08 ± 1.61 c | 8.44 ± 1.76 c | 8.23 ± 2.11 c | 14.35 ± 2.15 bc | 19.62 ± 2.37 ab | 14.35 ± 1.72 bc | |
| Total antioxidant activity(FeSO4·7H2O eq mmol/L) | CFS | 1.19 ± 0.06 a | 1.00 ± 0.04 c | 1.21 ± 0.05 a | 1.14 ± 0.01 ab | 1.15 ± 0.01 ab | 1.15 ± 0.02 ab | 0.98 ± 0.02 cd | 0.85 ± 0.03 d | 1.06 ± 0.04 bc |
| IC | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| CFE | 1.25 ± 0.01 a | 1.24 ± 0.05 a | 1.19 ± 0.02 a | 1.28 ± 0.05 a | 1.03 ± 0.01 b | 1.30 ± 0.06 a | 1.28 ± 0.01 a | 1.19 ± 0.02 a | 1.29 ± 0.02 a | |
| Hydroxyl radical scavenging activity(%) | CFS | 41.12 ± 0.51 e | 39.12 ± 0.49 f | 64.81 ± 0.10 a | 52.96 ± 0.26 c | 60.40 ± 0.26 b | 49.86 ± 0.68 d | 61.43 ± 0.42 b | 41.74 ± 0.44 e | 51.79 ± 1.09 c |
| IC | 22.24 ± 1.12 b | 21.07 ± 0.94 b | 13.09 ± 0.39 cd | 8.75 ± 0.49 e | 14.05 ± 0.51 c | 11.43 ± 0.52 d | 11.09 ± 0.83 de | 21.01 ± 0.19 b | 30.58 ± 0.73 a | |
| CFE | 45.45 ± 0.77 b | 45.73 ± 0.83 b | 41.94 ± 0.45 c | 40.91 ± 0.51 c | 45.94 ± 0.49 b | 50.69 ± 0.85 a | 50.14 ± 0.35 a | 46.97 ± 0.35 b | 46.49 ± 0.58 b | |
| SOD activity (%) | CFS | 35.01 ± 0.78 a | 28.60 ± 1.56 e | 33.35 ± 0.61 ab | 32.36 ± 1.18 bc | 32.75 ± 0.25 b | 32.00 ± 0.26 bcd | 29.91 ± 0.25 de | 32.59 ± 0.96 bc | 30.42 ± 0.85 cde |
| IC | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| CFE | 22.27 ± 0.3 a | 17.53 ± 0.36 b | 23.45 ± 0.57 a | 15.57 ± 0.84 b | 17.30 ± 1.23 b | 22.09 ± 1.81 a | 17.62 ± 0.67 b | 15.85 ± 1.06 b | 18.03 ± 0.39 b |
| Strain | AMP | CHL | ERY | TET | PEN | STR | GEN | KAN |
|---|---|---|---|---|---|---|---|---|
| L. plantarum | S | S | S | S | S | R | R | R |
| L. rhamnosus | S | S | S | S | S | R | R | R |
| L. acidophilus | S | S | S | S | S | R | R | R |
| L. delbrueckii | S | S | S | S | S | R | I | R |
| L. paracasei | S | S | S | S | S | R | R | R |
| L. casei | S | S | S | S | R | R | R | R |
| S. thermophilus | S | S | S | S | S | S | R | R |
| L. mesenteroides | S | S | S | S | S | S | R | R |
| L. lactis | S | S | S | S | R | R | R | R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, L. Comprehensive Evaluation of Probiotic Property, Hypoglycemic Ability and Antioxidant Activity of Lactic Acid Bacteria. Foods 2022, 11, 1363. https://doi.org/10.3390/foods11091363
Wang H, Li L. Comprehensive Evaluation of Probiotic Property, Hypoglycemic Ability and Antioxidant Activity of Lactic Acid Bacteria. Foods. 2022; 11(9):1363. https://doi.org/10.3390/foods11091363
Chicago/Turabian StyleWang, Hongyu, and Liang Li. 2022. "Comprehensive Evaluation of Probiotic Property, Hypoglycemic Ability and Antioxidant Activity of Lactic Acid Bacteria" Foods 11, no. 9: 1363. https://doi.org/10.3390/foods11091363
APA StyleWang, H., & Li, L. (2022). Comprehensive Evaluation of Probiotic Property, Hypoglycemic Ability and Antioxidant Activity of Lactic Acid Bacteria. Foods, 11(9), 1363. https://doi.org/10.3390/foods11091363






