Comparative Study on Physicochemical and Nutritional Qualities of Kiwifruit Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagent
2.2. Methods
2.2.1. Determinations of Pectin and Moisture Content
2.2.2. Determinations of Chlorophyll a Content (CAC), Chlorophyll B Content (CBC), Total Carotenoid Content (TCC)
2.2.3. Determination of Total Flavonoid Content (TFC)
2.2.4. Determinations of Energy, Carbohydrates, Protein, Lipid and Dietary Fiber
2.2.5. Determinations of Total Sugar and Total Acid Content
2.2.6. Determinations of Soluble Sugar and Organic Acid Content
2.2.7. Determinations of Vitamin C (VC), Vitamin B1 (VB1), Vitamin B2 (VB2), Vitamin B6 (VB6) and Vitamin E (VE)
2.2.8. Determination of Aroma
2.3. Statistical Analysis
3. Results
3.1. Pectin and Moisture
3.2. CAC, CBC, TCC and TFC
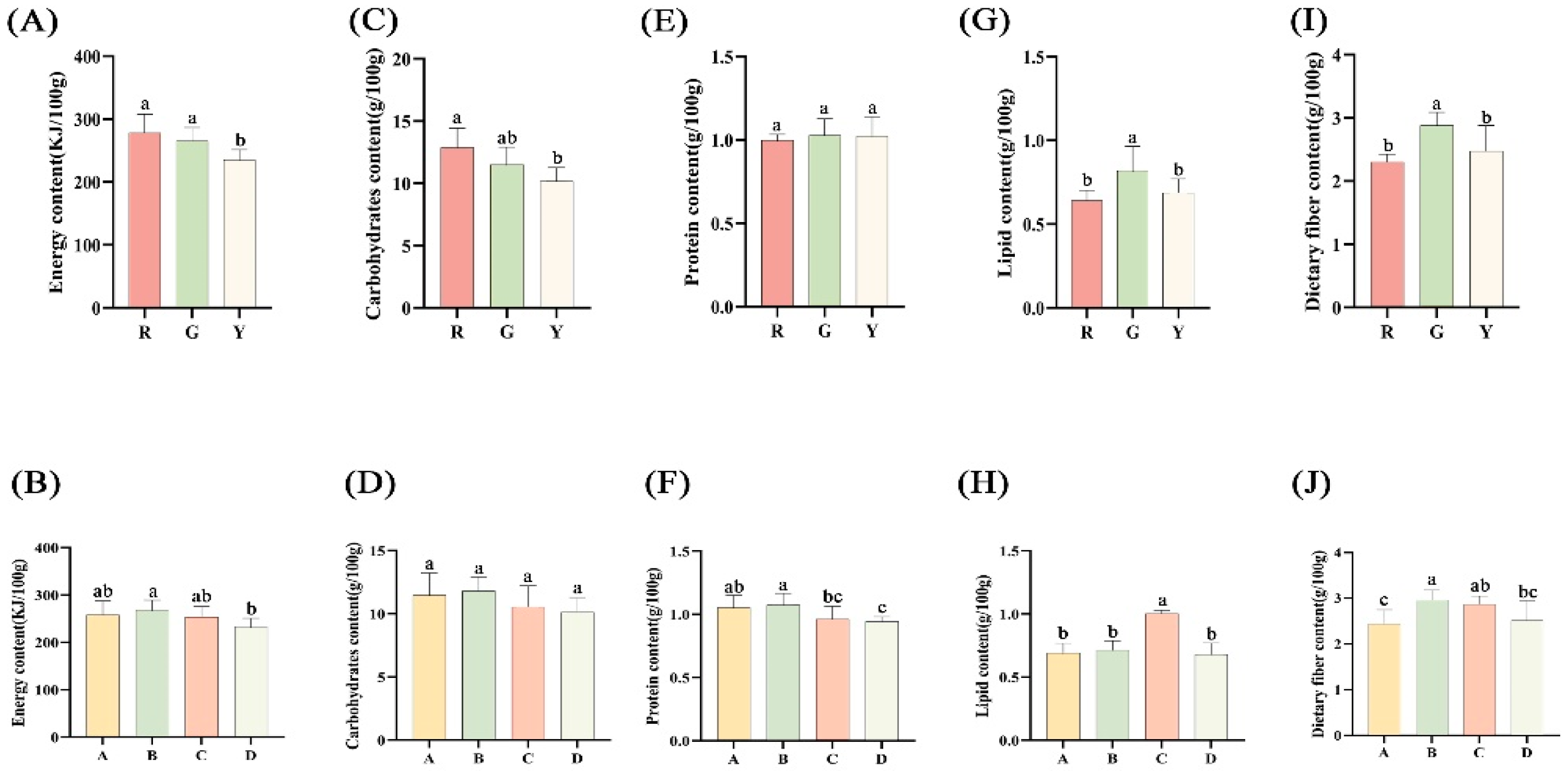
3.3. Energy, Carbohydrates, Protein, Lipid and Dietary Fiber
3.4. Total Sugar Content, Sucrose, Glucose and Fructose Content
3.5. Total Acid Content, Malic Acid Content, Citric Acid Content, Tartaric Acid Content and Quinic Acid Content
3.6. Sugar: Acid Ratio
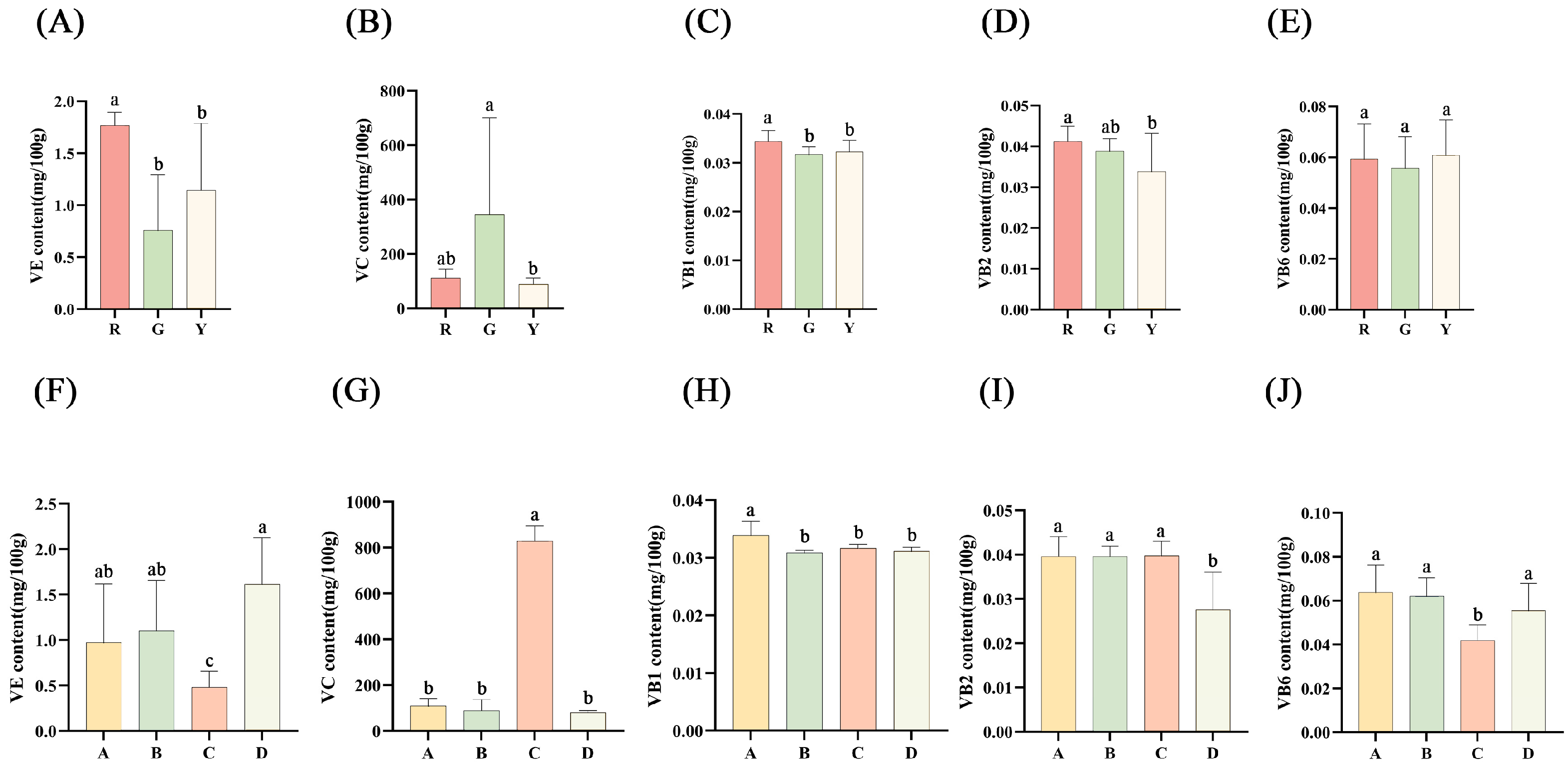
3.7. Vitamin
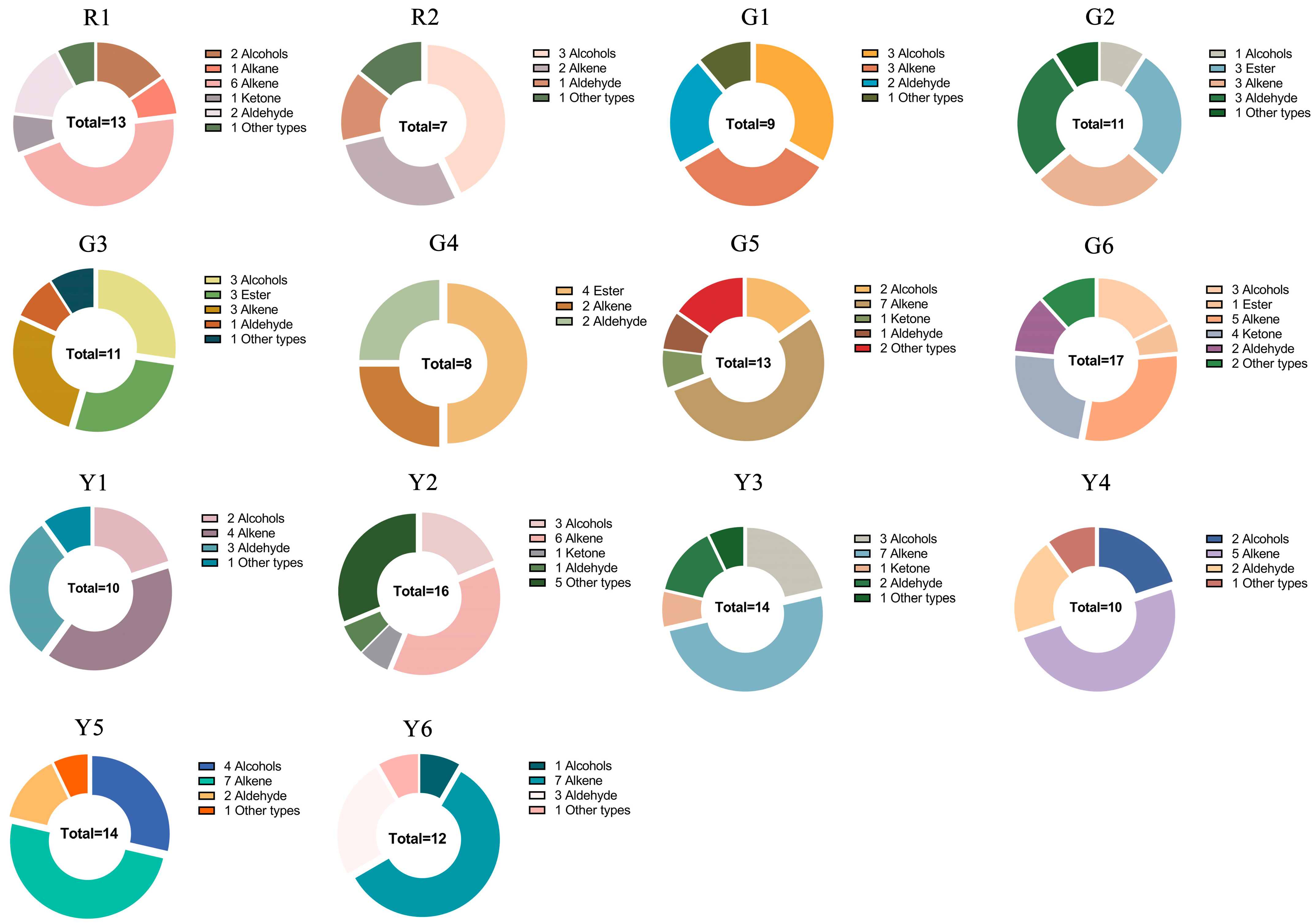
3.8. Aroma Substance
3.9. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.W.; Li, J.Q.; Soejarto, D.D. New synonyms in Actinidiaceae from China. Acta Phytotaxon. Sin. 2007, 45, 633–660. [Google Scholar] [CrossRef]
- Huang, H.W. Actinidia Jaxonomy Germplasm Domestication Cultivation; Botany, Zhang, Y.B., Eds.; China Science Publishing & Media Ltd.: Beijing, China, 2013; Volume 40, p. 539. [Google Scholar]
- Zhong, C.H.; Wang, S.M.; Jiang, Z.W.; Huang, H.W. ‘Jinyan’, an Interspecific Hybrid Kiwifruit with Brilliant Yellow Flesh and Good Storage Quality. Hortscience 2012, 47, 1187–1190. [Google Scholar] [CrossRef]
- Han, M.L.; Zhang, Z.Y.; Zhao, G.; Chen, L.P.; Li, Y.D. Research Advance and Prospect of Red-fleshed Kiwifruit Breeding in China. North. Hortic. 2014, 1, 182–187. [Google Scholar]
- Wang, M.Z. Sustainable Breeding Research of Actinidia Chinensis Var. Rufopulpa. Resour. Dev. Mark. 2003, 19, 309–310. [Google Scholar]
- Zhong, C.; Han, F.; Li, D.; Liu, X.; Zhang, Q.; Jiang, Z.; Huang, H. Breeding of red-fleshed kiwifruit cultivar Donghong. J. Fruit Sci. 2016, 33, 1596–1599. [Google Scholar]
- Cano, A.; Medina, A.; Bermejo, A. Bioactive compounds in different citrus varieties. Discrimination among cultivars. J. Food Compos. Anal. 2008, 21, 377–381. [Google Scholar] [CrossRef]
- Pu, D.D.; Duan, W.; Huang, Y.; Zhang, L.L.; Zhang, Y.Y.; Sun, B.G.; Tang, Y.Z. Characterization of the dynamic texture perception and the impact factors on the bolus texture changes during oral processing. Food Chem. 2021, 339, 128078. [Google Scholar] [CrossRef]
- Li, Z.H.; Jin, R.N.; Yang, Z.X.; Wang, X.; You, G.Y.; Guo, J.J.; Pan, S.Y. Comparative study on physicochemical, nutritional and enzymatic properties of two Satsuma mandarin (Citrus unshiu Marc.) varieties from different regions. J. Food Compos. Anal. 2021, 95, 103614. [Google Scholar] [CrossRef]
- Yuan, H.L.; Zhou, Y.L.; Chen, Y.S.; Yao, C.L.; He, Q.S.; Liu, L.; Yu, X. The Analysis and Evaluation on Yellow-fleshed Kiwifruit Quality of Different Variety. J. Hefei Norm. Univ. 2016, 34, 50–52. [Google Scholar]
- Burdon, J.N. Kiwifruit Biology: The Commercial Implications of Fruit Maturation. Hortic. Rev. 2019, 46, 385–421. [Google Scholar]
- Li, Q.; Yao, S.; Deng, L.; Zeng, K. Changes in biochemical properties and pectin nanostructures of juice sacs during the granulation process of pomelo fruit (Citrus grandis). Food Chem. 2022, 376, 131876. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J. Cloning and Expression Analysis of Genes Involved in Chlorophyll Synthesis in ‘Ganmi6′ kiwifruit (Actinidia eriantha Benth). Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2016. [Google Scholar]
- Yang, M.; Xu, Y.Y.; Yang, G.; Hao, M.W.; Liu, C.M. Optimization of the process for the extraction of total flavonoids in stem of Lentinus edodes by ultrasonic assisted enzymatic method. J. Food Saf. Qual. 2017, 8, 202–209. [Google Scholar]
- Huang, W.J.; Jiang, C.Y.; Chen, M.Y.; Liu, X.L.; Zhang, Q.; Yan, C.L.; Zhong, C.H. Changes in postharvest physiology and fruit quality of Actinidia chinensis Planch ‘Jinmei’ from three different production regions during cool storage and shelf life. Plant Sci. J. 2020, 38, 687–695. [Google Scholar]
- Ma, B.Q.; Zhao, S.; Wu, B.H.; Wang, D.M.; Peng, Q.; Owiti, A.; Fang, T.; Liao, L.; Ogutu, C.; Korban, S.S.; et al. Construction of a high density linkage map and its application in the identification of QTLs for soluble sugar and organic acid components in apple. Tree Genet. Genomes 2016, 12, 1. [Google Scholar] [CrossRef]
- Ma, T.T.; Sun, X.Y.; Zhao, J.M.; You, Y.L.; Lei, Y.S.; Gao, G.T.; Zhan, J.C. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Zhang, Q.; Zhong, C.H.; Guo, M.Q. Volatile fingerprints and biomarkers of three representative kiwifruit cultivars obtained by headspace solid-phase microextraction gas chromatography mass spectrometry and chemometrics. Food Chem. 2019, 271, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Mujumdar, A.S.; Jin, X.W.; Liu, Z.L.; Zhang, Y.; Xiao, H.W. Effects of postharvest ripening on physicochemical properties, microstructure, cell wall polysaccharides contents (pectin, hemicellulose, cellulose) and nanostructure of kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 118, 106808. [Google Scholar] [CrossRef]
- Drummond, L. The composition and nutritional value of kiwifruit. Adv. Food Nutr. Res. 2013, 68, 33–57. [Google Scholar]
- Arivalagan, M.; Karunakaran, G.; Roy, T.K.; Dinsha, M.; Sindhu, B.C.; Shilpashree, V.M.; Shivashankara, K.S. Biochemical and nutritional characterization of dragon fruit (Hylocereus species). Food Chem. 2021, 353, 129426. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abu-Serie, M.M.; Abd El-Aziz, N.M.; El-Sohaimy, S.A. Nutritional, phytochemical, and in vitro anticancer potential of sugar apple (Annona squamosa) fruits. Sci. Rep. 2021, 11, 6224. [Google Scholar] [CrossRef]
- Jahromi, K.G.; Koochi, Z.H.; Kavoosi, G.; Shahsavar, A. Manipulation of fatty acid profile and nutritional quality of Chlorella vulgaris by supplementing with citrus peel fatty acid. Sci. Rep. 2022, 12, 8151. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Ling, C.; Li, J.H. Extraction and isolation of chlorophyll from kiwi fruit pulp. Teach. Biol. Second. Sch. 2019, 21, 63–66. [Google Scholar]
- Von Wettstein, D.; Gough, S.; Kannangara, C.G. Chlorophyll Biosynthesis. Plant Cell 1995, 7, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, S.X.; Tian, C.Y.; Wang, Y. Effects of Temperature on the Chlorophyll Fluorescence Parameters and Growth of Phaeodactylum tricornutum. Period. Ocean Univ. China 2008, 38, 377–383. [Google Scholar]
- Zhao, T.H.; Shen, X.Y.; Yang, D.G.; Ma, X.F. Effects on Chlorophyll Content and Photosynthetic Rate of Maize Leaves under Water Stress and Rewatering. Rain Fed. Crops 2003, 1, 33–35. [Google Scholar]
- Giuffrida, D.; Dugo, P.; Salvo, A.; Saitta, M.; Dugo, G. Free carotenoid and carotenoid ester composition in native orange juices of different varieties. Fruits 2010, 65, 277–284. [Google Scholar] [CrossRef]
- Cozzolino, R.; De Giulio, B.; Petriccione, M.; Martignetti, A.; Malorni, L.; Zampella, L.; Pellicano, M.P. Comparative analysis of volatile metabolites, quality and sensory attributes of Actinidia chinensis fruit. Food Chem. 2020, 316, 126340. [Google Scholar] [CrossRef]
- Xu, Z.R.; Li, J.; Ma, Z.; Li, J.P. Dynamic changes of dietary fiber content in tomato fruits of different varieties. Jiangxi Agric. 2020, 8, 92–94. [Google Scholar]
- Guo, L.L.; Pang, R.L.; Wang, R.P.; Qiao, C.K.; Tian, F.J.; Wang, C.X.; Xie, H.Z. Comprehensive Trait Evaluation for kiwifruit Nutritional Quality. J. Fruit Sci. 2022, 1–14. [Google Scholar]
- Li, Y.H.; Ran, M.Q.; Xu, M.H.; Chen, L.; You, Y.D.; Zhao, Y.; Jiao, Y.Z. Comparison and comprehensive evaluation of fruit quality of different varieties of kiwifruit. Food Ferment. Ind. 2020, 46, 162–168. [Google Scholar]
- Cheng, B.; Zhang, J.; Xu, H.; Hou, L.; Wang, Y.; Zhang, Y.; Wang, C. Analysis of Organic Acid Contents in Wine Grape from Different Cultivars. Food Sci. 2013, 34, 223–228. [Google Scholar]
- Liang, J.J.; Ren, Y.P.; Wang, Y.; Han, M.Z.; Yue, T.L.; Wang, Z.L.; Gao, Z.P. Physicochemical, nutritional, and bioactive properties of pulp and peel from 15 kiwifruit cultivars. Food Biosci. 2021, 42, 101157. [Google Scholar] [CrossRef]
- Garcia, C.V.; Quek, S.Y.; Stevenson, R.J.; Winz, R.A. Kiwifruit flavour: A review. Trends Food Sci. Technol. 2012, 24, 82–91. [Google Scholar] [CrossRef]
- Guo, L.; Wang, H.; Ma, S.; Zhong, C.; Qu, H. Effect of refrigeration on aroma components of postharvest Jinyan kiwifruit. Sci. Technol. Food Ind. 2013, 34, 304–308. [Google Scholar]


| Cultivar | Abbr. | Flesh Color | Species |
|---|---|---|---|
| Hongyang | R1 | Red | A.chinensis |
| Donghong | R2 | Red | A.chinensis |
| Cuiyu | G1 | Green | A.chinensis |
| Xuxiang | G2 | Green | A.chinensis var. deliciosa |
| Hayward | G3 | Green | A.chinensis var. deliciosa |
| Jinkui | G4 | Green | A.chinensis var. deliciosa |
| MHYX | G5 | Green | A.eriantha |
| Huate | G6 | Green | A.eriantha |
| Hort16A | Y1 | Yellow | A.chinensis |
| G3 | Y2 | Yellow | A.chinensis |
| Jintao | Y3 | Yellow | A.chinensis |
| Jinyuan | Y4 | Yellow | A.eriantha × A.chinensis |
| Jinmei | Y5 | Yellow | A.eriantha × A.chinensis |
| Jinyan | Y6 | Yellow | A.eriantha × A.chinensis |
| PCA | Eigenvalues | Variance Contribution Rate (%) | Cumulative Variance Contribution Rate (%) |
|---|---|---|---|
| 1 | 5.51 | 50.07 | 50.07 |
| 2 | 2.07 | 18.86 | 68.93 |
| 3 | 1.22 | 11.05 | 79.98 |
| Quality Indicators | PCA | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Chlorophyll | 0.95 | 0.16 | 0.01 |
| Carotenoids | 0.73 | −0.11 | 0.15 |
| Flavonoids | −0.81 | 0.44 | 0.13 |
| Energy | −0.13 | 0.80 | 0.37 |
| Protein | −0.22 | −0.13 | 0.80 |
| Lipid | 0.87 | 0.25 | 0.20 |
| Dietary Fiber | −0.39 | −0.58 | 0.32 |
| Soluble Sugars | −0.56 | 0.77 | 0.07 |
| Organic Acids | 0.95 | 0.23 | −0.05 |
| VC | 0.93 | 0.30 | −0.07 |
| Pectin | 0.61 | −0.25 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Zheng, H.; Fan, J.; Liu, F.; Li, J.; Zhong, C.; Zhang, Q. Comparative Study on Physicochemical and Nutritional Qualities of Kiwifruit Varieties. Foods 2023, 12, 108. https://doi.org/10.3390/foods12010108
Yuan X, Zheng H, Fan J, Liu F, Li J, Zhong C, Zhang Q. Comparative Study on Physicochemical and Nutritional Qualities of Kiwifruit Varieties. Foods. 2023; 12(1):108. https://doi.org/10.3390/foods12010108
Chicago/Turabian StyleYuan, Xinyu, Hao Zheng, Jiangtao Fan, Fengxia Liu, Jitao Li, Caihong Zhong, and Qiong Zhang. 2023. "Comparative Study on Physicochemical and Nutritional Qualities of Kiwifruit Varieties" Foods 12, no. 1: 108. https://doi.org/10.3390/foods12010108
APA StyleYuan, X., Zheng, H., Fan, J., Liu, F., Li, J., Zhong, C., & Zhang, Q. (2023). Comparative Study on Physicochemical and Nutritional Qualities of Kiwifruit Varieties. Foods, 12(1), 108. https://doi.org/10.3390/foods12010108







