Preparation of Gum Arabic–Maltose–Pea Protein Isolate Complexes for 1−Octacosanol Microcapsule: Improved Storage Stability, Sustained Release in the Gastrointestinal Tract, and Its Effect on the Lipid Metabolism of High−Fat−Diet−Induced Obesity Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Octa−GA−Malt−PPI Microcapsule Preparation
2.2.1. Emulsion Preparation
2.2.2. pH Condition Optimization
2.2.3. Temperature Condition Optimization
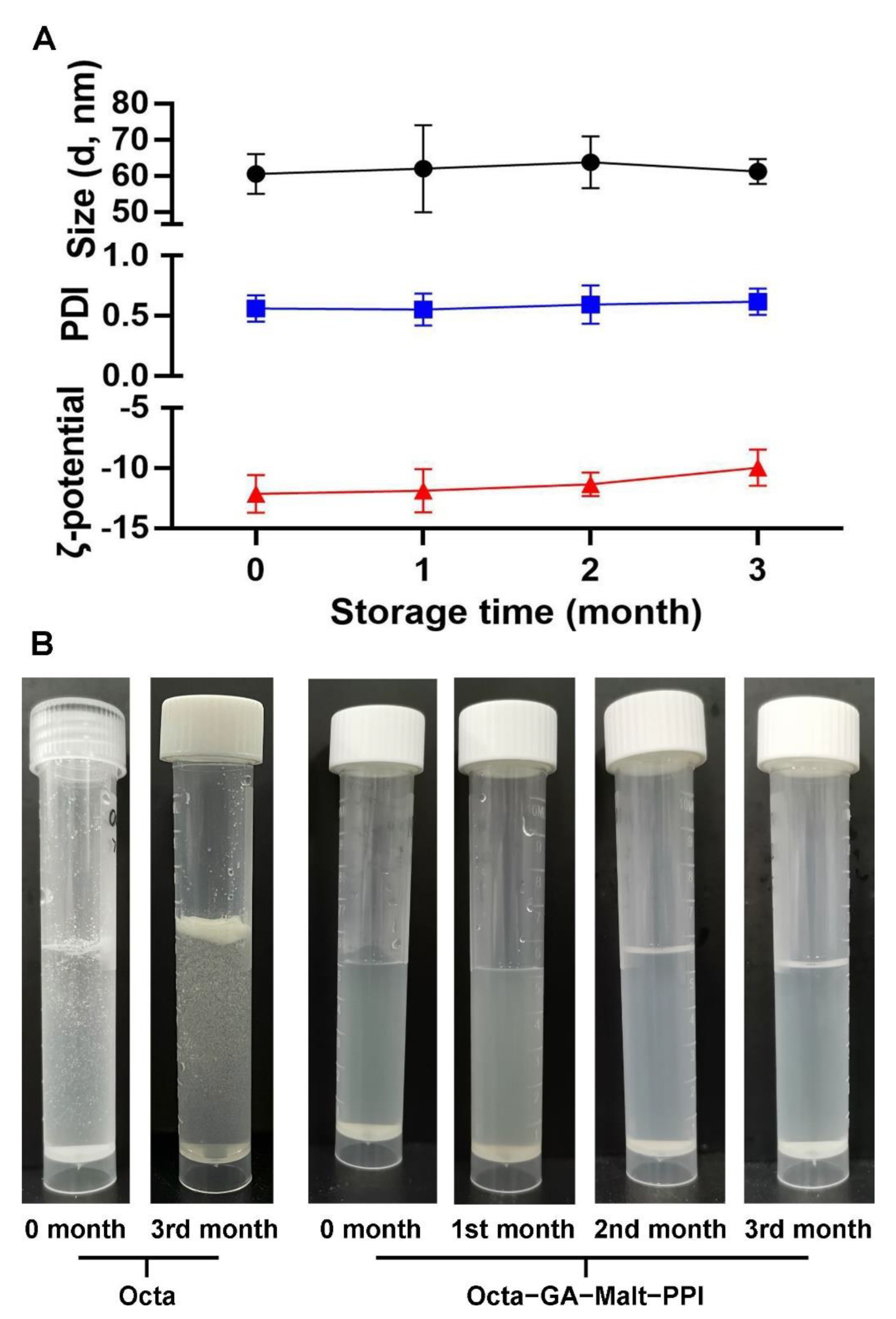
2.3. Determination of Particle Size and the ζ−Potential
2.4. Determination of the EE and Loading Amount (LA)
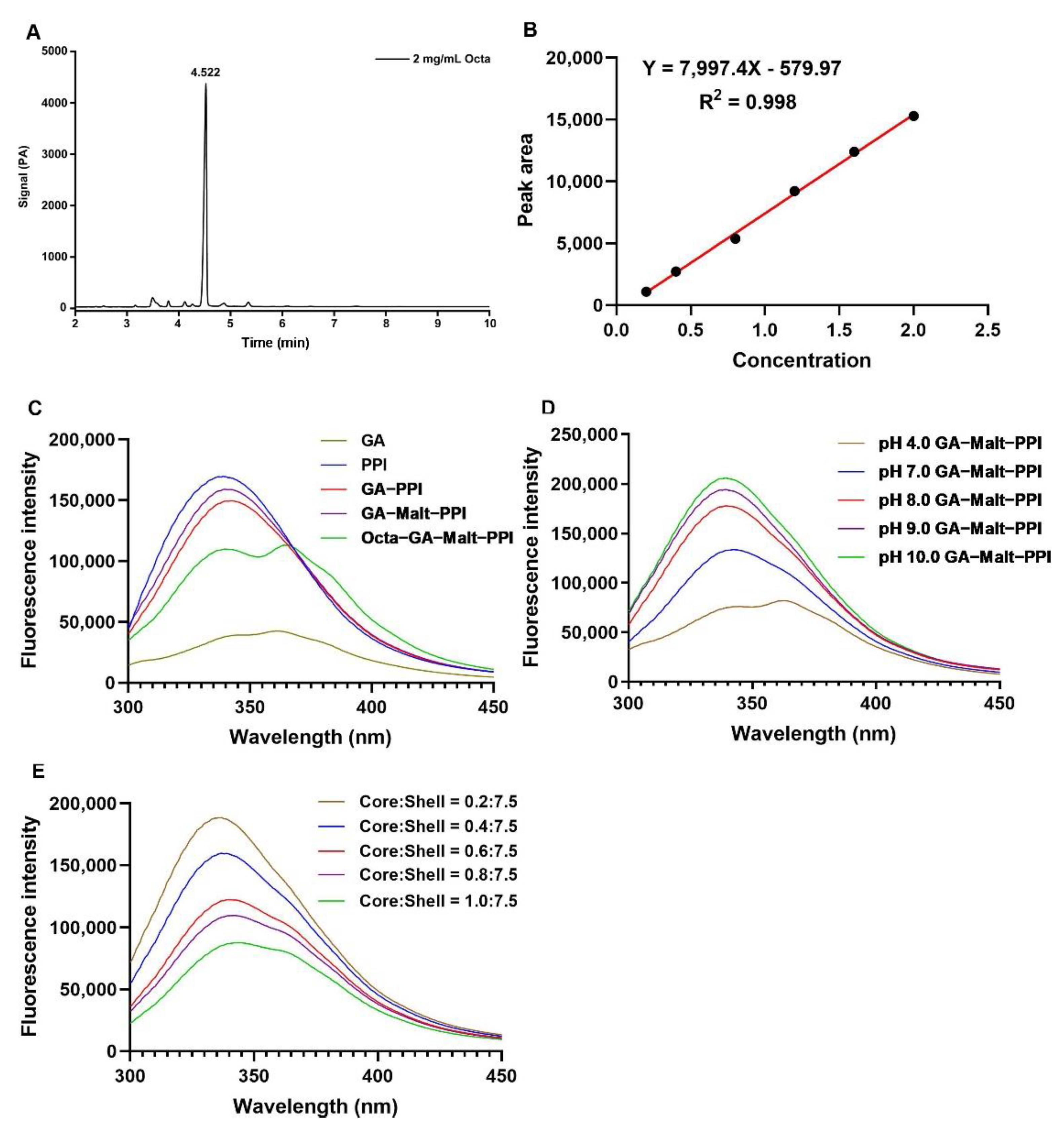
2.5. Octa Content Determination by Gas Chromatography (GC)
2.6. Digestion Experiment using the Simulated Gastrointestinal Tract
2.6.1. Preparation of Storage Solution, Simulated Gastric Fluid (SGF) and Simulated Intestinal Fluid (SIF)
2.6.2. Preparation of Octa−GA−Malt−PPI Stock Solution and Octa Stock Solution
2.6.3. Sustained Release of Octa from the Octa−GA−Malt−PPI Microcapsule in the Simulated Gastrointestinal Tract
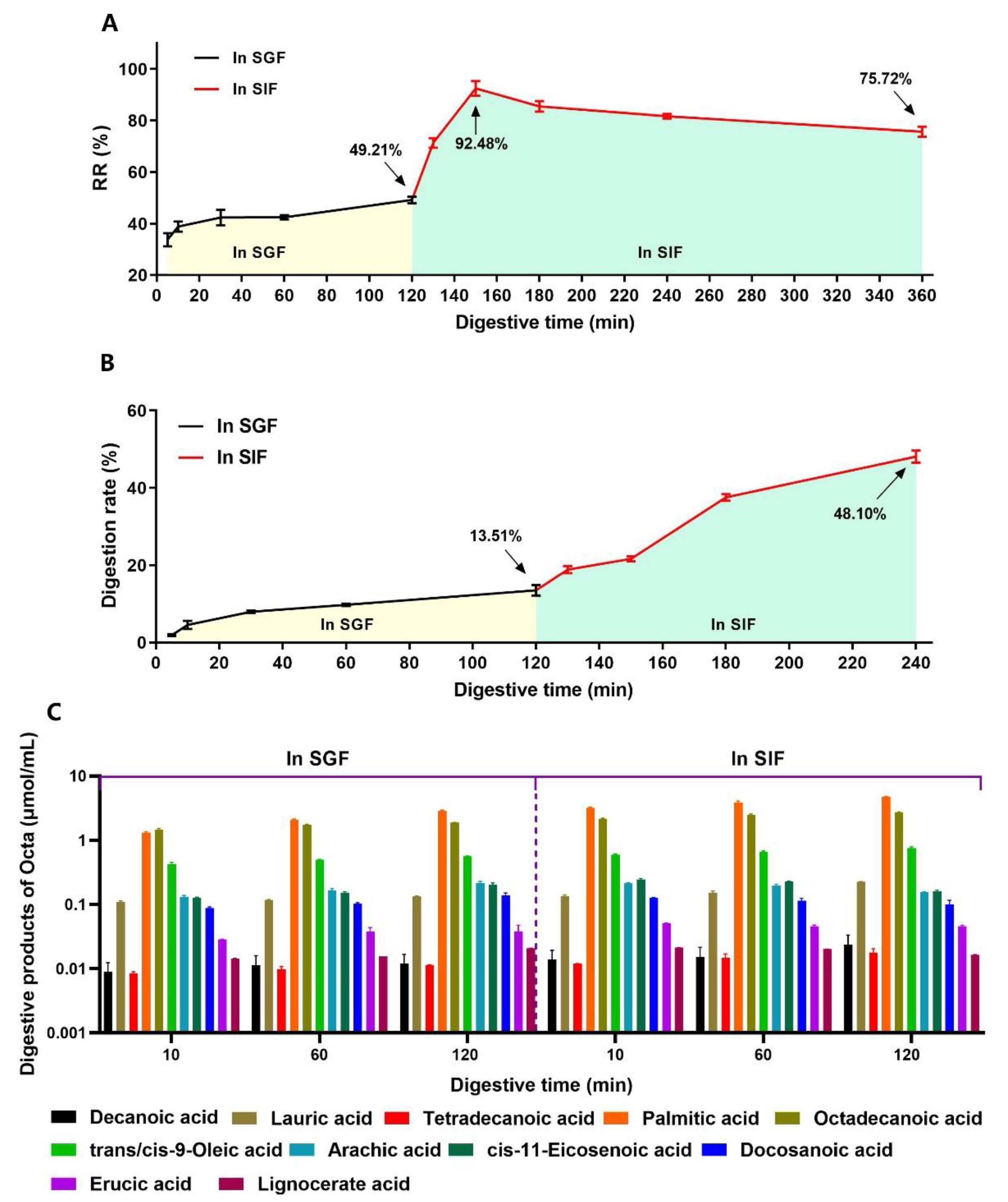
2.6.4. The Release Rate (RR) of the Octa from Octa−GA−Malt−PPI Microparticles in the SGF or SIF
2.6.5. The Analysis of Digestive Products of the Octa Monomer in the Simulated Gastrointestinal Tract
2.7. Fluorescence Spectroscopy
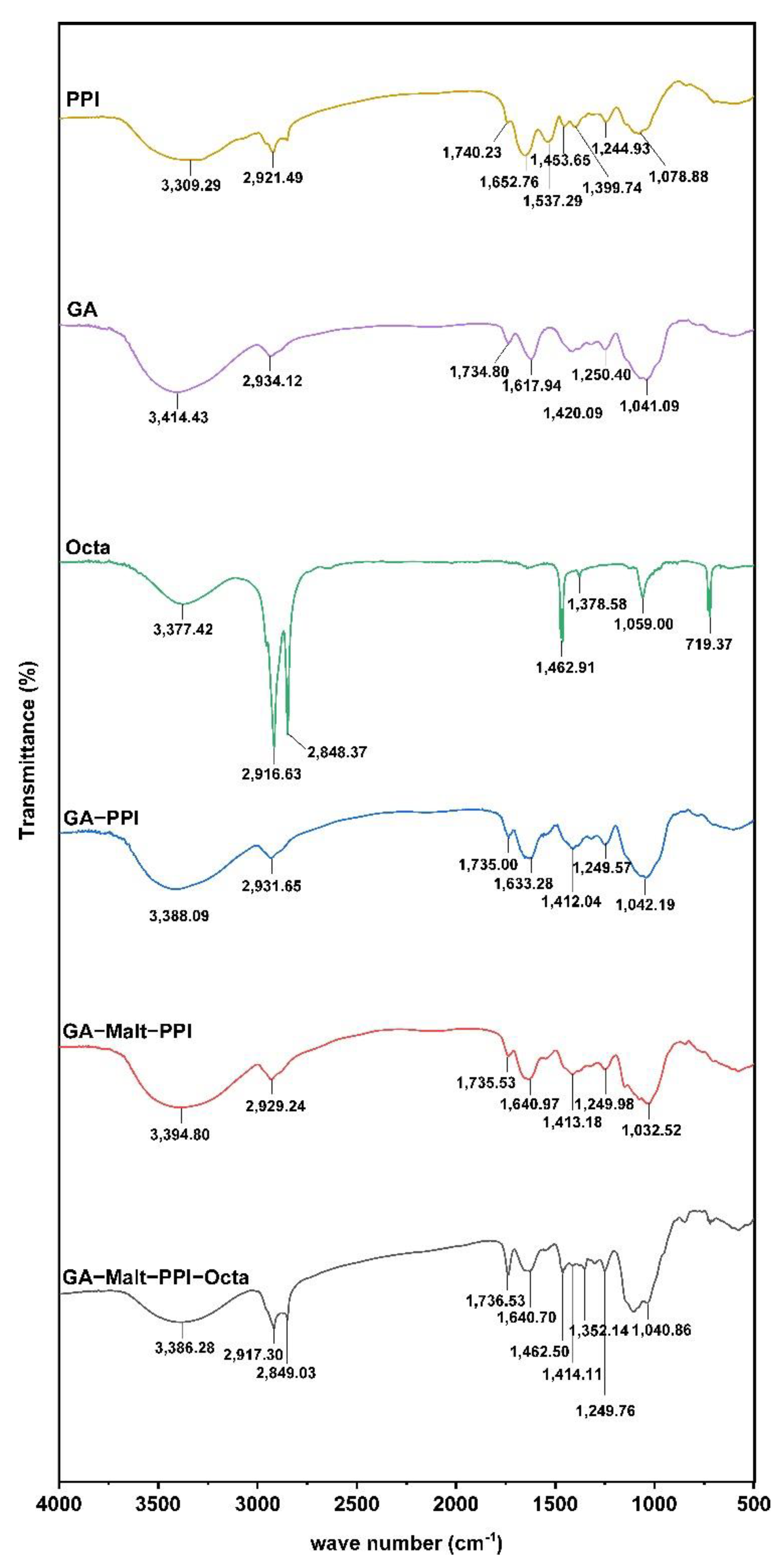
2.8. Fourier Transform Infrared (FTIR) Spectra
2.9. Animal Experiment
2.9.1. Animals and Sample Collections
2.9.2. Blood Pressure Determination
2.9.3. Lipid Metabolism Index Measurement
2.9.4. Histopathological Analysis of Liver
2.9.5. Total RNA Isolation from Liver and Quantitative RT−PCR (qRT−PCR)
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Optimization Conditions for the Octa−Loaded Core–Shell Complexes Preparation
3.1.1. Condition Optimization for Emulsifying pH
3.1.2. Condition Optimization for Emulsifying Temperature
3.2. Molecular Mechanism for Underlying Octa Encapsulation by GA−Malt−PPI Complexes
3.2.1. The Fluorescence Quenching of the Complexes
3.2.2. The Interaction between Octa and GA−Malt−PPI Complexes by FTIR
3.3. Encapsulation Improved the Stability of Octa in Different Storage Periods
3.4. The Digestive Behavior of Octa and the Sustained Release of Octa from the Microcapsule in a Simulated Gastrointestinal Tract
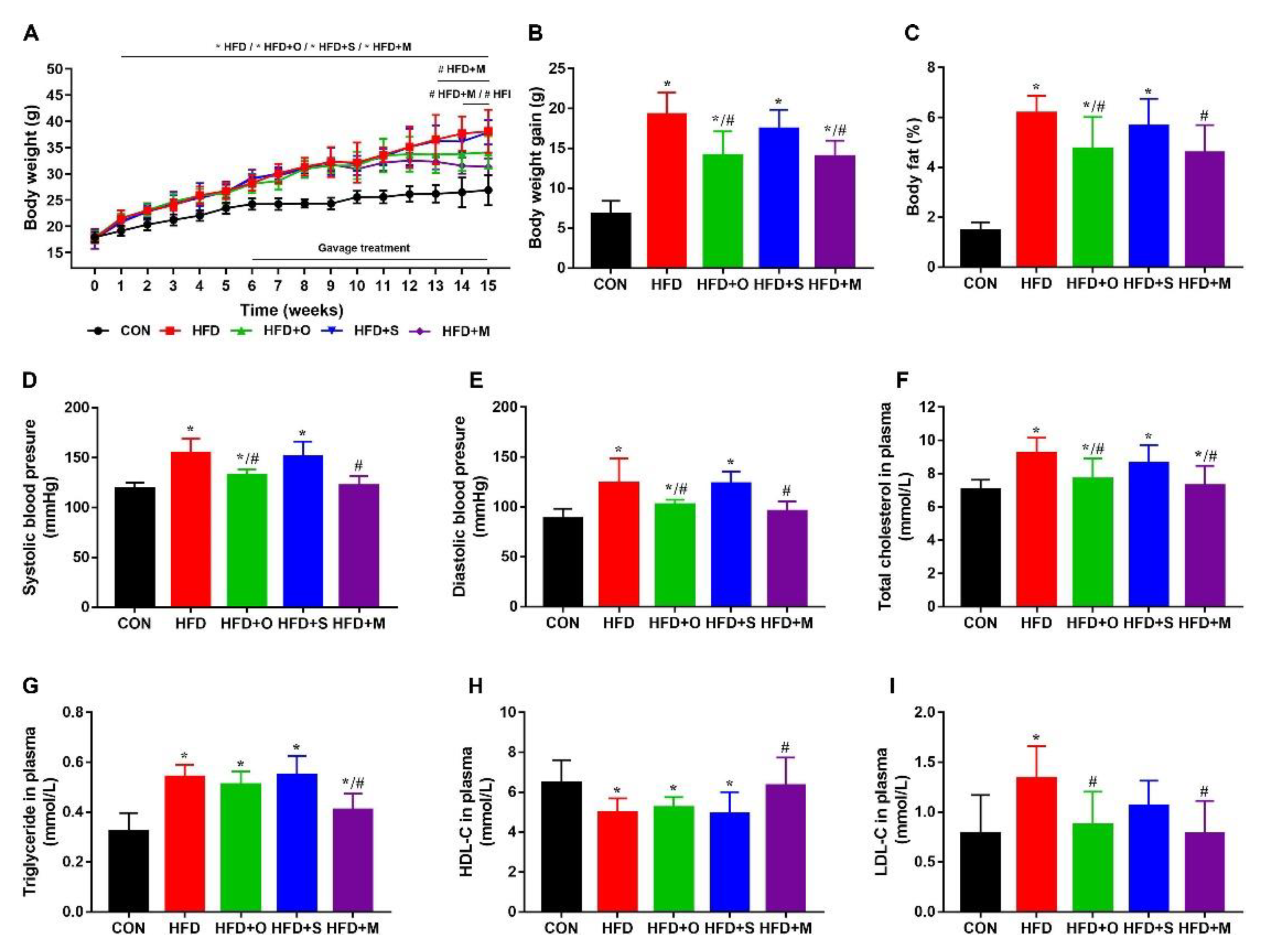
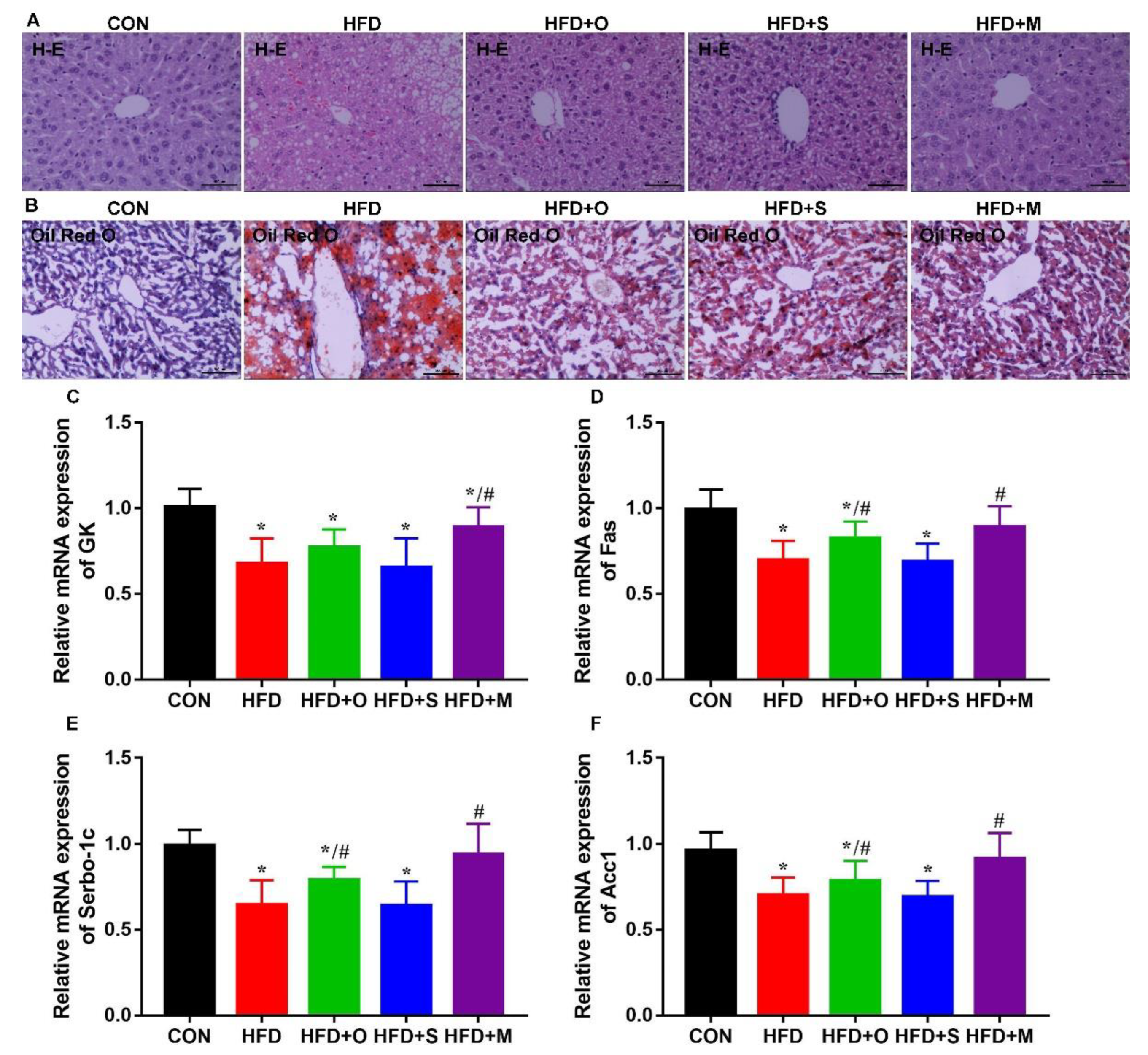
3.5. Octa and Octa−GA−Malt−PPI Microcapsule Improved the Lipid Metabolism in HFD−Induced Obesity Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuevas, M.S.; Crevelin, E.J.; de Moraes, L.A.B.; Oliveira, A.L.; Rodrigues, C.E.C.; Meirelles, A.J.A. Solubility of commercial octacosanol in organic solvents and their correlation by thermodynamic models at different temperatures. J. Chem. Thermodyn. 2017, 110, 186–192. [Google Scholar] [CrossRef]
- Sharma, R.; Matsuzaka, T.; Kaushik, M.K.; Sugasawa, T.; Ohno, H.; Wang, Y.; Motomura, K.; Shimura, T.; Okajima, Y.; Mizunoe, Y.; et al. Octacosanol and policosanol prevent high-fat diet-induced obesity and metabolic disorders by activating brown adipose tissue and improving liver metabolism. Sci. Rep. 2019, 9, 5169. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Scott, S.D.; Pekas, E.J.; Lee, J.G.; Park, S.Y. Improvement of lipids and reduction of oxidative stress with octacosanol after taekwondo training. Int. J. Sport. Physiol. 2019, 14, 1297–1303. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Garcia-Gimenez, M.D.; Angel-Martin, M.; Perez-Camino, M.C.; Fernandez Arche, A. Long-chain fatty alcohols from evening primrose oil inhibit the inflammatory response in murine peritoneal macrophages. J. Ethnopharmacol. 2014, 151, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Rapport, L.; Lockwood, G.B. Octacosanol in human health. Nutrition 2003, 19, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Irmak, S.; Dunford, N.T. Policosanol contents and compositions of wheat varieties. J. Agric. Food Chem. 2005, 53, 5583–5586. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.M.; Luo, Z.S.; Zeng, F.F.; Liu, S.B.; Khan, Z.U. Effect of water, metallic ions, fatty acid and temperature on oxidative stability of 1-octacosanol from sugarcane rind. Food Chem. 2015, 182, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Menendez, R.; Marrero, D.; Mas, R.; Fernandez, I.; Gonzalez, L.; Gonzalez, R.M. In vitro and in vivo study of octacosanol metabolism. Arch. Med. Res. 2005, 36, 113–119. [Google Scholar] [CrossRef]
- Farahani, Z.K.; Mousavi, M.; Ardebili, M.S.; Bakhoda, H. The effects of Ziziphus jujuba extract-based sodium alginate and proteins (whey and pea) beads on characteristics of functional beverage. J. Food Meas. Charact. 2022, 16, 2782–2788. [Google Scholar] [CrossRef]
- Wang, J.; Nickerson, M.T.; Low, N.H.; Van Kessel, A.G. Efficacy of pea protein isolate–alginate encapsulation on viability of a probiotic bacterium in the porcine digestive tract. Can. J. Anim. Sci. 2017, 97, 214–222. [Google Scholar] [CrossRef]
- Varankovich, N.; Martinez, M.F.; Nickerson, M.T.; Korber, D.R. Survival of probiotics in pea protein-alginate microcapsules with or without chitosan coating during storage and in a simulated gastrointestinal environment. Food Sci. Biotechnol. 2017, 26, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, G.; Zhang, H.; Qi, X. The soy protein isolate-Octacosanol-polysaccharides nanocomplex for enhanced physical stability in neutral conditions: Fabrication, characterization, thermal stability. Food Chem. 2020, 322, 126638. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Wu, G.; Li, P.; Zhang, H.; Qi, X.; Wang, L.; Qian, H. The characterization and stability of the soy protein isolate/1-Octacosanol nanocomplex. Food Chem. 2019, 297, 124766. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, C.; Shi, J.; Ni, F.; Shen, Q.; Xie, H.; Wang, K.; Lei, Q.; Fang, W.; Ren, G. The regulation of sodium alginate on the stability of ovalbumin-pectin complexes for VD3 encapsulation and in vitro simulated gastrointestinal digestion study. Food Res. Int. 2021, 140, 110011. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zeng, X.; Yi, J.; Zhang, Y. Fabrication of pea protein nanoparticles with calcium-induced cross-linking for the stabilization and delivery of antioxidative resveratrol. Int. J. Biol. Macromol. 2020, 152, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Mao, Y.Z.; Xiang, C.Y.; Cao, M.N.; Ren, G.R.; Wang, K.W.; Ma, X.J.; Wu, D.; Xie, H.J. Preparation of ll-lactoglobulin/gum arabic complex nanoparticles for encapsulation and controlled release of EGCG in simulated gastrointestinal digestion model. Food Chem. 2021, 354, 129516. [Google Scholar] [CrossRef]
- Hosseini, S.M.H.; Emam-Djomeh, Z.; Sabatino, P.; Van der Meeren, P. Nanocomplexes arising from protein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocolloid. 2015, 50, 16–26. [Google Scholar] [CrossRef]
- Yin, B.; Deng, W.; Xu, K.; Huang, L.; Yao, P. Stable nano-sized emulsions produced from soy protein and soy polysaccharide complexes. J. Colloid Interface. Sci. 2012, 380, 51–59. [Google Scholar] [CrossRef]
- Xiang, C.Y.; Gao, J.; Ye, H.X.; Ren, G.R.; Ma, X.J.; Xie, H.J.; Fang, S.; Lei, Q.F.; Fang, W.J. Development of ovalbumin-pectin nanocomplexes for vitamin D-3 encapsulation: Enhanced storage stability and sustained release in simulated gastrointestinal digestion. Food Hydrocolloid. 2020, 106, 105926. [Google Scholar] [CrossRef]
- Chen, F.P.; Ou, S.Y.; Tang, C.H. Core-shell soy protein-soy polysaccharide complex (nano)particles as carriers for improved stability and sustained release of curcumin. J. Agric. Food Chem. 2016, 64, 5053–5059. [Google Scholar] [CrossRef]
- Sneharani, A.H.; Karakkat, J.V.; Singh, S.A.; Rao, A.G. Interaction of curcumin with beta-lactoglobulin-stability, spectroscopic analysis, and molecular modeling of the complex. J. Agric. Food Chem. 2010, 58, 11130–11139. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fu, D.; Zhang, X.; Chai, J.; Tian, S.; Han, J. Development and validation of a new artificial gastric digestive system. Food Res. Int. 2019, 122, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ye, A.; Han, F.; Han, J. Advances and challenges in liposome digestion: Surface interaction, biological fate, and GIT modeling. Adv. Colloid Interface. Sci. 2019, 263, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–11124. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.R.; Sorgentini, D.A.; Anon, M.C. Relation between solubility and surface hydrophobicity as an indicator of modifications during preparation processes of commercial and laboratory-prepared soy protein isolates. J. Agric. Food Chem. 2000, 48, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, C.; Peinado, I.; McClements, D.J. Formation of protein nanoparticles by controlled heat treatment of lactoferrin: Factors affecting particle characteristics. Food Hydrocolloid. 2011, 25, 1354–1360. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Wang, W.; Dong, K. Modification of soy protein isolate by glutaminase for nanocomplexation with curcumin. Food Chem. 2018, 268, 504–512. [Google Scholar] [CrossRef]
- Liu, C.Z.; Lv, N.; Ren, G.R.; Wu, R.B.; Wang, B.J.; Cao, Z.X.; Xie, H.J. Explore the interaction mechanism between zein and EGCG using multi-spectroscopy and molecular dynamics simulation methods. Food Hydrocolloid. 2021, 120, 106906. [Google Scholar] [CrossRef]
- Kraft, C.A.; Garrido, J.L.; Leiva-Vega, L.; Romero, G. Quantitative analysis of protein-lipid interactions using tryptophan fluorescence. Sci. Signal. 2009, 2, pl4. [Google Scholar] [CrossRef]
- Hu, Y.J.; Liu, Y.; Sun, T.Q.; Bai, A.M.; Lu, J.Q.; Pi, Z.B. Binding of anti-inflammatory drug cromolyn sodium to bovine serum albumin. Int. J. Biol. Macromol. 2006, 39, 280–285. [Google Scholar] [CrossRef]
- Fang, B.; Zhang, M.; Jiang, L.; Jing, H.; Ren, F.Z. Influence of pH on the structure and oleic acid binding ability of bovine alpha-lactalbumin. Protein J. 2012, 31, 564–572. [Google Scholar] [CrossRef]
- Hu, S.Q.; Wang, T.R.; Fernandez, M.L.; Luo, Y.C. Development of tannic acid cross-linked hollow zein nanoparticles as potential oral delivery vehicles for curcumin. Food Hydrocolloid. 2016, 61, 821–831. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocolloid. 2012, 27, 175–184. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Shahamirian, M.; John, D.; Ramaswamy, H. Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 94, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Nakanaga, T. Observation of the high-resolution spectrum of the N–H bending vibration of ketenimine CH2CNH. J. Mol. Spectrosc. 2010, 264, 100–104. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Sandoval-Castilla, O.; Vazquez-Torres, H.; Vernon-Carter, E.; Lobato-Calleros, C. Determination of the gum Arabic–chitosan interactions by Fourier Transform Infrared Spectroscopy and characterization of the microstructure and rheological features of their coacervates. Carbohyd. Polym. 2010, 79, 541–546. [Google Scholar] [CrossRef]
- Fang, S.; Zhao, X.J.; Liu, Y.M.; Liang, X.R.; Yang, Y.X. Fabricating multilayer emulsions by using OSA starch and chitosan suitable for spray drying: Application in the encapsulation of beta-carotene. Food Hydrocolloid. 2019, 93, 102–110. [Google Scholar] [CrossRef]
- Mo, X.P.; Peng, X.L.; Liang, X.R.; Fang, S.; Xie, H.J.; Chen, J.; Meng, Y.C. Development of antifungal gelatin-based nanocomposite films functionalized with natamycin-loaded zein/casein nanoparticles. Food Hydrocolloid. 2021, 113, 106506. [Google Scholar] [CrossRef]
- Huang, G.Q.; Sun, Y.T.; Xiao, J.X.; Yang, J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012, 135, 534–539. [Google Scholar] [CrossRef]
- Zou, B.; Xue, J.; Zhao, Y.; Zheng, X. Effect of the weak intermolecular C-H...O=C hydrogen bonding, solvent polarity and concentration on the frequency of the C=O stretch mode of 2-thiophenecarboxaldehyde. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2021, 255, 119651. [Google Scholar] [CrossRef]
- Zhang, L.T.; Zhang, F.; Fang, Y.P.; Wang, S.Y. Alginate-shelled SPI nanoparticle for encapsulation of resveratrol with enhanced colloidal and chemical stability. Food Hydrocolloid. 2019, 90, 313–320. [Google Scholar] [CrossRef]
- Galinato, M.G.I.; Bowman, S.E.J.; Kleingardner, J.G.; Martin, S.; Zhao, J.Y.; Sturhahn, W.; Alp, E.E.; Bren, K.L.; Lehnert, N. Effects of protein structure on iron-polypeptide vibrational dynamic coupling in cytochrome c. Biochemistry 2015, 54, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wu, K.R.; Cui, W.N.; Fu, D.Y.; Han, J.Z.; Liu, W.L. Tracking the digestive performance of different forms of dairy products using a dynamic artificial gastric digestive system. Food Struct. 2021, 29, 100194. [Google Scholar] [CrossRef]
- Wang, X.; Ye, A.Q.; Lin, Q.Q.; Han, J.Z.; Singh, H. Gastric digestion of milk protein ingredients: Study using an in vitro dynamic model. J. Dairy Sci. 2018, 101, 6842–6852. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, K.; Wang, Z.; Park, H.G.; Wang, D.; Kitano, R.; Brenna, T. Fatty acid desaturase 2 (FADS2) actions on branched chain and normal odd chain saturated fatty acids. Curr. Dev. Nutr. 2020, 4, 1261. [Google Scholar] [CrossRef]
- Iwase, M.; Tokiwa, S.; Seno, S.; Mukai, T.; Yeh, Y.S.; Takahashi, H.; Nomura, W.; Jheng, H.F.; Matsumura, S.; Kusudo, T.; et al. Glycerol kinase stimulates uncoupling protein 1 expression by regulating fatty acid metabolism in beige adipocytes. J. Biol. Chem. 2020, 295, 7033–7045. [Google Scholar] [CrossRef]
- Saleh Al-Maamari, J.N.; Rahmadi, M.; Panggono, S.M.; Prameswari, D.A.; Pratiwi, E.D.; Ardianto, C.; Balan, S.S.; Suprapti, B. The effects of quercetin on the expression of SREBP-1c mRNA in high-fat diet-induced NAFLD in mice. J. Basic. Clin. Physiol. Pharmacol. 2021, 32, 637–644. [Google Scholar] [CrossRef]
- Nogoy, K.M.C.; Kim, H.J.; Lee, D.H.; Smith, S.B.; Seong, H.A.; Choi, S.H. Oleic acid in Angus and Hanwoo (Korean native cattle) fat reduced the fatty acid synthase activity in rat adipose tissues. J. Anim. Sci. Technol. 2021, 63, 380–393. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef]
| pH | ζ Potential (mV) | EE (%) | LA (mg/mg) | Z−Average Diameter (μm) | PDI |
|---|---|---|---|---|---|
| 7.0 | −25.17 ± 1.08 a | 40.43 ± 3.68 a | 0.33 ± 0.06 a | 80.30 ± 6.24 a | 0.70 ± 0.07 a |
| 8.0 | −25.43 ± 3.59 a | 47.67 ± 3.46 a | 0.41 ± 0.05 ab | 81.77 ± 0.2 a | 0.68 ± 0.01 a |
| 9.0 | −31.37 ± 1.97 b | 58.07 ± 2.36 b | 0.51 ± 0.04 b | 138.27 ± 1.81 c | 0.56 ± 0.05 b |
| 10.0 | −28.53 ± 1.21 ab | 55.90 ± 2.01 b | 0.45 ± 0.04 b | 131.83 ± 6.27 bc | 0.63 ± 0.03 ab |
| 11.0 | −28.87 ± 1.90 ab | 47.70 ± 2.19 a | 0.40 ± 0.04 ab | 763.47 ± 20.06 d | 0.64 ± 0.03 ab |
| Temperature | ζ Potential (mV) | EE (%) | LA (mg/mg) | Z−Average Diameter (μm) | PDI |
|---|---|---|---|---|---|
| 50 °C | −20.17 ± 1.00 a | 56.02 ± 2.00 a | 0.36 ± 0.02 ab | 318.90 ± 3.57 a | 0.29 ± 0.01 a |
| 60 °C | −21.70 ± 1.00 a | 56.25 ± 1.68 a | 0.36 ± 0.04 ab | 291.43 ± 3.28 b | 0.30 ± 0.01 a |
| 70 °C | −22.37 ± 0.67 a | 60.99 ± 5.98 a | 0.48 ± 0.07 b | 259.47 ± 4.76 c | 0.24 ± 0.02 b |
| 80 °C | −16.60 ± 0.87 b | 51.02 ± 5.92 a | 0.45 ± 0.05 b | 273.70 ± 5.20 d | 0.35 ± 0.01 c |
| 90 °C | −10.08 ± 0.58 c | 34.10 ± 4.63 b | 0.28 ± 0.04 a | 86.10 ± 1.45 e | 0.50 ± 0.01 d |
| PPI | GA−PPI | GA−Malt−PPI | Octa−GA−Malt−PPI | |
|---|---|---|---|---|
| β−sheet (%) | 34.07 ± 1.39 c | 37.57 ± 1.00 b | 39.66 ± 0.62 a | 39.37 ± 1.27 b |
| Random coil (%) | 14.29 ± 2.28 ab | 11.18 ± 1.16 b | 7.36 ± 0.67 c | 7.32 ± 0.75 a |
| α−helix (%) | 18.53 ± 2.80 b | 23.64 ± 1.48 a | 24.27 ± 0.15 a | 26.38 ± 1.63 b |
| β−turn (%) | 33.11 ± 1.19 a | 27.60 ± 1.33 b | 27.89 ± 0.37 b | 27.68 ± 0.64 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.-Y.; Pan, Y.; Zhang, W.; Sheng, Y.; Cao, Y.; Gu, Z.; Shen, Q.; Wang, Q.; Chen, X. Preparation of Gum Arabic–Maltose–Pea Protein Isolate Complexes for 1−Octacosanol Microcapsule: Improved Storage Stability, Sustained Release in the Gastrointestinal Tract, and Its Effect on the Lipid Metabolism of High−Fat−Diet−Induced Obesity Mice. Foods 2023, 12, 112. https://doi.org/10.3390/foods12010112
Ding Y-Y, Pan Y, Zhang W, Sheng Y, Cao Y, Gu Z, Shen Q, Wang Q, Chen X. Preparation of Gum Arabic–Maltose–Pea Protein Isolate Complexes for 1−Octacosanol Microcapsule: Improved Storage Stability, Sustained Release in the Gastrointestinal Tract, and Its Effect on the Lipid Metabolism of High−Fat−Diet−Induced Obesity Mice. Foods. 2023; 12(1):112. https://doi.org/10.3390/foods12010112
Chicago/Turabian StyleDing, Yin-Yi, Yuxiang Pan, Wanyue Zhang, Yijing Sheng, Yanyun Cao, Zhenyu Gu, Qing Shen, Qingcheng Wang, and Xi Chen. 2023. "Preparation of Gum Arabic–Maltose–Pea Protein Isolate Complexes for 1−Octacosanol Microcapsule: Improved Storage Stability, Sustained Release in the Gastrointestinal Tract, and Its Effect on the Lipid Metabolism of High−Fat−Diet−Induced Obesity Mice" Foods 12, no. 1: 112. https://doi.org/10.3390/foods12010112
APA StyleDing, Y.-Y., Pan, Y., Zhang, W., Sheng, Y., Cao, Y., Gu, Z., Shen, Q., Wang, Q., & Chen, X. (2023). Preparation of Gum Arabic–Maltose–Pea Protein Isolate Complexes for 1−Octacosanol Microcapsule: Improved Storage Stability, Sustained Release in the Gastrointestinal Tract, and Its Effect on the Lipid Metabolism of High−Fat−Diet−Induced Obesity Mice. Foods, 12(1), 112. https://doi.org/10.3390/foods12010112








