Improved Stabilization and In Vitro Digestibility of Mulberry Anthocyanins by Double Emulsion with Pea Protein Isolate and Xanthan Gum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PPI-XG Hydrocolloids
2.3. Production of the Double Emulsion Encapsulated MAs
2.4. Encapsulation Efficiency and Creaming Stability of Double Emulsion
2.5. Particle Size, Zeta Potential and Microstructure
2.6. In Vitro Simulated Digestion of Double Emulsions Encapsulated MAs
2.7. Thermal Degradation of Mas from Double Emulsions at Different Temperatures
2.8. Release of MAs in Double Emulsions during In Vitro Simulated Digestion
2.9. Oxidative Stability of Double Emulsions during In Vitro Simulated Digestion
2.10. Statistical Analysis
3. Results
3.1. Effects of Various XG Concentrations on the Physical Properties of PPI-XG Double Emulsions Encapsulated MAs
3.1.1. Particle Size and Microstructure
3.1.2. Zeta Potential
3.1.3. Encapsulation Efficiency
3.1.4. Creaming Index
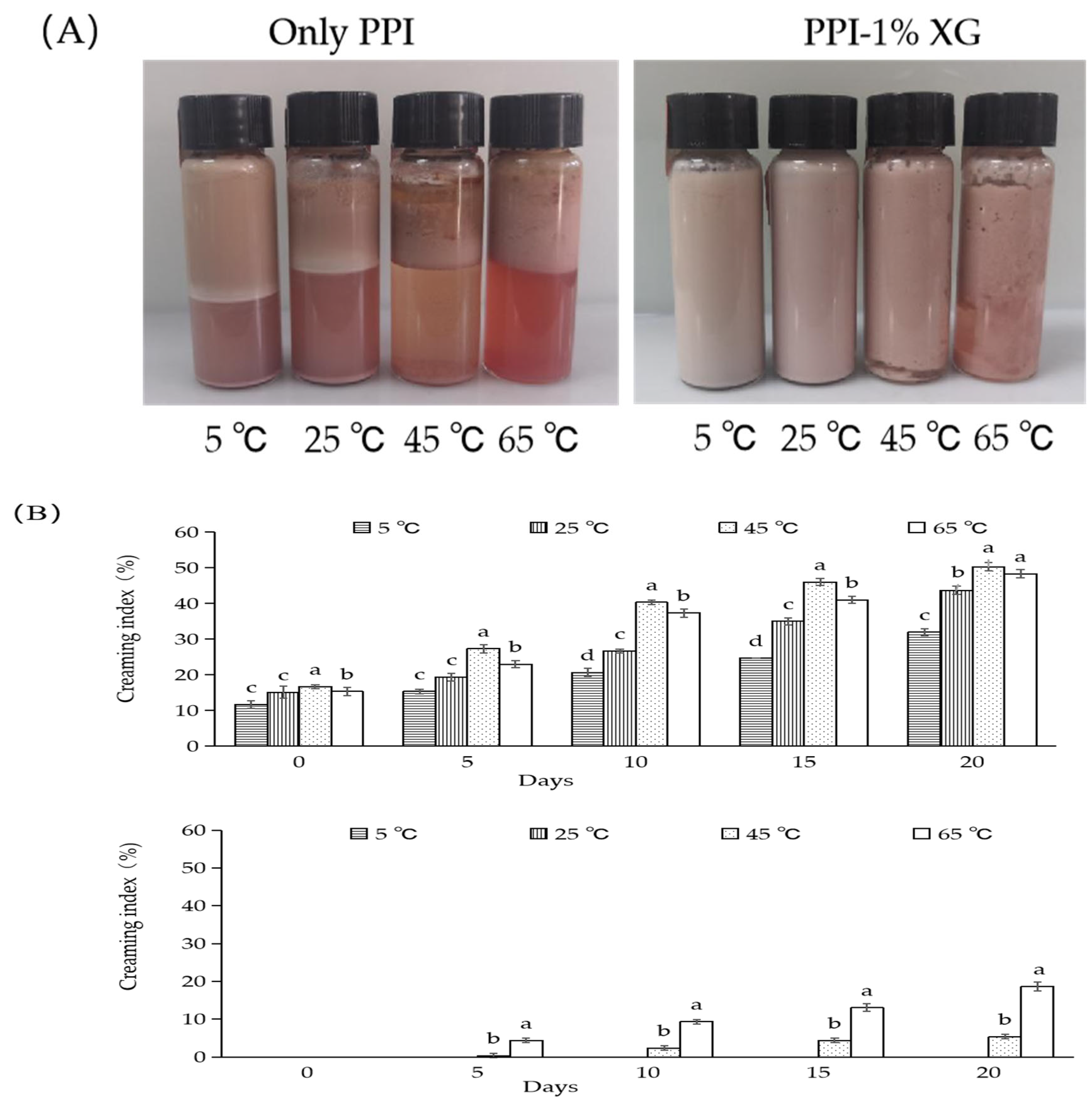
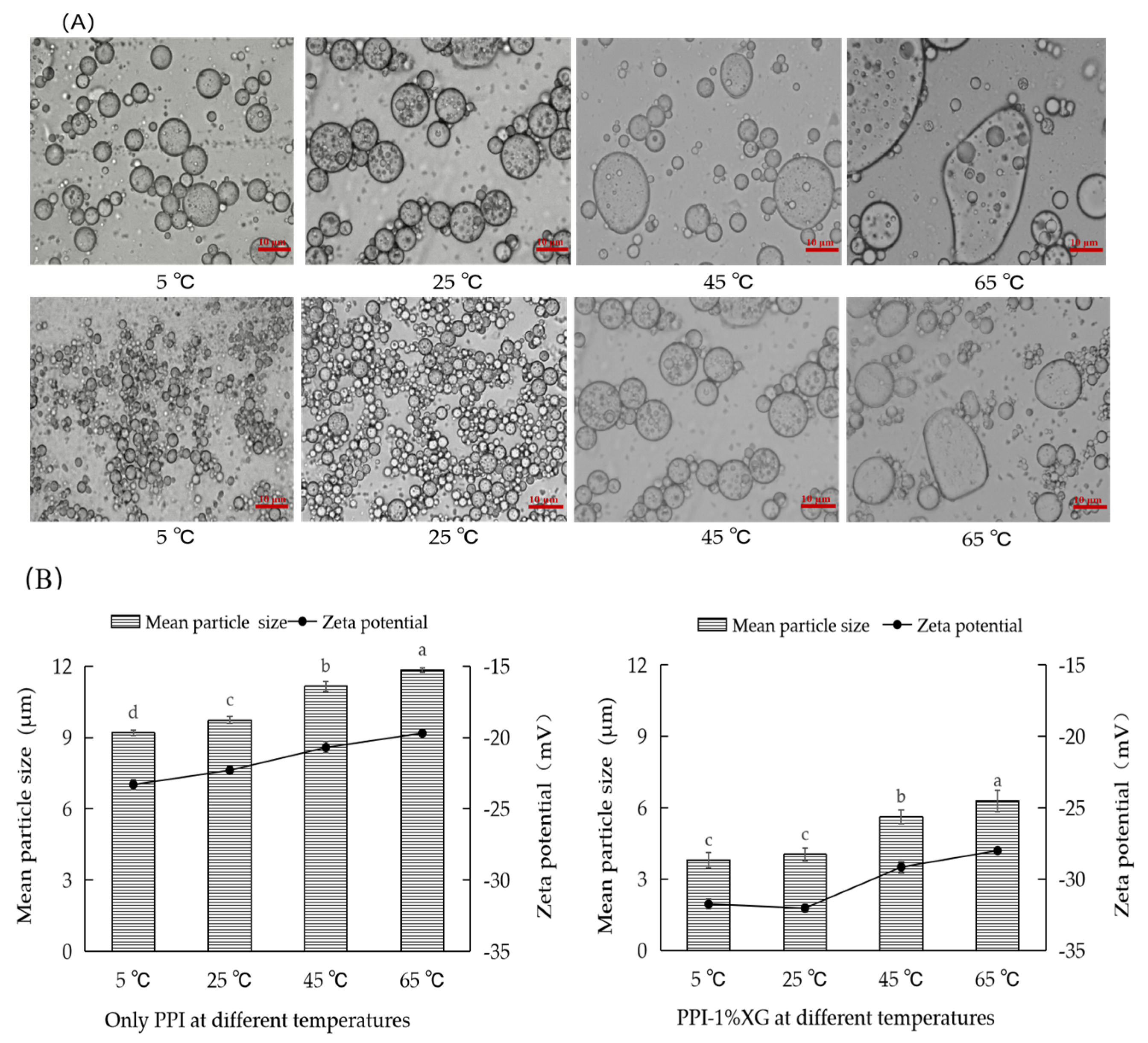
3.2. Effect of Different Temperatures on the Storage Stability of Double Emulsions
3.2.1. Emulsion Morphology and Creaming Index
3.2.2. Microstructure, Particle Size and Zeta Potential
3.3. Thermal Degradation of MAs at Different Temperatures
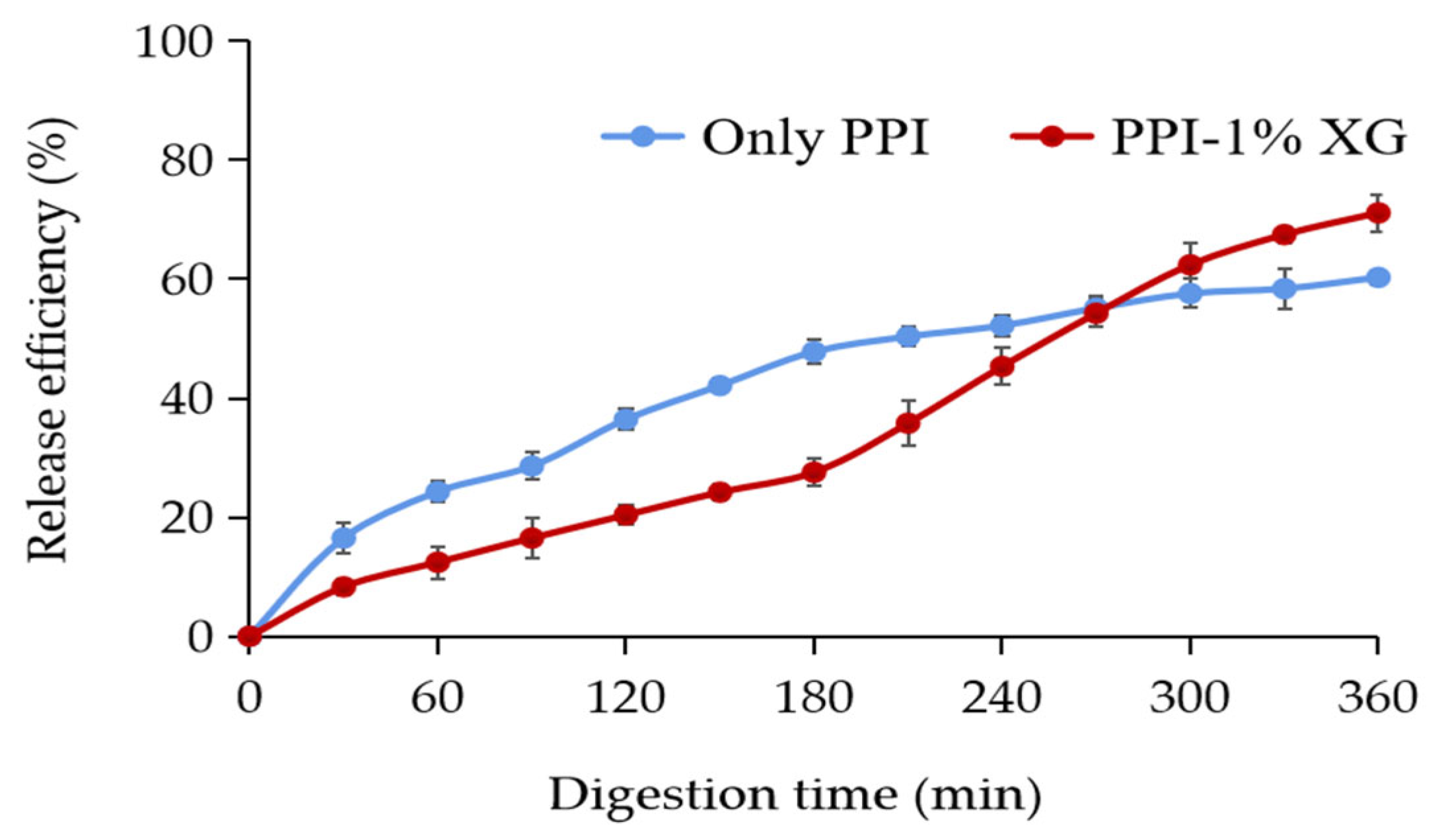
3.4. In Vitro Digestive Properties of Double Emulsions
3.4.1. Particle Size and Micromorphology
3.4.2. Zeta Potential
3.4.3. Release Properties of MAs for In Vitro Simulated Digestion
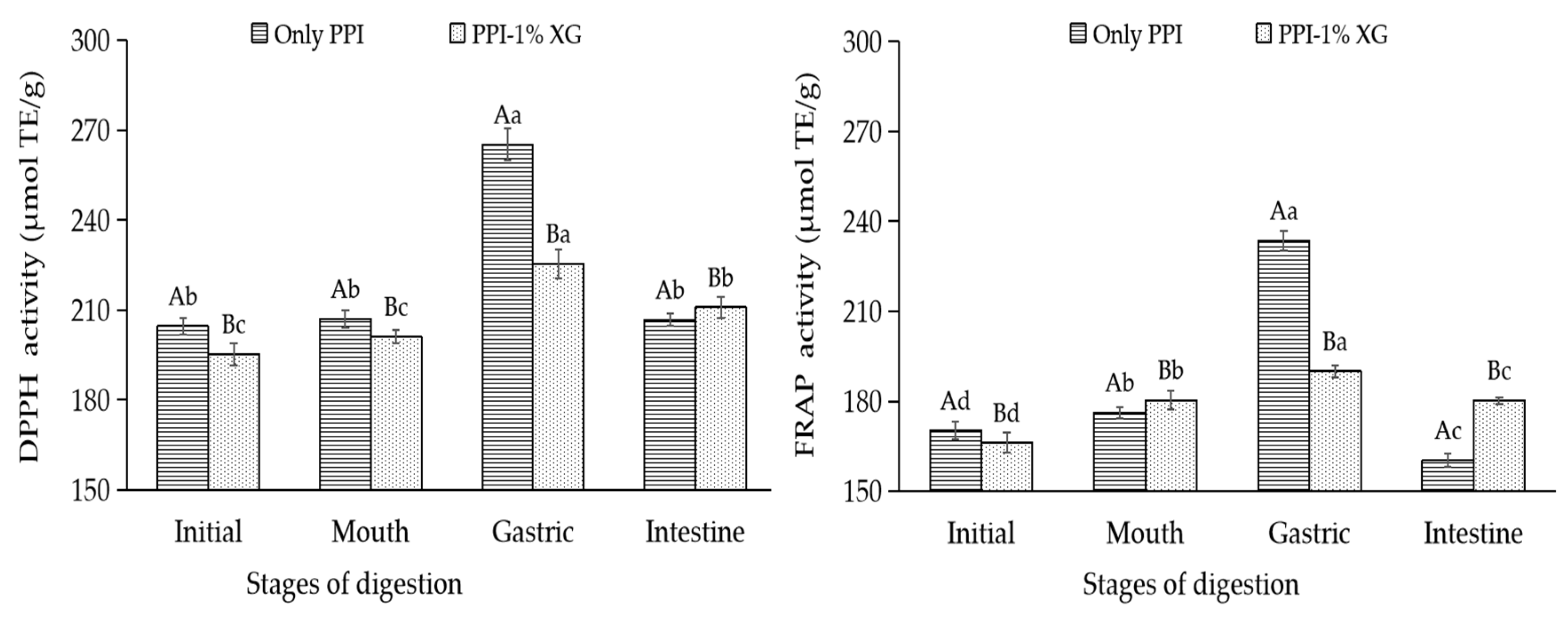
3.4.4. Evaluation of Oxidative Stability of Double Emulsions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N. Structures, bioactivities and future prospective of polysaccharides from Morus alba (white mulberry): A review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zheng, J.; Xu, Y. Composition of anthocyanins in mulberry and their antioxidant activity. J. Food Compos. Anal. 2008, 21, 390–395. [Google Scholar] [CrossRef]
- Celia, C.; Senem, K.; Charlotte, G.; John, V.C.; Marc, H. Co-Ingestion of Black Carrot and Strawberry. Effects on Anthocyanin Stability, Bioaccessibility and Uptake. Food Chem. 2020, 9, 15–95. [Google Scholar]
- Casta, N.A.; Pacheco-Hern’andez, M.D.; P’aez-Hern’andez, M.E.; Rodríguez, J.A.; Gal’an-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar]
- Fornasaro, S.; Ziberna, L.; Gasperotti, M.; Tramer, F.; Vrhovsek, U.; Mattivi, F.; Passamonti, S. Determination of cyanidin 3-glucoside in rat brain, liver and kidneys by UPLC/MS-MS and its application to a short-term pharmacokinetic study. Sci. Rep. 2016, 6, 22815. [Google Scholar] [CrossRef]
- Cournarie, F.; Savelli, M.-P.; Rosilio, V.; Bretez, F.; Vauthier, C.; Grossiord, J.-L.; Seiller, M. Insulin-loaded W/O/W multiple emulsions: Comparison of the performances of systems prepared with medium-chain-triglycerides and fish oil. Eur. J. Pharm. Biopharm. 2004, 58, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Charles, F.; Lim, L.F.T.; Xu, W.; Xu, G. Coencapsulation of Polyphenols and Anthocyanins from Blueberry Pomace by Double Emulsion Stabilized by Whey Proteins: Effect of Homogenization Parameters. Molecules 2018, 23, 2525. [Google Scholar] [CrossRef] [Green Version]
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems Preparation, stability, and application. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef] [Green Version]
- Mcclements, J.D. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 4, 611–649. [Google Scholar] [CrossRef]
- Chen, Q.; Li, T.; Zhao, Z.; Zhang, G.; Mao, W.; Feng, X.; Yang, L. Bio-transformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; FathiAchachlouei, B.; Huang, Q. Double emulsion followed by complex coacervation as a promising method for protection of black raspberry anthocyanins. Food Hydrocoll. 2018, 77, 803–816. [Google Scholar] [CrossRef]
- Donald, A.M. Aggregation in β-lactoglobulin. Soft Matter 2008, 4, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Chen, B.; Rao, J. Pea protein isolate–high methoxyl pectin soluble complexes for improving pea protein functionality: Effect of pH, biopolymer ratio and concentrations. Food Hydrocoll. 2018, 80, 245–253. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef]
- Davidsson, L.; Dimitriou, T.; Walczyk, T. Iron absorption from experimental infant formulas based on pea (Pisum sativum)-protein isolate: The effect of phytic acid and ascorbic acid. Brit. J. Nutr. 2001, 85, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Tang, C. pH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Selomulya, C. Impact of sodium alginate on binary whey/pea protein-stabilised emulsions. J. Food Eng. 2022, 321, 110978. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Darani, K. Effective variables on production andstructure of xanthan gum and its food applications: A review. Biocatal. Agric. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Ge, J.; Yu, X.; Wang, S.; Chi, J.; Liang, J.; Sun, Y.; Yue, P. Nanocomplexes composed of chitosan derivatives and beta-Lactoglobulin as a carrier for anthocyanins: Preparation, stability and bioavailability in vitro. Food Res. Int. 2019, 116, 336–345. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, X.; Ding, L.; Wang, S. Effect of pH, ionic strength, chitosan deacetylation on the stability and rheological properties of O/W emulsions formulated with chitosan/ casein complexes. Food Hydrocoll. 2020, 111, 106211. [Google Scholar] [CrossRef]
- Rabelo, C.A.S.; Taarji, N.; Khalid, N. Formulation and characterization of water-in-oil nanoemulsions loaded with acai berry anthocyanins: Insights of degradation kinetics and stability evaluation of anthocyanins and nanoemulsions. Food Res. Int. 2018, 106, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wan, Z.; Yang, X. Multiple Water-in-Oil-in-Water Emulsion Gels Based on Self-Assembled Saponin Fibrillar Network for Photosensitive Cargo Protection. J. Agric. Food Chem. 2017, 65, 9735–9743. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 11–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moura, S.C.; Berling, C.L.; Germer, S.P.M. Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: Pigment stability during storage of microparticles. Food Chem. 2018, 241, 317–327. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Y.; Xue, S.J.; Shi, J.; Lim, L.; Forney, C.; Xu, G.; Bamba, S.B.B. Effect of In Vitro Digestion on Water-in-Oil-in-Water Emulsions Containing Anthocyanins from Grape Skin Powder. Molecules 2018, 23, 2808. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Song, H.S.; Ukeda, H. Radical scavenging activities of citrus essential oils and their components: Detection using 1,1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.H.; Mcclements, D.J. Theoretical stability maps for guiding preparation of emulsions stabilized by protein-polysaccharide interfacial complexes. Langmuir 2009, 25, 649–657. [Google Scholar] [CrossRef]
- Abdolmaleki, K.; Alizadeh, L.; Hosseini, S.M. Concentrated o/w emulsions formulated by binary and ternary mixtures of sodium caseinate, xanthan and guar gums: Rheological properties, microstructure, and stability. Food Sci. Biotechnol. 2020, 29, 1685–1693. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Zhao, Q.; Selomulya, C.; Zhu, X.; Xiong, H. Physical and oxidative stabilities of O/W emulsions formed with rice dreg protein hydrolysate: Effect of xanthan gum rheology. Food Bioprocess Technol. 2016, 9, 1380–1390. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, Z.; Mclements, D.J. Influence of aqueous phase emulsifiers on lipid oxidation in water-in-walnut oil emulsions. J. Agric. Food Chem. 2014, 62, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Boonlao, N.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of whey protein-xanthan gum stabilized emulsion on stability and in vitro digestibility of encapsulated astaxanthin. J. Food Eng. 2020, 272, 109859. [Google Scholar] [CrossRef]
- Zhao, J.J.; Wei, T.; Wei, Z.H. Influence of soybean polysaccharides and beet pectin on the physicochemical properties of lactoferrin-coated orange oil emulsion. Food Hydrocoll. 2015, 44, 443–452. [Google Scholar] [CrossRef]
- Kanha, N.; Surawang, S.; Pitchakarn, P.; Regenstein, J.M.; Laokuldilok, T. Copigmentation of Cyanidin 3-O-glucoside with phenolics: Thermodynamic data and thermal stability. Food Biosci. 2019, 30, 100–419. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S.; Richards, M.P. Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Food Hydrocoll. 2007, 21, 555–564. [Google Scholar] [CrossRef]
- Protonotariou, S.; Evageliou, V.; Yanniotis, S.; Mandala, I. The influence of different stabilizers and salt addition on the stability of model emulsions containing olive or sesame oil. J. Food Eng. 2013, 117, 124–132. [Google Scholar] [CrossRef]
- Xu, X.; Luo, L.; Liu, C.; McClements, D.J. Utilization of anionic polysaccharides to improve the stability of rice glutelin emulsions: Impact of polysaccharide type, pH, salt, and temperature. Food Hydrocoll. 2017, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Soumia, S.; Moulai-Mostefa, N. Formulation and Characterization of Double Emulsions Stabilized by Sodium Caseinate–Xanthan Mixtures. Effect of pH and Biopolymer Concentration. J. Dispers. Sci. Technol. 2015, 36, 51–60. [Google Scholar] [CrossRef]
- Sun, C.; Sundaram, G.; Mark, R.P. Effect of xantham gum on physiochemical properties of whey protein isolate stabilized oilin-water emulsion. J. Food Eng. 2014, 122, 15–27. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Qiu, L.; Sun, Y. Improvement of the solubility and emulsifying properties of rice bran protein by phosphorylation with sodium trimetaphosphate. Food Hydrocoll. 2019, 96, 288–299. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Q.; Hu, Z.Z. Maillard-reacted whey protein isolates and epigallocatechin gallate complex enhance the thermal stability of the Pickering emulsion delivery of curcumin. J. Agric. Food Chem. 2019, 67, 5212–5220. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Zhao, M.; Liu, N. Physicochemical properties of peanut oil-based diacylglycerol and their derived oil-inwater emulsions stabilized by sodium caseinate. Food Chem. 2015, 184, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Murray, B.S.; Afeisume, E.I.; Khew, S.H. Encapsulation of flavonoid in multiple emulsion using spinning disc reactor technology. Food Hydrocoll. 2014, 34, 62–67. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios, J.D.J.; Powers, J.R.; Tang, T. Thermal degradation of anthocyanins from purple potato (cv. purple majesty) and impact on antioxidant capacity. J. Agric. Food Chem. 2011, 59, 11040–11049. [Google Scholar] [CrossRef]
- Aihara, K.; Kajimoto, O.; Hirata, H.; Takahashi, R.; Nakamura, Y. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J. Am. Coll. Nutr. 2005, 24, 257–265. [Google Scholar] [CrossRef]
- Tako, M.; Teruya, T.; Tamaki, Y.; Ohkawa, K. Co-gelation mechanism of xanthan and galactomannan. Colloid Polym. Sci. 2010, 288, 1161–1166. [Google Scholar] [CrossRef]
- Vingerhoeds, M.H.; Blijdenstein, T.B.J.; Zoet, F.D.; Aken, G.A.V. Emulsion flocculation induced by saliva and mucin. Food Hydrocoll. 2005, 19, 915–922. [Google Scholar] [CrossRef]
- Somaratne, G.; Ferrua, M.J.; Ye, A.; Nau, F.; Floury, J.; Dupont, D.; Singh, J. Food material properties as determining factors in nutrient release during human gastric digestion: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3753–3769. [Google Scholar] [CrossRef]
- Krstonosic, V.; Dokic, L.; Dokic, P.; Dapcevic, T. Effects of Xanthan Gum on Physicochemical Properties and Stability of Corn Oil-in-Water Emulsions Stabilized by Polyoxyethylene (20) Sorbitan Monooleate. Food Hydrocoll. 2009, 23, 2212–2218. [Google Scholar] [CrossRef]
- Mao, Y.; McClements, D.J. Influence of electrostatic heteroaggregation of lipid droplets on their stability and digestibility under simulated gastrointestinal conditions. Food Funct. 2012, 3, 1025–1034. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog. Lipid Res. 2009, 48, 92–100. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan-a review. J Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rayment, P.; Wright, P.; Hoad, C.; Ciampi, E.; Haydock, D.; Gowland, P.; Butler, M.F. Investigation of alginate beads for gastro-intestinal functionality, Part 1: In vitro characterisation. Food Hydrocoll. 2009, 23, 816–822. [Google Scholar] [CrossRef]
- Papalamprou, E.M.; Makri, E.A.; Kiosseoglou, V.D. Effect of medium molecular weight xanthan gum in rheology and stability of oil-in-water emulsion stabilized with legume proteins. J. Sci. Food Agric. 2005, 85, 1967–1973. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Q.; Kong, J.; Zhao, M. Effect of xanthan gum on the stability of sodium caseinate emulsion. Sci. Technol. Food Ind. 2012, 33, 83–86. [Google Scholar]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Wang, S.; Ye, X.; Sun, Y.; Liang, J.; Yue, P.; Gao, X. Nanocomplexes derived from chitosan and whey protein isolate enhance the thermal stability and slow the release of anthocyanins in simulated digestion and prepared instant coffee. Food Chem. 2020, 336, 127707. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef]
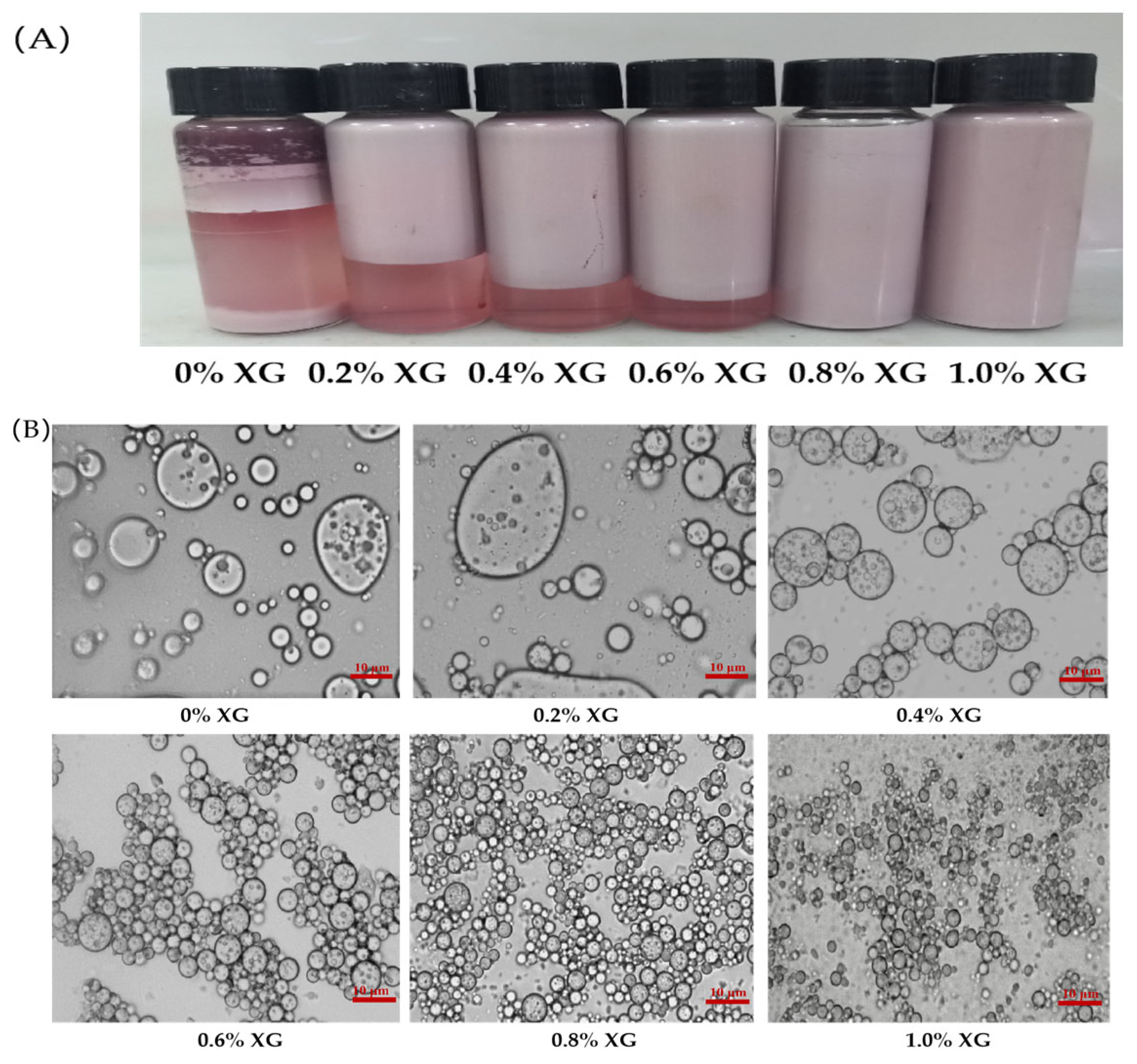


| 0% XG | 0.2% XG | 0.4% XG | 0.6% XG | 0.8% XG | 1% XG | |
|---|---|---|---|---|---|---|
| Mean particle size (μm) | 9.20 ± 0.21 b | 9.83 ± 0.17 b | 7.40 ± 0.23 c | 5.70 ± 0.31 d | 4.20 ± 0.24 e | 3.80 ± 0.33 e |
| Zeta potential (mV) | −25.23 ± 0.21 a | −27.60 ± 0.36 b | −28.50 ± 0.30 c | −29.83 ± 0.15 d | −31.73 ± 0.31 e | −32.58 ± 0.31 f |
| Encapsulation efficiency (%) | 44.57 ± 0.93 f | 51.50 ± 0.87 e | 62.66 ± 0.79 d | 68.52 ± 0.81 c | 83.02 ± 0.70 b | 88.52 ± 2.05 a |
| Creaming index (%) | 74.33 ± 1.53 a | 45.00 ± 1.00 b | 22.67 ± 1.53 c | 12.33 ± 1.15 d | 0 | 0 |
| Temperature | k × 10−2 (day−1) | t1/2 (day) | R2 | |||
|---|---|---|---|---|---|---|
| PPI | PPI-1% XG | PPI | PPI-1% XG | PPI | PPI-1% XG | |
| 5 °C | 2.29 ± 0.05 d | 0.89 ± 0.05 d | 30.32 ± 0.69 a | 78.07 ± 4.72 a | 0.9716 | 0.9991 |
| 25 °C | 2.75 ± 0.06 c | 1.18 ± 0.03 c | 25.21 ± 0.56 c | 60.25 ± 1.33 b | 0.9804 | 0.9936 |
| 45 °C | 3.80 ± 0.07 b | 1.94 ± 0.10 b | 18.25 ± 0.34 d | 35.73 ± 1.86 c | 0.9826 | 0.9685 |
| 65 °C | 4.48 ± 0.04 a | 2.37 ± 0.20 a | 15.40 ± 0.06 b | 29.30 ± 2.05 d | 0.9803 | 0.9949 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniya; Cao, Y.; Liu, C.; Lu, S.; Fujii, Y.; Jin, J.; Xia, Q. Improved Stabilization and In Vitro Digestibility of Mulberry Anthocyanins by Double Emulsion with Pea Protein Isolate and Xanthan Gum. Foods 2023, 12, 151. https://doi.org/10.3390/foods12010151
Aniya, Cao Y, Liu C, Lu S, Fujii Y, Jin J, Xia Q. Improved Stabilization and In Vitro Digestibility of Mulberry Anthocyanins by Double Emulsion with Pea Protein Isolate and Xanthan Gum. Foods. 2023; 12(1):151. https://doi.org/10.3390/foods12010151
Chicago/Turabian StyleAniya, Yan Cao, Chenxing Liu, Shengming Lu, Yoshiharu Fujii, Jiaxiu Jin, and Qile Xia. 2023. "Improved Stabilization and In Vitro Digestibility of Mulberry Anthocyanins by Double Emulsion with Pea Protein Isolate and Xanthan Gum" Foods 12, no. 1: 151. https://doi.org/10.3390/foods12010151
APA StyleAniya, Cao, Y., Liu, C., Lu, S., Fujii, Y., Jin, J., & Xia, Q. (2023). Improved Stabilization and In Vitro Digestibility of Mulberry Anthocyanins by Double Emulsion with Pea Protein Isolate and Xanthan Gum. Foods, 12(1), 151. https://doi.org/10.3390/foods12010151







