An Accurate and Rapid Way for Identifying Food Geographical Origin and Authenticity: Editable DNA-Traceable Barcode
Abstract
:1. Introduction
2. Materials and Methods
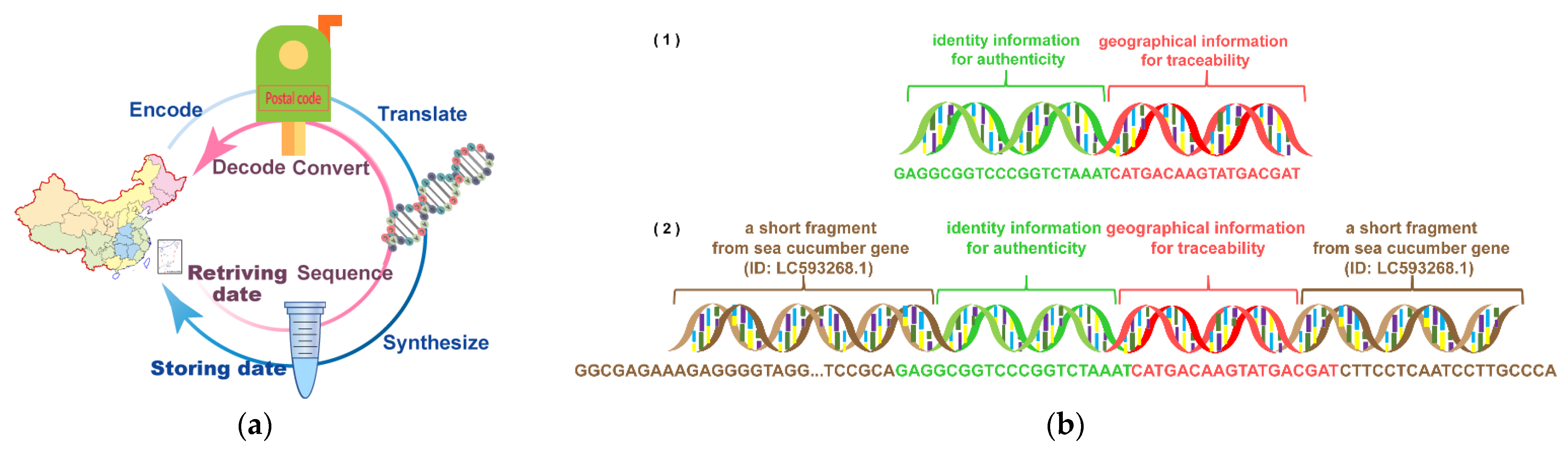
2.1. Designing and Synthesizing DNA-Traceable Barcodes
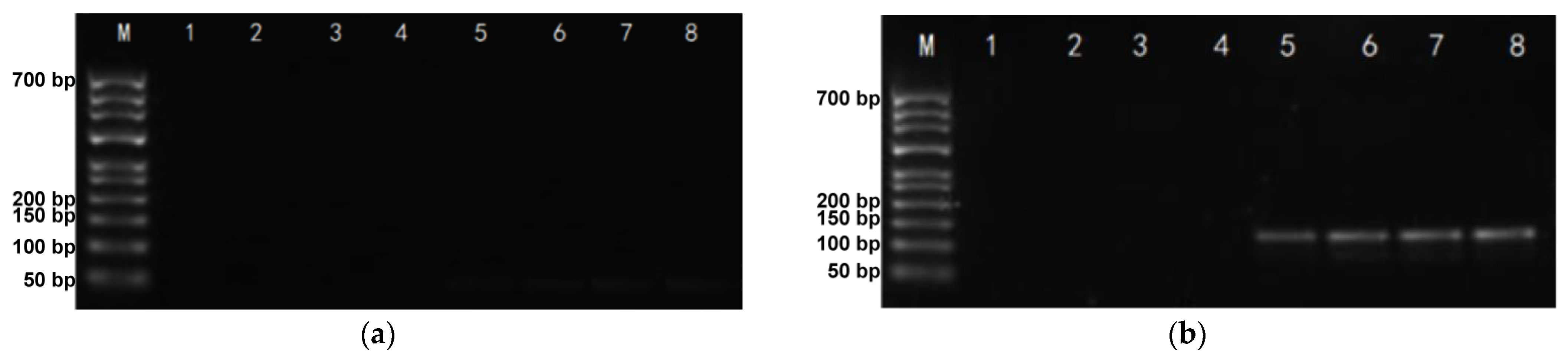
2.2. DNA-Traceable Barcode Vector Sequencing and Identification Primer Screening
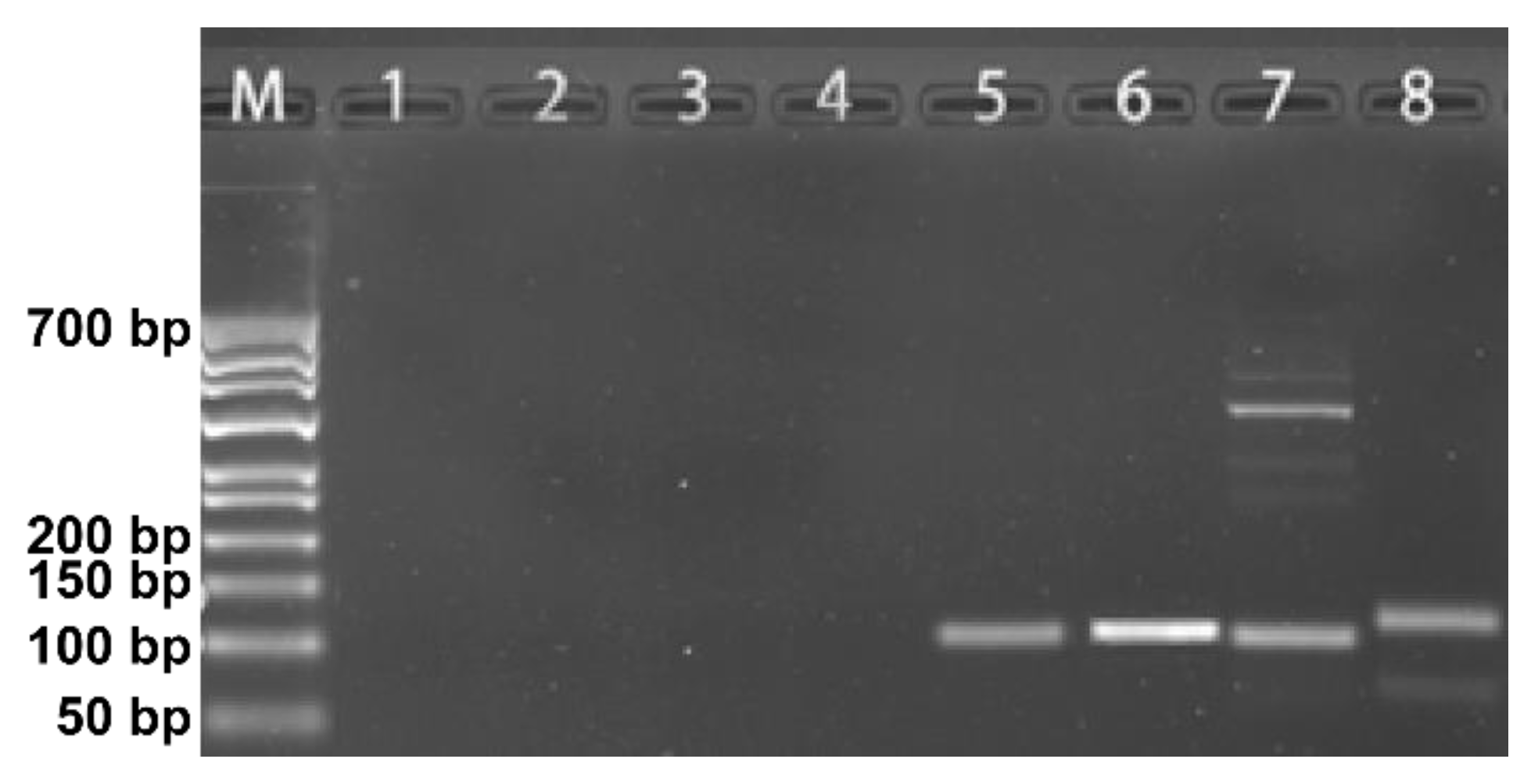
2.3. DNA-Traceable Barcodes Encapsulation and Post-Recycling Identification
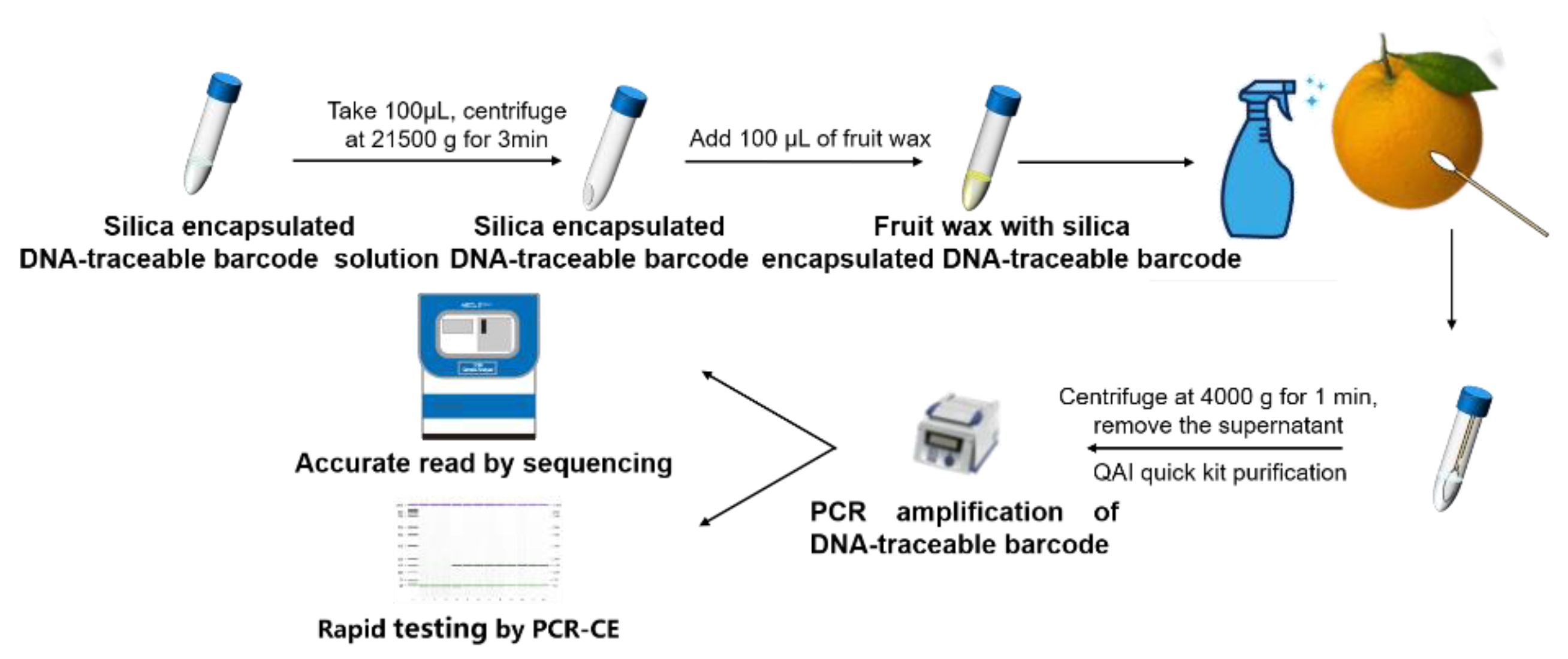
2.4. Using Silica-Encapsulated DNA-Traceable Barcode to Identify Citrus Sinensis
3. Results and Discussion
3.1. Comparison of DNA-Traceable Barcode Composition Schemes
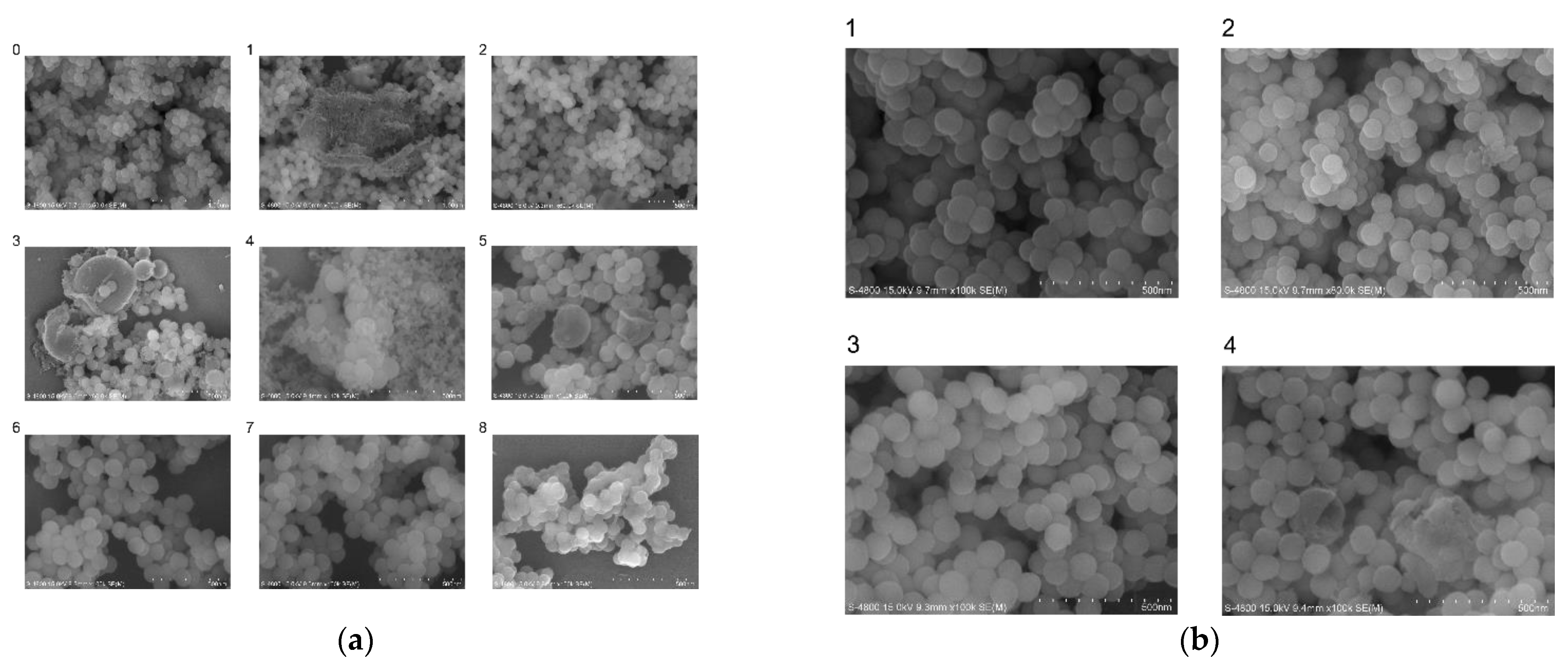
3.2. Generation and Validation of Silica Particles with Encapsulated DNA-Traceable Barcode
3.3. Using DNA-Traceable Barcodes to Label Citrus Sinensis and Detecting DNA-Traceable Barcodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wadood, S.A.; Guo, B.L.; Zhang, X.W.; Hussain, I.; Wei, Y.M. Recent development in the application of analytical techniques for the traceability and authenticity of food of plant origin. Microchem. J. 2020, 152, 104295. [Google Scholar] [CrossRef]
- Sun, S.N.; Wang, X.P. Promoting traceability for food supply chain with certification. J. Clean. Prod. 2019, 217, 658–665. [Google Scholar] [CrossRef]
- Krajnc, B.; Bontempo, L.; Luis Araus, J.; Giovanetti, M.; Alegria, C.; Lauteri, M.; Augusti, A.; Atti, N.; Smeti, S.; Taous, F.; et al. Selective Methods to Investigate Authenticity and Geographical Origin of Mediterranean Food Products. Food Rev. Int. 2021, 37, 656–682. [Google Scholar] [CrossRef]
- Spink, J.; Moyer, D.C. Defining the public health threat of food fraud. J. Food Sci. 2011, 76, R157–R163. [Google Scholar] [CrossRef]
- Meiser, L.C.; Nguyen, B.H.; Chen, Y.-J.; Nivala, J.; Strauss, K.; Ceze, L.; Grass, R.N. Synthetic DNA applications in information technology. Nat. Commun. 2022, 13, 352. [Google Scholar] [CrossRef]
- Behnke, K.; Janssen, M. Boundary conditions for traceability in food supply chains using blockchain technology. Int. J. Inf. Manag. 2020, 52, 101969. [Google Scholar] [CrossRef]
- Kendall, H.; Kuznesof, S.; Dean, M.; Chan, M.-Y.; Clark, B.; Home, R.; Stolz, H.; Zhong, Q.; Liu, C.; Brereton, P.; et al. Chinese consumer’s attitudes, perceptions and behavioural responses towards food fraud. Food Control 2019, 95, 339–351. [Google Scholar] [CrossRef]
- Dimitrakopoulou, M.E.; Vantarakis, A. Does Traceability Lead to Food Authentication? A Systematic Review from A European Perspective. Food Rev. Int. 2021, 1–23. [Google Scholar] [CrossRef]
- Gill, P.; Ivanov, P.L.; Kimpton, C.; Piercy, R.; Sullivan, K. Identification of the remains of the Romanov family by DNA analysis. Nat. Genet. 1994, 6, 130–135. [Google Scholar] [CrossRef]
- Cusa, M.; Glew, K.S.; Trueman, C.; Mariani, S.; Buckley, L.; Neat, F.; Longo, C. A future for seafood point-of-origin testing using DNA and stable isotope signatures. Rev. Fish Biol. Fish. 2022, 32, 597–621. [Google Scholar] [CrossRef]
- Fanelli, V.; Mascio, I.; Miazzi, M.M.; Savoia, M.A.; De Giovanni, C.; Montemurro, C. Molecular Approaches to Agri-Food Traceability and Authentication: An Updated Review. Foods 2021, 10, 1644. [Google Scholar] [CrossRef]
- Liu, S.L.; Lang, D.D.; Meng, G.L.; Hu, J.H.; Tang, M.; Zhou, X. Tracing the origin of honey products based on metagenomics and machine learning. Food Chem. 2022, 371, 131066. [Google Scholar] [CrossRef]
- Puddu, M.; Paunescu, D.; Stark, W.J.; Grass, R.N. Magnetically Recoverable, Thermostable, Hydrophobic DNA/Silica Encapsulates and Their Application as Invisible Oil Tags. ACS Nano 2014, 8, 2677–2685. [Google Scholar] [CrossRef]
- Koch, J.; Gantenbein, S.; Masania, K.; Stark, W.J.; Erlich, Y.; Grass, R.N. A DNA-of-things storage architecture to create materials with embedded memory. Nat. Biotechnol. 2020, 38, 39–43. [Google Scholar] [CrossRef]
- Erlich, Y.; Zielinski, D. DNA Fountain enables a robust and efficient storage architecture. Science 2017, 355, 950–953. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.P.; Dai, B.Y.; Wang, B.G.; Zha, Y.; Song, Q. Traceability in food processing: Problems, methods, and performance evaluations-a review. Crit. Rev. Food Sci. Nutr. 2022, 62, 679–692. [Google Scholar] [CrossRef]
- Qian, J.; Lu, Z.X.; Mancuso, C.P.; Jhuang, H.Y.; Springer, M. Barcoded microbial system for high-resolution object provenance. Science 2020, 368, 1135–1140. [Google Scholar] [CrossRef]
- Zografos, A.; Farquar, G.R. DNA Based Bar Code for Improved Food Traceability. WO 2016114808A1, 21 July 2016. [Google Scholar]
- Lalitha, S. Primer Premier 5. Biotech Softw. Internet Rep. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Xing, R.-R.; Hu, R.-R.; Wang, N.; Zhang, J.-K.; Ge, Y.-Q.; Chen, Y. Authentication of sea cucumber products using NGS-based DNA mini-barcoding. Food Control 2021, 129, 108199. [Google Scholar] [CrossRef]
- Paunescu, D.; Puddu, M.; Soellner, J.O.B.; Stoessel, P.R.; Grass, R.N. Reversible DNA encapsulation in silica to produce ROS-resistant and heat-resistant synthetic DNA ‘fossils’. Nat. Protoc. 2013, 8, 2440–2448. [Google Scholar] [CrossRef]
- Handy, S.M.; Mueller, S.; Jacob, S.M.; Paul, S.Z.; Garrett, S.D.; Deeds, J.R. Evaluation of the Agilent Technologies bioanalyzer-based DNA fish identification solution. Food Control 2017, 73, 627–633. [Google Scholar] [CrossRef]
- Yeo, D.; Srivathsan, A.; Meier, R. Longer is Not Always Better: Optimizing Barcode Length for Large-Scale Species Discovery and Identification. Syst. Biol. 2020, 69, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Tanita, I.; Nishihama, S.; Hayashibara, T. Identification of species of teatfish (Holothuroidea: Holothuriida) in Japan based on mitochondrial cytochrome oxidase subunit I (COI) sequences, morphology, and ossicles. Plankton Benthos Res. 2021, 16, 200–209. [Google Scholar] [CrossRef]
- Lin, K.; Chavalarias, D.; Panahi, M.; Yeh, T.; Takimoto, K.; Mizoguchi, M. Mobile-based traceability system for sustainable food supply networks. Nat. Food 2020, 1, 673–679. [Google Scholar] [CrossRef]
- Feng, H.; Wang, X.; Duan, Y.; Zhang, J.; Zhang, X. Applying blockchain technology to improve agri-food traceability: A review of development methods, benefits and challenges. J. Clean. Prod. 2020, 260, 121031. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, S.; Zhang, Y.; Hu, B. Construct Food Safety Traceability System for People’s Health Under the Internet of Things and Big Data. IEEE Access 2021, 9, 70571–70583. [Google Scholar] [CrossRef]
- Tarjan, L.; Šenk, I.; Tegeltija, S.; Stankovski, S.; Ostojic, G. A readability analysis for QR code application in a traceability system. Comput. Electron. Agric. 2014, 109, 1–11. [Google Scholar] [CrossRef]
- Yu, W.; Huang, S. Traceability of Food Safety Based on Block Chain and RFID Technology. In Proceedings of the 2018 11th International Symposium on Computational Intelligence and Design (ISCID), Hangzhou, China, 8–9 December 2018; pp. 339–342. [Google Scholar]
- Bai, H.; Zhou, G.; Hu, Y.; Sun, A.; Xu, X.; Liu, X.; Lu, C. Traceability technologies for farm animals and their products in China. Food Control 2017, 79, 35–43. [Google Scholar] [CrossRef]
- Avoine, G.; Oechslin, P. RFID traceability: A multilayer problem. In Proceedings of the International Conference on Financial Cryptography and Data Security, Roseau, Dominica, 28 February–3 March 2005; pp. 125–140. [Google Scholar]
- Gautam, R.; Singh, A.; Karthik, K.; Pandey, S.; Scrimgeour, F.; Tiwari, M.K. Traceability using RFID and its formulation for a kiwifruit supply chain. Comput. Ind. Eng. 2017, 103, 46–58. [Google Scholar] [CrossRef]
- Feng, T. An agri-food supply chain traceability system for China based on RFID & blockchain technology. In Proceedings of the 2016 13th International Conference on Service Systems and Service Management (ICSSSM), Kunming, China, 24–26 June 2016; pp. 1–6. [Google Scholar]
- Paunescu, D.; Stark, W.J.; Grass, R.N. Particles with an identity: Tracking and tracing in commodity products. Powder Technol. 2016, 291, 344–350. [Google Scholar] [CrossRef]
- Sharief, S.A.; Chahal, P.; Alocilja, E. Application of DNA sequences in anti-counterfeiting: Current progress and challenges. Int. J. Pharm. 2021, 602, 120580. [Google Scholar] [CrossRef] [PubMed]
- Paunescu, D.; Fuhrer, R.; Grass, R.N. Protection and Deprotection of DNA—High-Temperature Stability of Nucleic Acid Barcodes for Polymer Labeling. Angew. Chem. Int. Ed. 2013, 52, 4269–4272. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.-T.; Tsai, M.-C. Effects of the perception of traceable fresh food safety and nutrition on perceived health benefits, affective commitment, and repurchase intention. Food Qual. Prefer. 2019, 78, 103723. [Google Scholar] [CrossRef]
- Antkowiak, P.L.; Koch, J.; Nguyen, B.H.; Stark, W.J.; Strauss, K.; Ceze, L.; Grass, R.N. Integrating DNA Encapsulates and Digital Microfluidics for Automated Data Storage in DNA. Small 2022, 18, 2107381. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Li, X.; Wu, D. A self-monitored fluorescence DNA anti-counterfeiting system based on silica coated SYBR Green I/DNA gelatin nanoparticles. J. Mater. Chem. C 2017, 5, 5939–5948. [Google Scholar] [CrossRef]
- Bisht, B.; Bhatnagar, P.; Gururani, P.; Kumar, V.; Tomar, M.S.; Sinhmar, R.; Rathi, N.; Kumar, S. Food irradiation: Effect of ionizing and non-ionizing radiations on preservation of fruits and vegetables—A review. Trends Food Sci. Technol. 2021, 114, 372–385. [Google Scholar] [CrossRef]
- Bloch, M.S.; Paunescu, D.; Stoessel, P.R.; Mora, C.A.; Stark, W.J.; Grass, R.N. Labeling Milk along Its Production Chain with DNA Encapsulated in Silica. J. Agric. Food Chem. 2014, 62, 10615–10620. [Google Scholar] [CrossRef]
- Banal, J.L.; Bathe, M. Scalable Nucleic Acid Storage and Retrieval Using Barcoded Microcapsules. ACS Appl. Mater. Interfaces 2021, 13, 49729–49736. [Google Scholar] [CrossRef]
- Ding, S.; Wang, L.; He, Z.; Sui, Z.; Wang, G.; Takarada, T.; Maeda, M.; Liang, X. Identifying Exogenous DNA in Liquid Foods by Gold Nanoparticles: Potential Applications in Traceability. ACS Food Sci. Technol. 2021, 1, 605–613. [Google Scholar] [CrossRef]
- Authority, E.F.S. Calcium silicate and silicon dioxide/silicic acid gel added for nutritional purposes to food supplements. EFSA J. 2009, 7, 1132. [Google Scholar]
- Organick, L.; Nguyen, B.H.; McAmis, R.; Chen, W.D.; Kohll, A.X.; Ang, S.D.; Grass, R.N.; Ceze, L.; Strauss, K. An Empirical Comparison of Preservation Methods for Synthetic DNA Data Storage. Small Methods 2021, 5, 2001094. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Zhao, F.; Hamada, S.; Yang, P.; Xu, H.; Luo, D.; Yang, D. DNA-based engineering system for improving human and environmental health: Identification, detection, and treatment. Nano Today 2020, 35, 100958. [Google Scholar] [CrossRef]
- Zhao, D.; Tian, Z.; Cai, J.; He, J. Microbial spore genetic marker technology, a potential technology for traditional Chinese medicine traceability system. Chin. Med. 2022, 17, 61. [Google Scholar] [CrossRef]
- Mora, C.A.; Paunescu, D.; Grass, R.N.; Stark, W.J. Silica particles with encapsulated DNA as trophic tracers. Mol. Ecol. Resour. 2015, 15, 231–241. [Google Scholar] [CrossRef]
- Katerinopoulou, K.; Kontogeorgos, A.; Salmas, C.E.; Patakas, A.; Ladavos, A. Geographical Origin Authentication of Agri-Food Products: A Review. Foods 2020, 9, 489. [Google Scholar] [CrossRef]
- Díaz, R.; Pozo, O.J.; Sancho, J.V.; Hernández, F. Metabolomic approaches for orange origin discrimination by ultra-high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Chem. 2014, 157, 84–93. [Google Scholar] [CrossRef]
- Francois, G.; Fabrice, V.; Didier, M. Traceability of fruits and vegetables. Phytochemistry 2020, 173, 112291. [Google Scholar] [CrossRef]
- El Sheikha, A.F. How to Determine the Geographical Origin of Food by Molecular Techniques. In Molecular Techniques in Food Biology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 1–26. [Google Scholar]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous silica and organosilica nanoparticles: Physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [Green Version]
- Croissant, J.G.; Brinker, C.J. Chapter Eight—Biodegradable Silica-Based Nanoparticles: Dissolution Kinetics and Selective Bond Cleavage. In The Enzymes; Tamanoi, F., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 43, pp. 181–214. [Google Scholar]
- Finnie, K.S.; Waller, D.J.; Perret, F.L.; Krause-Heuer, A.M.; Lin, H.Q.; Hanna, J.V.; Barbé, C.J. Biodegradability of sol–gel silica microparticles for drug delivery. J. Sol-Gel Sci. Technol. 2009, 49, 12–18. [Google Scholar] [CrossRef]
- Wetmur, J.G.; Davidson, N. Kinetics of renaturation of DNA. J. Mol. Biol. 1968, 31, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Shaik, C. Preventing counterfeit products using cryptography, qr code and webservice. Comput. Sci. Eng. Int. J. 2021, 11, 1. [Google Scholar] [CrossRef]
- Ullrich, C.; Luescher, A.M.; Koch, J.; Grass, R.N.; Sax, H. Silica nanoparticles with encapsulated DNA (SPED) to trace the spread of pathogens in healthcare. Antimicrob. Resist. Infect. Control. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.N.; Yeh, Q.-J.; Huang, C.-Y. Understanding consumer’ switching intention toward traceable agricultural products: Push-pull-mooring perspective. Int. J. Consum. Stud. 2022, 46, 870–888. [Google Scholar] [CrossRef]
- Nivala, J. Follow the barcoded microbes. Science 2020, 368, 1058–1059. [Google Scholar] [CrossRef]
- Doroschak, K.; Zhang, K.; Queen, M.; Mandyam, A.; Strauss, K.; Ceze, L.; Nivala, J. Rapid and robust assembly and decoding of molecular tags with DNA-based nanopore signatures. Nat. Commun. 2020, 11, 5454. [Google Scholar] [CrossRef]
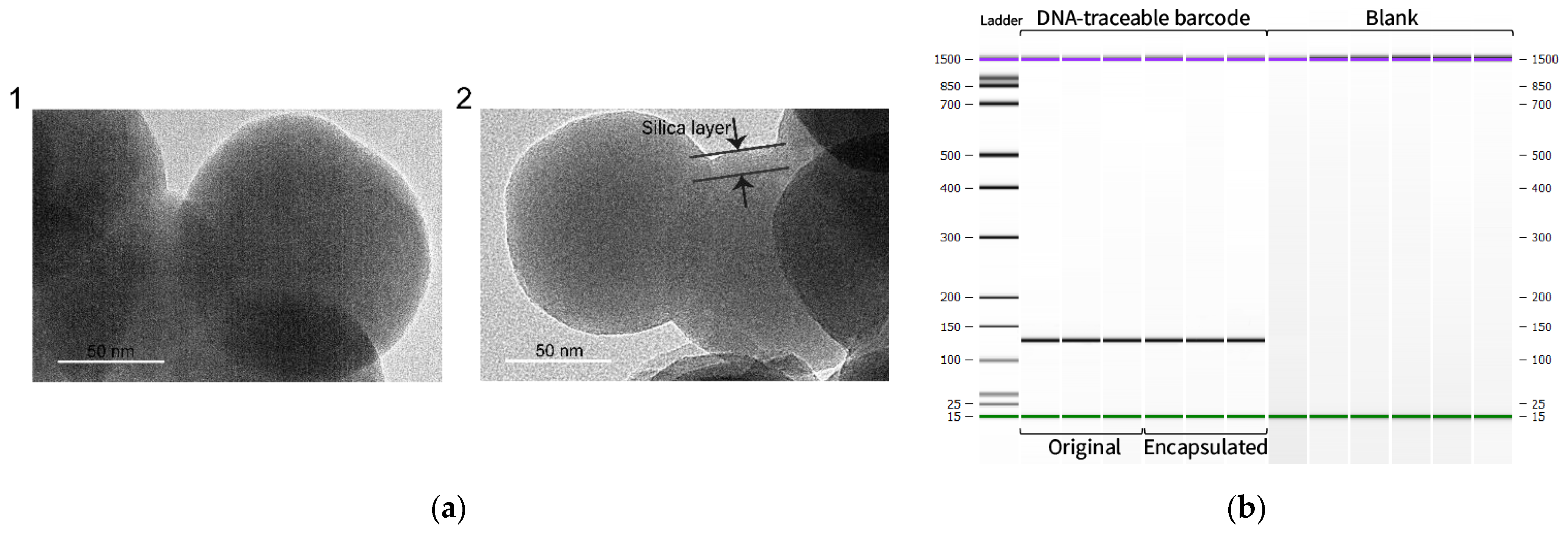
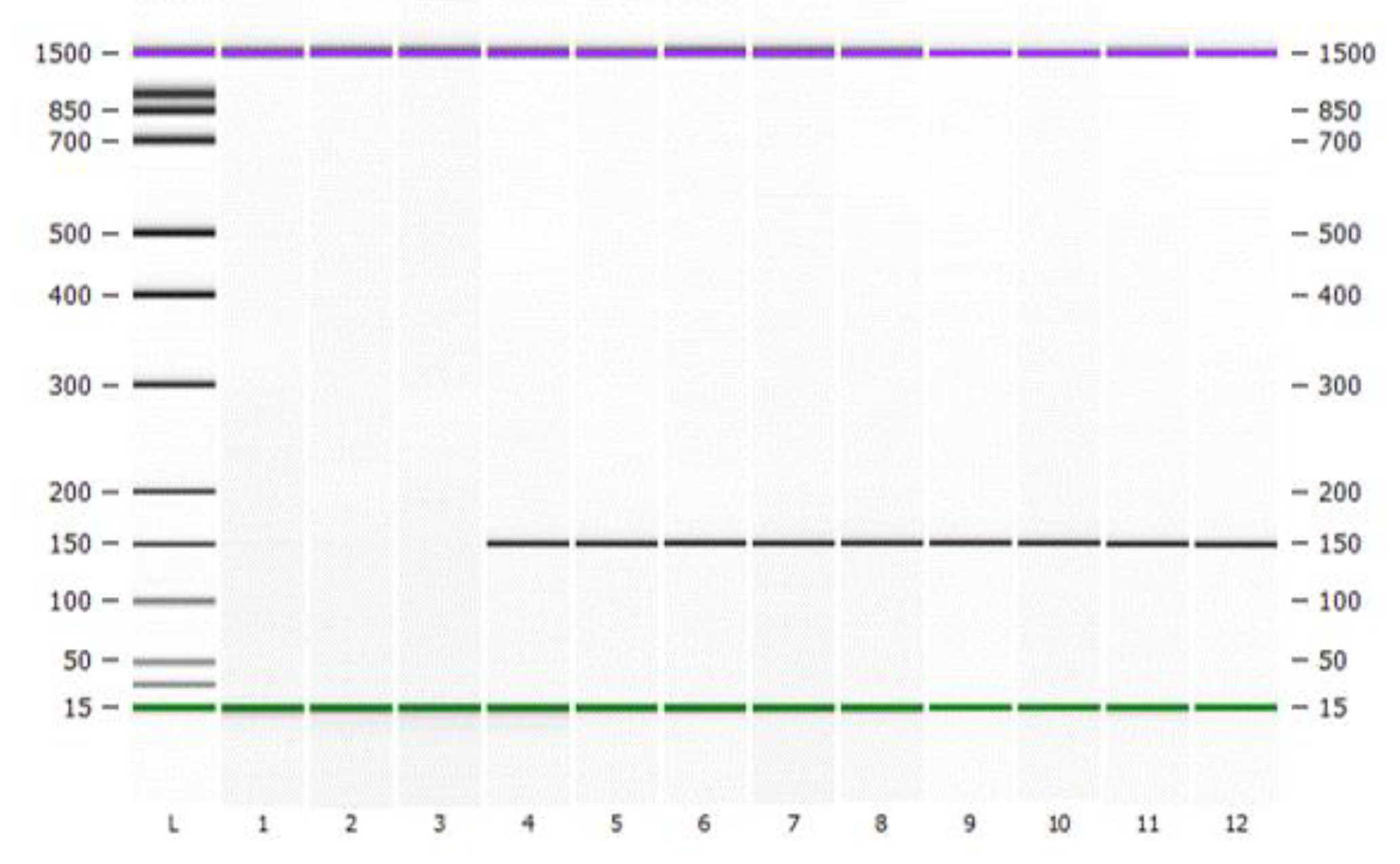
| Base Sequence | Length | Primer Name | Direction | Sequence (5′-3′) | Annealing Temperature (°C) | |
|---|---|---|---|---|---|---|
| Amplification solutions | GGCGTAGAAAGAGGGGTAGGACCCCCATCCTTTATTCTCCTTCTAGCCTCCGCAGAGGCGGTCCCGGTCTAAATCATGACAAGTATGACGATCTTCCTCAATCCT TGCCTCA | 112 bp | CAI-BF | Forward | GGCGTAGAAAGAGGGGTAGG | 60 °C |
| CAI-BR | Reverse | TTGAGGCAAGGATTGAGGAA | ||||
| Identification solutions | TAGAGATTGGCGTAGAAAGAGGGGTAGGACCCCCATCCTTTATTCTCCTTCTAGCCTCCGCAGAGGCGGTCCCGGTCTAAATCATGACAAGTATGACGATCTTCCTCAATCCT TGCCTCA | 120 bp | CAI-AF | Forward | GGCGTAGAAAGAGGGGTAGG | 60 °C |
| CAI-AR | Reverse | TTGAGGCAAGGATTGAGGAA | ||||
| CAI-BF | Forward | TTTGAGGCAAGGATTGAGGA | 62 °C | |||
| CAI-BR | Reverse | GTGGGAACAGGCTGAACTATATACC | ||||
| CAI-CF | Forward | TTTGAGGCAAGGATTGAGGA | / 1 | |||
| CAI-CR | Reverse | GTGGGAACAGGCTGAACTAT | ||||
| CAI-DF | Forward | TAGAGATTGGCGTAGAAGAGGGGT | 58 °C | |||
| CAI-DR | Reverse | TTGAGGCAAGGATTGAGGAAG | ||||
| M13-47 | Forward | AGCGGTAACAATTTCACACAGGA | 58 °C | |||
| RV-M | Reverse | CGCCAGGGTTTTCCCAGTCAGAC |
| Water (μL) | DNA Solution (μL) | Functionalized Particle Solution (μL) | Release of DNA-Traceable Barcode Concentration (ng/µL) | Release of DNA-Traceable Barcode Purity | ||
|---|---|---|---|---|---|---|
| A | 820 | 190 | 10 | / 1 | / 1 | / 1 |
| B | 820 | 190 | 20 | 10.3 ± 0.8 | 1.21 ± 0.86 | 1.82 ± 0.02 |
| C | 820 | 190 | 20 | 17.3 ± 5.8 | 1.83 ± 0.02 | 1.56 ± 0.04 |
| D | 820 | 190 | 40 | 16.7 ± 2.4 | 1.55 ± 0.02 | 1.86 ± 0.03 |
| E | 820 | 190 | 50 | 42.3 ± 4.7 | 1.84 ± 0.03 | 1.87 ± 0.10 |
| F | 820 | 190 | 60 | 54.2 ± 4.8 | 1.82 ± 0.06 | 2.10 ± 0.09 |
| G | 820 | 190 | 70 | 67.2 ± 13.5 | 1.85 ± 0.04 | 1.91 ± 0.16 |
| H | 820 | 190 | 80 | 26.9 ± 5.2 | 1.72 ± 0.09 | 1.38 ± 0.05 |
| G1 | 820 | 220 | 70 | 82.6 ± 6.6 | 1.82 ± 0.06 | 2.16 ± 0.15 |
| G2 | 820 | 250 | 70 | 91.9 ± 1.8 | 1.83 ± 0.05 | 2.14 ± 0.14 |
| G3 | 820 | 280 | 70 | 92.1 ± 9.6 | 1.74 ± 0.03 | 1.80 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Xing, R.; Sun, R.; Ge, Y.; Chen, Y. An Accurate and Rapid Way for Identifying Food Geographical Origin and Authenticity: Editable DNA-Traceable Barcode. Foods 2023, 12, 17. https://doi.org/10.3390/foods12010017
Liu K, Xing R, Sun R, Ge Y, Chen Y. An Accurate and Rapid Way for Identifying Food Geographical Origin and Authenticity: Editable DNA-Traceable Barcode. Foods. 2023; 12(1):17. https://doi.org/10.3390/foods12010017
Chicago/Turabian StyleLiu, Kehan, Ranran Xing, Ruixue Sun, Yiqiang Ge, and Ying Chen. 2023. "An Accurate and Rapid Way for Identifying Food Geographical Origin and Authenticity: Editable DNA-Traceable Barcode" Foods 12, no. 1: 17. https://doi.org/10.3390/foods12010017




