The Milk Active Ingredient, 2′-Fucosyllactose, Inhibits Inflammation and Promotes MUC2 Secretion in LS174T Goblet Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Cellular Inflammatory Model Construction by TNF-α Treatment
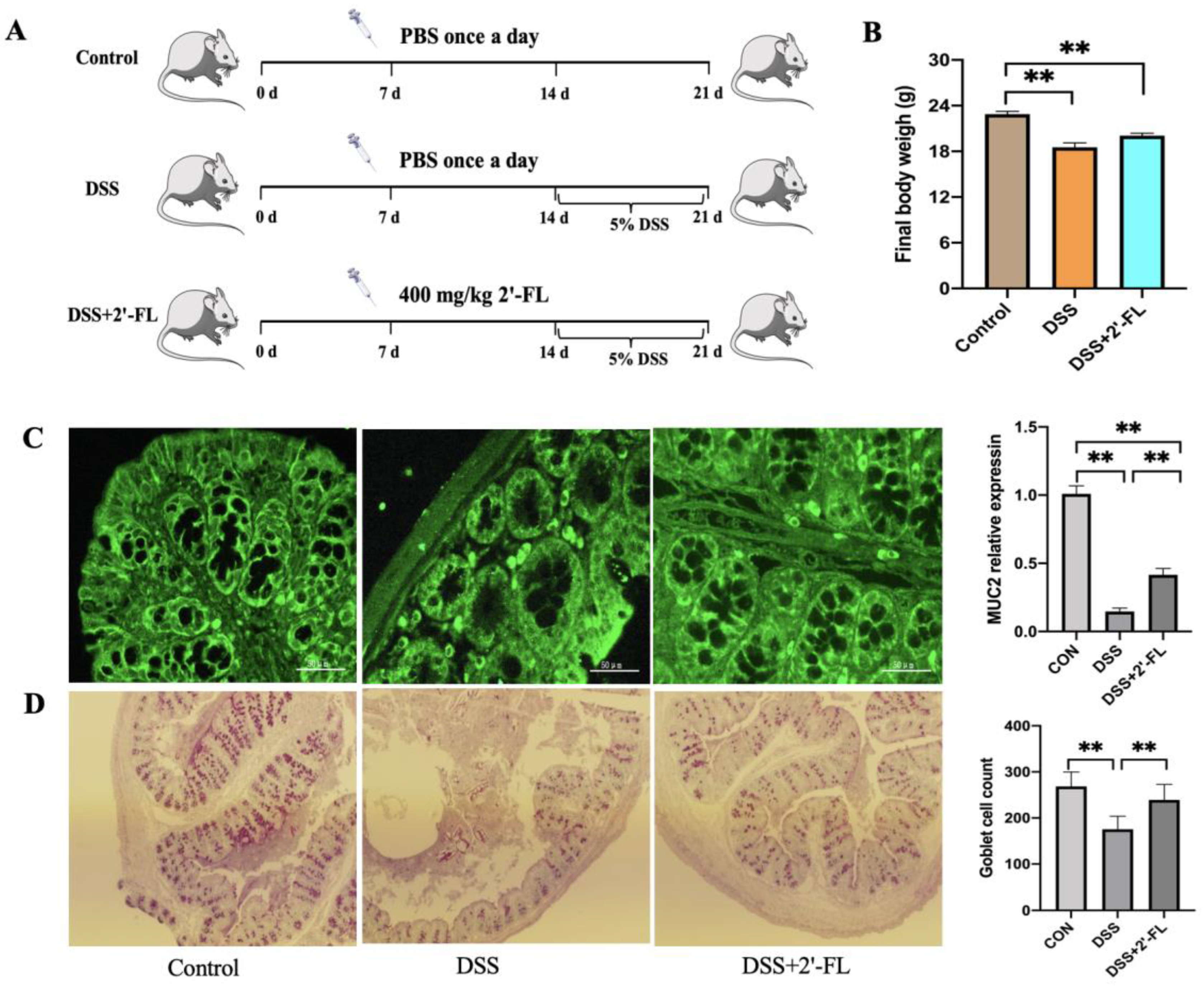
2.4. Animal Model
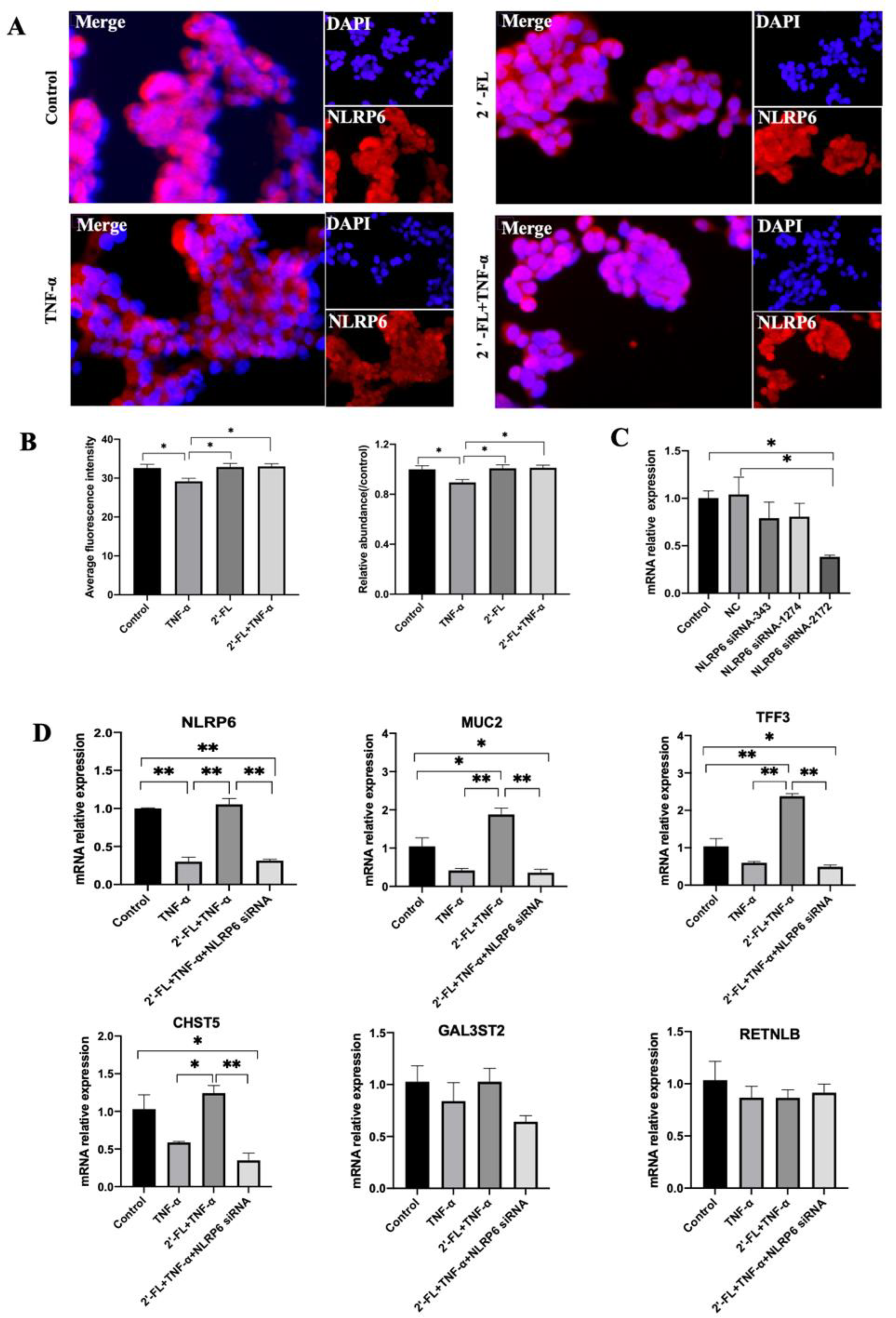
2.5. siRNA Gene Treatment
2.6. Cell Immunofluorescence Staining
2.7. Total RNA Extraction and Gene Expression Detection
2.8. Statistical Analysis
3. Results
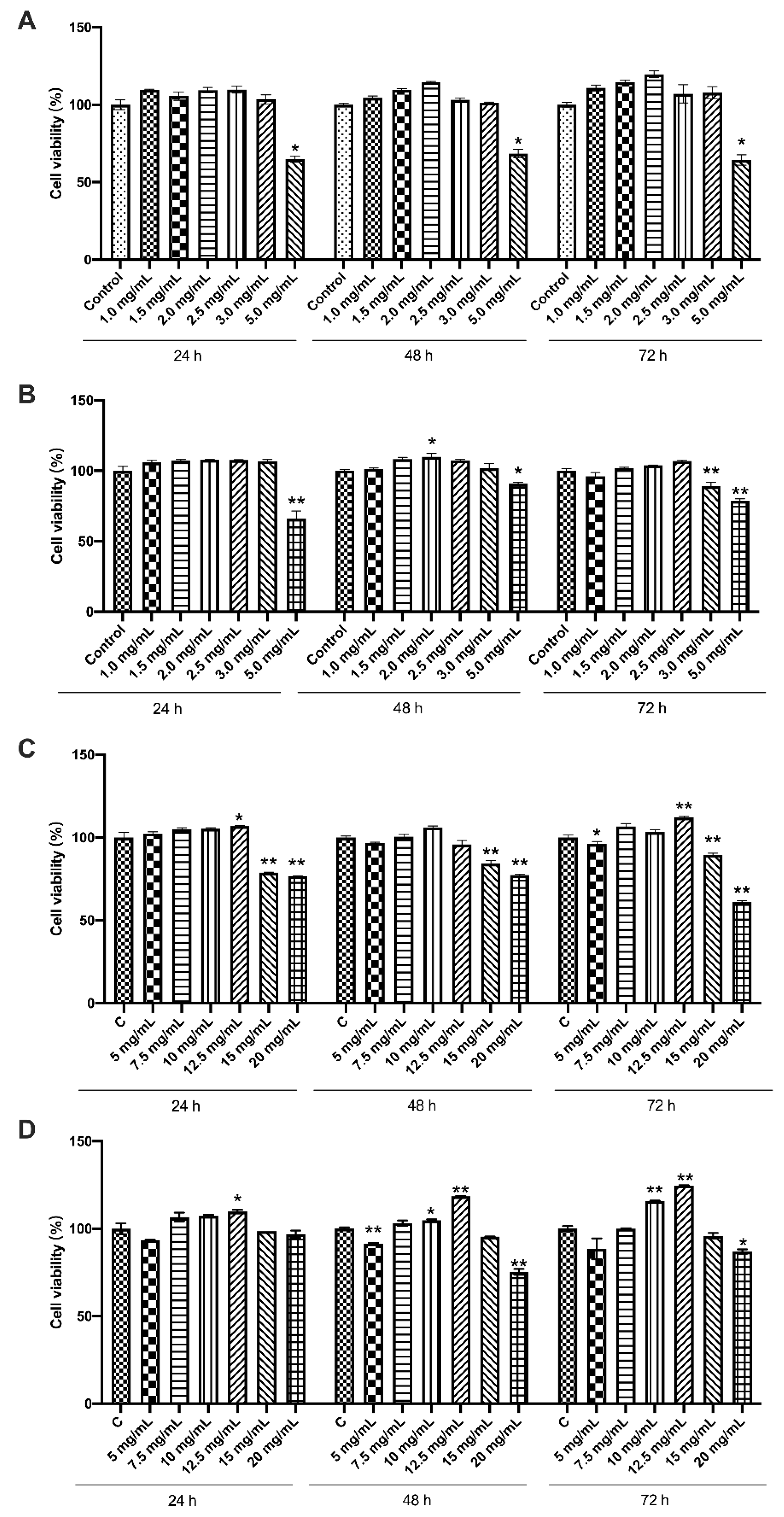
3.1. Effects of the Four Chemicals on Cell Viability of LS174T Cells
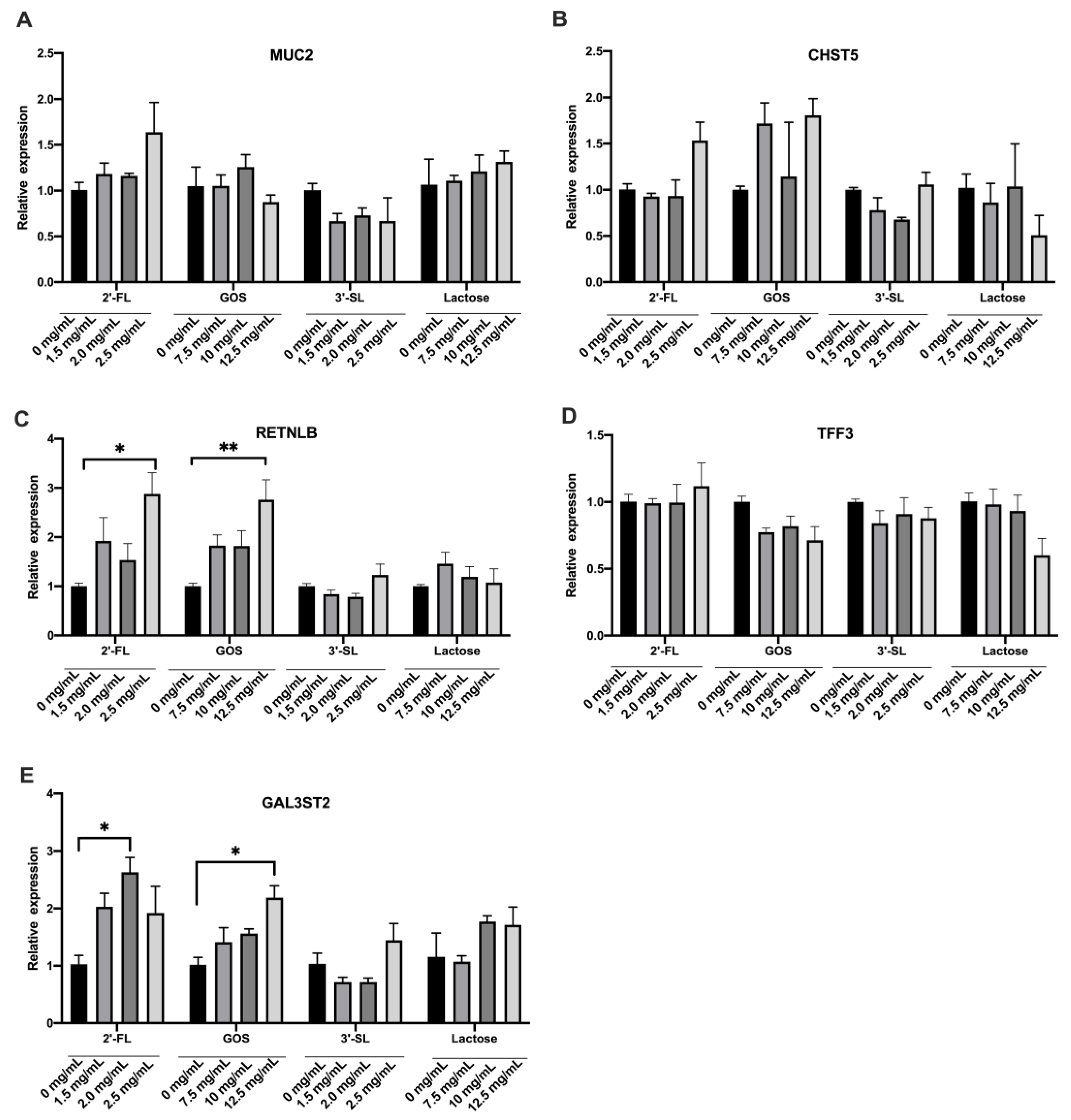
3.2. Effects of the Four Chemicals on Mucin Secretion of LS174T Cells under Normal Condition
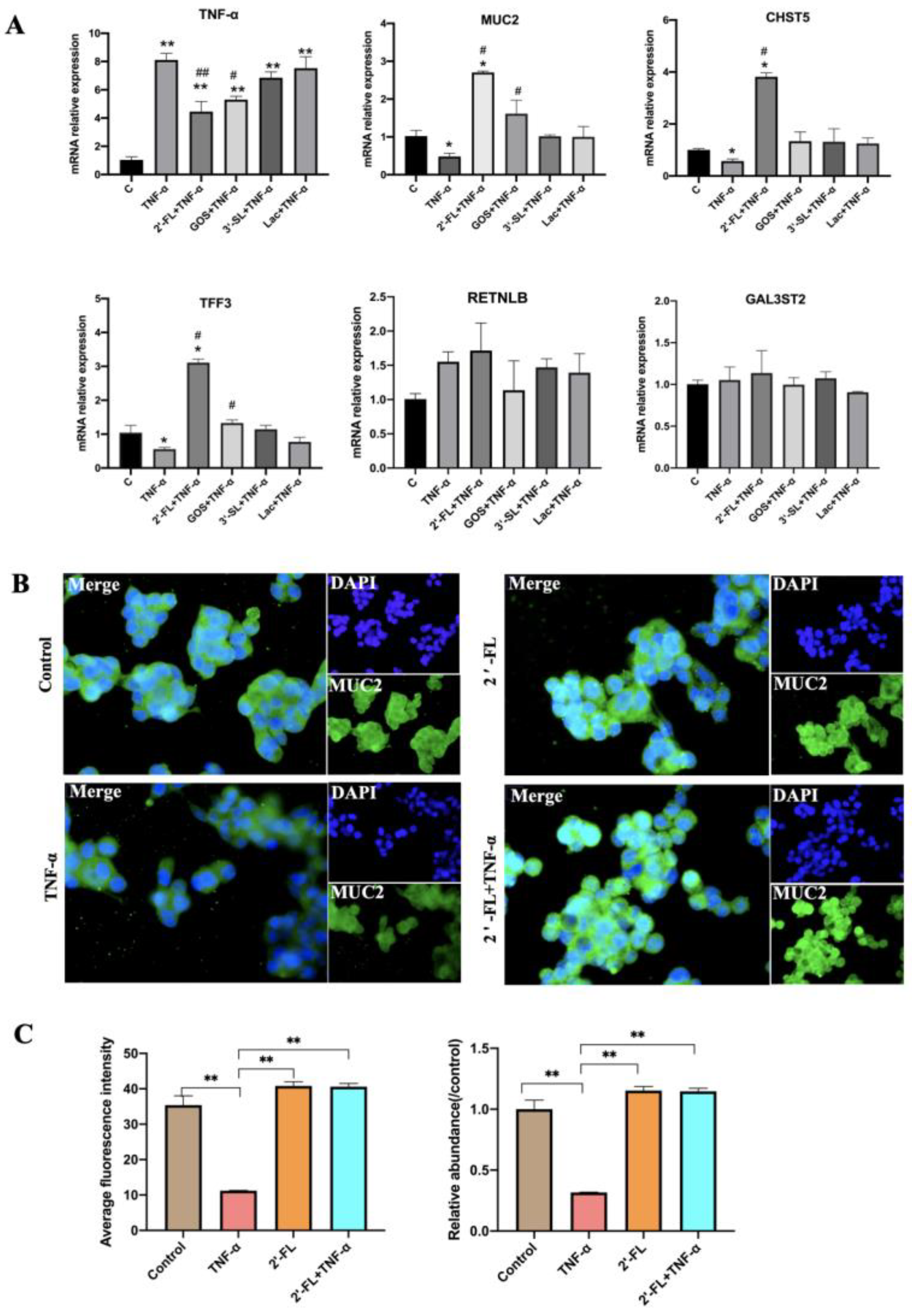
3.3. Effects of the Four Chemicals on MUC2-Related Gene Expression under an Inflammatory Condition
3.4. 2′-FL Enhanced MUC2 Secretion in DSS-Induced Colitis Mice
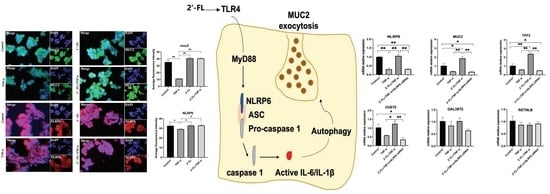
3.5. NLRP6 Is Necessary for MUC2 Secretion
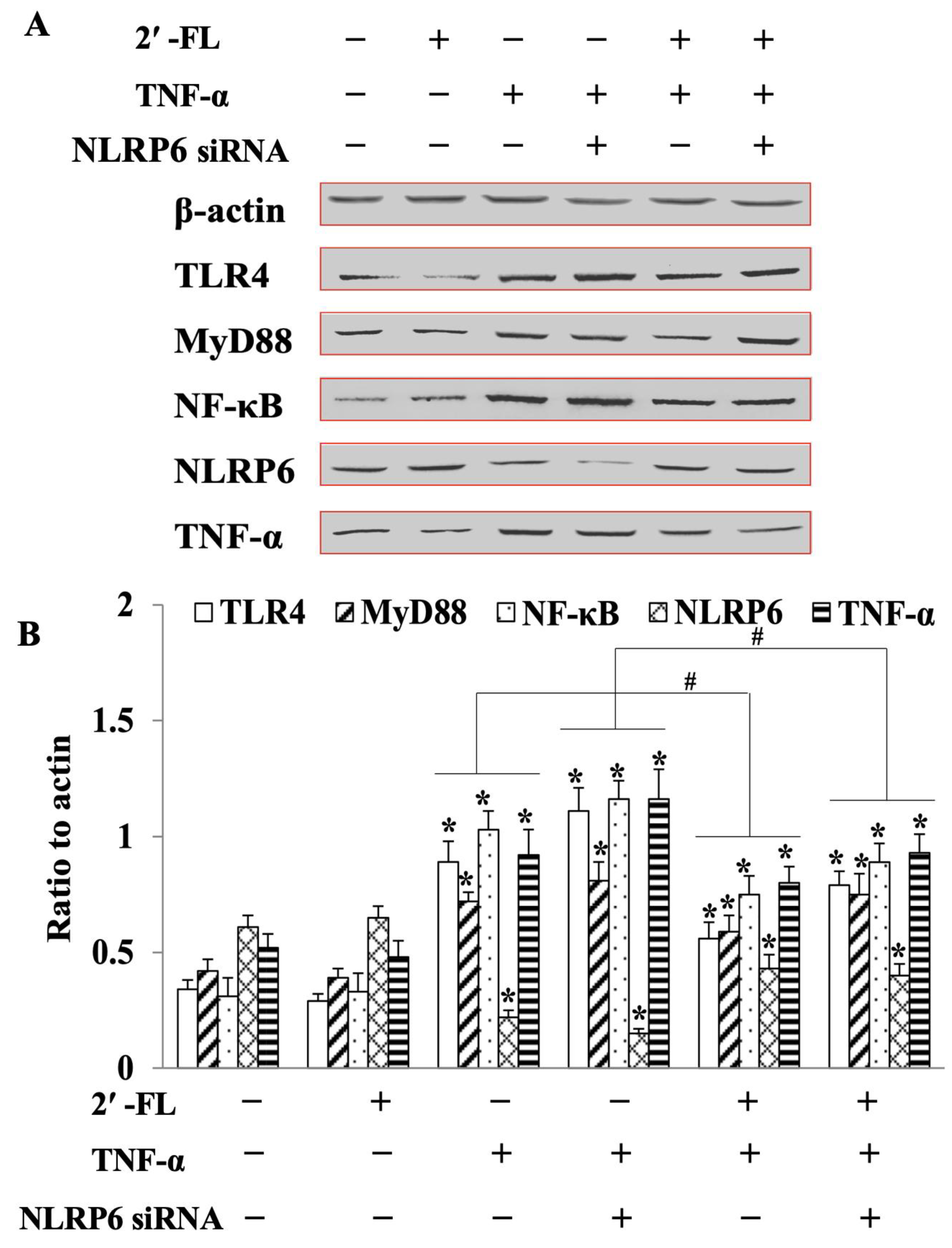
3.6. 2′-FL Suppression of TNF-Induced Inflammation via Regulating the TLR4/MyD88/NF-κB Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Torres Roldan, V.D.; Urtecho, S.M.; Gupta, J.; Yonemitsu, C.; Cárcamo, C.P.; Bode, L.; Ochoa, T.J. Human milk oligosaccharides and their association with late-onset neonatal sepsis in Peruvian very-low-birth-weight infants. Am. J. Clin. Nutr. 2020, 112, 106–112. [Google Scholar] [CrossRef]
- Nolan, L.S.; Rimer, J.M.; Good, M. The role of human milk oligosaccharides and probiotics on the neonatal microbiome and risk of necrotizing enterocolitis: A narrative review. Nutrients 2020, 12, 3052. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Hirabayashi, J.; Sato, S.; Kobata, A. Human milk oligosaccharides as essential tools for basic and application studies on galectins. Trends Glycosci. Glycotechnol. 2018, 30, SE51–SE65. [Google Scholar] [CrossRef] [Green Version]
- Bering, S.B. Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates. Nutrients 2018, 10, 1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, M.; Sodhi, C.P.; Yamaguchi, Y.; Jia, H.; Lu, P.; Fulton, W.B.; Martin, L.Y.; Prindle, T.; Nino, D.F.; Zhou, Q.; et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental Necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 2016, 116, 1175–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thum, C.; Cookson, A.; McNabb, W.C.; Roy, N.C.; Otter, D. Composition and enrichment of caprine milk oligosaccharides from New Zealand Saanen goat cheese whey. J. Food Compos. Anal. 2015, 42, 30.e37. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Sandhu, Y.K.; Korzun, W.J.; Ghosh, S. Dietary supplementation with galactooligosaccharides attenuates high-fat, high-cholesterol diet-induced glucose intolerance and disruption of colonic mucin layer in C57BL/6 mice and reduces atherosclerosis in Ldlr-/-mice. J. Nutr. 2020, 150, 285–293. [Google Scholar] [CrossRef]
- Arike, L.; Hansson, G.C. The densely O-glycosylated MUC2 mucin protects the intestine and provides food for the commensal bacteria. J. Mol. Biol. 2016, 428, 3221–3229. [Google Scholar] [CrossRef] [Green Version]
- Blacher, E.; Levy, M.; Tatirovsky, E.; Elinav, E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J. Immunol. 2017, 198, 572–580. [Google Scholar] [CrossRef]
- Ge, H.; Gardner, J.; Wu, X.; Rulifson, I.; Wang, J.; Xiong, Y.; Ye, J.; Belouski, E.; Cao, P.; Tang, J.; et al. Trefoil Factor 3 (TFF3) is regulated by food intake, improves glucose tolerance and induces mucinous metaplasia. PloS ONE 2015, 10, e0126924. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.Z.; Dawson, P.A.; Lourie, R.; Hutson, P.; Tong, H.; Grencis, R.K.; McGuckin, M.A.; Thornton, D.J. Immune-driven alterations in mucin sulphation is an important mediator of Trichuris muris helminth expulsion. PLoS Pathog. 2017, 13, e1006218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morampudi, V.; Dalwadi, U.; Bhinder, G.; Sham, H.P.; Gill, S.K.; Chan, J.; Bergstrom, K.S.; Huang, T.; Ma, C.; Jacobson, K.; et al. The goblet cell-derived mediator RELM-β drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016, 9, 1218–1233. [Google Scholar] [CrossRef] [Green Version]
- Wlodarska, M.; Thaiss, C.A.; Nowarski, R.; Henao-Mejia, J.; Zhang, J.P.; Brown, E.M.; Frankel, G.; Levy, M.; Katz, M.N.; Philbrick, W.M.; et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 2014, 156, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Fan, L.; Zheng, N.; Blecker, C.; Delcenserie, V.; Li, H.; Wang, J. 2'-Fucosyllactose Ameliorates Inflammatory Bowel Disease by Modulating Gut Microbiota and Promoting MUC2 Expression. Front. Nutr. 2022, 9, 822020. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Lozano, S.; Akkerman, R.; Beukema, M.; van Leeuwen, S.S.; Dijkhuizen, L.; de Vos, P. 2′-Fucosyllactose impacts the expression of mucus-related genes in goblet cells and maintains barrier function of gut epithelial cells. J. Funct. Foods 2021, 85, 104630. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2'-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Kong, C.; Walvoort, M.T.C.; Faas, M.M.; de Vos, P. Human milk oligosaccharides differently modulate goblet cells under homeostatic, proinflammatory conditions and ER stress. Mol. Nutr. Food Res. 2019, 64, e1900976. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C.(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.; Nyström, E.E.; Johansson, M.E.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering NLRP6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef]
- Chambers, S.A.; Townsend, S.D. Like mother, like microbe: Human milk oligosaccharide mediated microbiome symbiosis. Biochem. Soc. Trans. 2020, 48, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Moubareck, C.A. Human milk microbiota and oligosaccharides: A glimpse into benefits, diversity, and correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.R.; Chow, J.M.; Buck, R.H. Multifunctional benefits of prevalent HMOs: Implications for infant health. Nutrients 2021, 13, 3364. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.Y.; Li, B.; Koike, Y.; Määttänen, P.; Miyake, H.; Cadete, M.; Johnson-Henry, K.C.; Botts, S.R.; Lee, C.; Abrahamsson, T.R.; et al. Human milk oligosaccharides increase mucin expression in experimental necrotizing enterocolitis. Mol. Nutr. Food Res. 2019, 63, e1800658. [Google Scholar] [CrossRef]
- Buda, A.; Jepson, M.A.; Pignatelli, M. Regulatory function of trefoil peptides (TFF) on intestinal cell junctional complexes. Cell Commun. Adhes. 2012, 19, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Pisa, E.; Martire, A.; Chiodi, V.; Traversa, A.; Caputo, V.; Hauser, J.; Macrì, S. Exposure to 3'Sialyllactose-poor milk during lactation impairs cognitive capabilities in adulthood. Nutrients 2021, 13, 4191. [Google Scholar] [CrossRef]
- Piotrowski, M.; Wultańska, D.; Pituch, H. The prebiotic effect of human milk oligosaccharides 3’- and 6’-sialyllactose on adhesion and biofilm formation by Clostridioides difficile-pilot study. Microbes and Infection 2022, 24, 104929. [Google Scholar] [CrossRef]
- Jeon, J.; Kang, L.J.; Lee, K.M.; Cho, C.; Song, E.K.; Kim, W.; Park, T.J.; Yang, S. 3'-Sialyllactose protects against osteoarthritic development by facilitating cartilage homeostasis. J. Cell. Mol. Med. 2018, 22, 57–66. [Google Scholar] [CrossRef]
- Kang, L.J.; Oh, E.; Cho, C.; Kwon, H.; Lee, C.G.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Nam, J.; et al. 3'-Sialyllactose prebiotics prevents skin inflammation via regulatory T cell differentiation in atopic dermatitis mouse models. Sci. Rep. 2020, 10, 5603. [Google Scholar] [CrossRef]
- Cederlund, A.; Kai-Larsen, Y.; Printz, G.; Yoshio, H.; Alvelius, G.; Lagercrantz, H.; Strömberg, R.; Jörnvall, H.; Gudmundsson, G.H.; Agerberth, B. Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS ONE 2013, 8, e53876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa-Lozano, S.; Ren, C.; Yin, H.; Pham, H.; van Leeuwen, S.; Dijkhuizen, L.; de Vos, P. The impact of oligosaccharide content, glycosidic linkages and lactose content of galacto-oligosaccharides (GOS) on the expression of mucus-related genes in goblet cells. Food Funct. 2020, 11, 3506–3515. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating nlrp6 inflammasome signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Hu, J.; Cheng, C.; Xu, F.; Au, R.; Zhu, L.; Shen, H. Baicalin ameliorates DSS-Induced colitis by protecting goblet cells through activating NLRP6 inflammasomes. Evid.-Based Complement. Altern. Med. 2022, 2022, 2818136. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, L.; Lu, P.; Li, X.; Tian, D.; Liu, M. NLRP6 exerts a protective role via NF-kB with involvement of CCL20 in a mouse model of alcoholic hepatitis. Biochem. Biophys. Res. Commun. 2020, 528, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Wipf, P.; Yamaguchi, Y.; Fulton, W.B.; Kovler, M.; Niño, D.F.; Zhou, Q.; Banfield, E.; Werts, A.D.; Ladd, M.R.; et al. The human milk oligosaccharides 2'-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr. Res. 2021, 89, 91–101. [Google Scholar] [CrossRef]
| Genes | Forward Primer Sequence (5′→3′) | Reverse Primer Sequence (5′→3′) |
|---|---|---|
| CHST5 | CCC AGT GAG GAA CTG GTC TTC | ATC TGT GTT CCA GGA AAG CC |
| GAL3ST2 | TGG GCG GCT TGC AGA GAT A | GCT CTA AGT CCG AGT GCA GGA |
| MUC2 | AAC ACA GTC CTG GTG GAA GG | CAT TGT CAG GTC CCA CAC AG |
| RETNLB | CAC CCA GGA GCT CAG AGA TCT AA | ACG GCC CCA TCC TGT ACA |
| TFF3 | CAT GTC ACC CCC AAG GAG TG | AGG TGC ATT CTG CTT CCT GC |
| GADPH | AAG ATC ATC AGC AAT GCC TCC TGC | ATG GAC TGT GGT CAT GAG TCC TTC |
| NLRP6-1374 | GCA GUU UGC CGA GAA GGA ATT | UUC CUU CUC GGC AAA CUG CTT |
| NLRP6-2142 | CCU UCU UCA UCC ACU CUU UTT | AAA GAG UGG AUG AAG AAG GTT |
| NLRP6-343 | GUG UCC GAG UAC AAG AAG ATT | UCU UCU UGU ACU CGG ACA CTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Q.; Li, H.; Gao, Y.; Zheng, N.; Delcenserie, V.; Wang, J. The Milk Active Ingredient, 2′-Fucosyllactose, Inhibits Inflammation and Promotes MUC2 Secretion in LS174T Goblet Cells In Vitro. Foods 2023, 12, 186. https://doi.org/10.3390/foods12010186
Yao Q, Li H, Gao Y, Zheng N, Delcenserie V, Wang J. The Milk Active Ingredient, 2′-Fucosyllactose, Inhibits Inflammation and Promotes MUC2 Secretion in LS174T Goblet Cells In Vitro. Foods. 2023; 12(1):186. https://doi.org/10.3390/foods12010186
Chicago/Turabian StyleYao, Qianqian, Huiying Li, Yanan Gao, Nan Zheng, Véronique Delcenserie, and Jiaqi Wang. 2023. "The Milk Active Ingredient, 2′-Fucosyllactose, Inhibits Inflammation and Promotes MUC2 Secretion in LS174T Goblet Cells In Vitro" Foods 12, no. 1: 186. https://doi.org/10.3390/foods12010186