Helium Atmospheric Pressure Plasma Jet Effects on Two Cultivars of Triticum aestivum L.
Abstract
1. Introduction
2. Materials and Methods
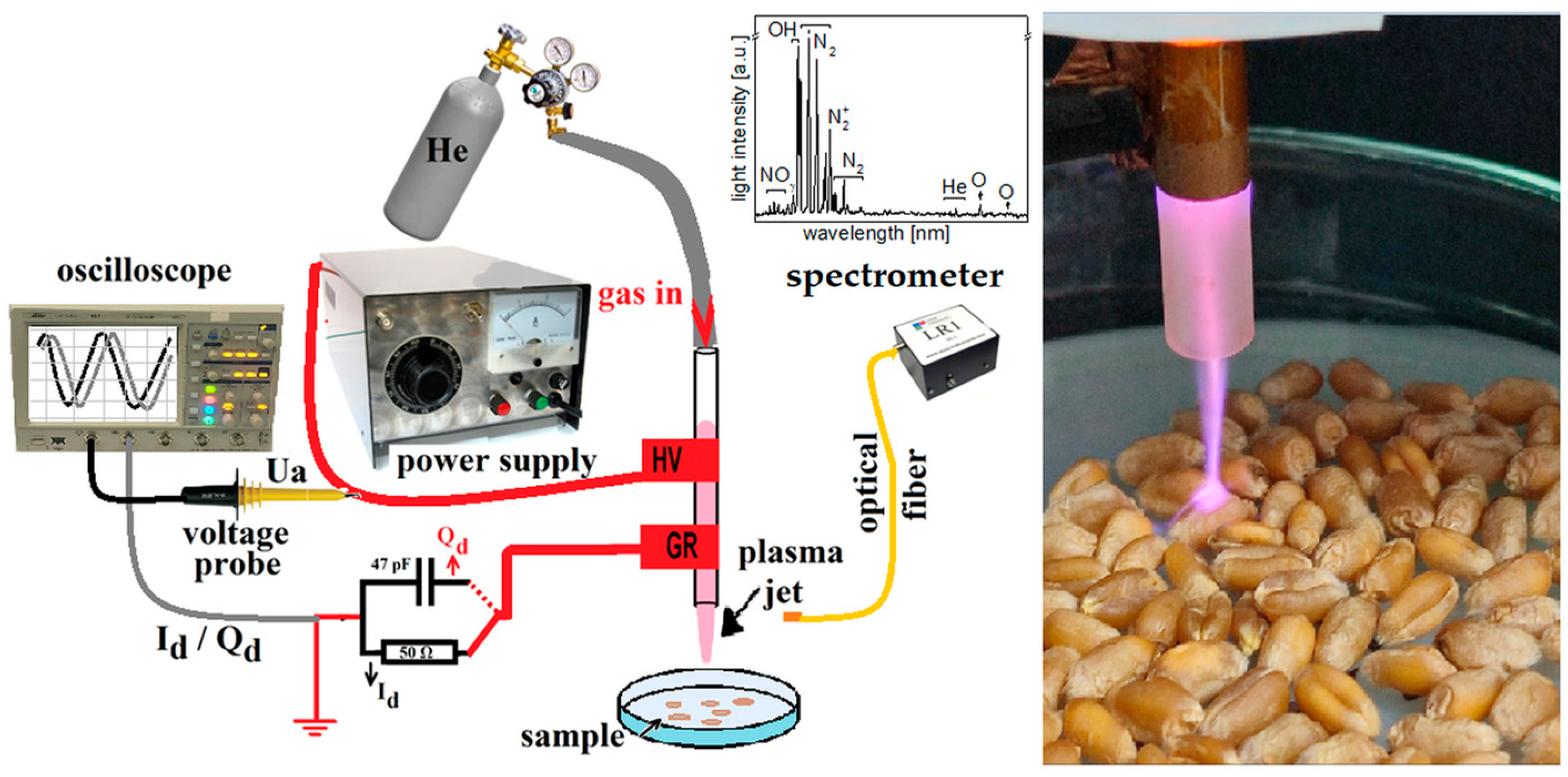
2.1. Plasma Source and Electro-Optical Diagnosis
2.2. Seed Characterization, Germination, and Plant Growth Parameters
2.2.1. Wheat Grass Characterization: Germination and Plant Growth
The Color Parameters of Wheat Grass
Nutritional Composition of the Wheat Grass
2.2.2. Wheat Seeds Characterization Techniques
Atomic Force Microscopy
Scanning Electron Microscopy
X-ray Computer Tomography of Wheat Seeds
Particle-Induced X-ray Emission
Statistical Analyses
3. Results
3.1. Plasma Source Electro-Optical Characterization
3.2. Micro-Morphological Characterization of Wheat Seeds Exposed to Atmospheric Pressure Plasma
3.2.1. AFM Surface Morphology
3.2.2. SEM-EDX Characterization
3.2.3. X-ray Computer Tomography
3.2.4. PIXE Elemental Analysis
3.3. Seed Germination and Plant Growth Parameters
3.4. Biochemical Composition of Wheat Grass
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khanal, U.; Wilson, C.; Rahman, S.; Lee, B.L.; Hoang, V.-N. Smallholder farmers’ adaptation to climate change and its potential contribution to UN’s sustainable development goals of zero hunger and no poverty. J. Clean. Prod. 2021, 281, 124999. [Google Scholar] [CrossRef]
- Saccone, D. Can the COVID-19 pandemic affect the achievement of the ‘Zero Hunger’ goal? Some preliminary reflections. Eur. J. Health Econ. 2021, 22, 1025–1038. [Google Scholar] [CrossRef]
- Ben Hassen, T.; El Bilali, H. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2017, 15, 1700174. [Google Scholar] [CrossRef]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Baturo-Cieśniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; Muhire, J.D.D.; et al. Can Cold Plasma Be Used for Boosting Plant Growth and Plant Protection in Sustainable Plant Production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of Plasma-Seed Treatments as a Potential Seed Processing Technology. Front. Phys. 2021, 9, 174. [Google Scholar] [CrossRef]
- Leti, L.-I.; Gerber, I.C.; Mihaila, I.; Galan, P.-M.; Strajeru, S.; Petrescu, D.-E.; Cimpeanu, M.-M.; Topala, I.; Gorgan, D.-L. The Modulatory Effects of Non-Thermal Plasma on Seed’s Morphology, Germination and Genetics—A Review. Plants 2022, 11, 2181. [Google Scholar] [CrossRef]
- Le, T.Q.X.; Nguyen, L.N.; Nguyen, T.T.; Choi, E.H.; Nguyen, Q.L.; Kaushik, N.K.; Dao, N.T. Effects of Cold Plasma Treatment on Physical Modification and Endogenous Hormone Regulation in Enhancing Seed Germination and Radicle Growth of Mung Bean. Appl. Sci. 2022, 12, 10308. [Google Scholar] [CrossRef]
- Grainge, G.; Nakabayashi, K.; Iza, F.; Leubner-Metzger, G.; Steinbrecher, T. Gas-Plasma-Activated Water Impact on Photo-Dependent Dormancy Mechanisms in Nicotiana tabacum Seeds. Int. J. Mol. Sci. 2022, 23, 6709. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Gerling, T. Cold Atmospheric Pressure Plasma Jet Operated in Ar and He: From Basic Plasma Properties to Vacuum Ultraviolet, Electric Field and Safety Thresholds Measurements in Plasma Medicine. Appl. Sci. 2022, 12, 644. [Google Scholar] [CrossRef]
- Huzum, R.; Nastuta, A.V. Helium Atmospheric Pressure Plasma Jet Source Treatment of White Grapes Juice for Winemaking. Appl. Sci. 2021, 11, 8498. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Sakiyama, Y.; Graves, D.B.; Zimmermann, J.L.; Morfill, G.E. The dynamics of ozone generation and mode transition in air surface micro-discharge plasma at atmospheric pressure. New J. Phys. 2012, 10, 103028. [Google Scholar] [CrossRef]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non-Thermal Atmospheric-Pressure Plasma. Front Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef]
- Mildaziene, V.; Sera, B. Effects of Non-Thermal Plasma Treatment on Plant Physiological and Biochemical Processes. Plants 2022, 11, 1018. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.F.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef]
- Mildaziene, V.; Pauzaite, G.; Naucienė, Z.; Malakauskiene, A.; Zukiene, R.; Januskaitiene, I.; Lyushkevich, V. Pre-sowing seed treatment with cold plasma and electromagnetic field increases secondary metabolite content in purple coneflower (Echinacea purpurea) leaves. Plasma Process. Polym. 2017, 15, 1700059. [Google Scholar] [CrossRef]
- Ghahremaninejad, F.; Hoseini, E.; Jalali, S. The cultivation and domestication of wheat and barley in Iran, brief review of a long history. Bot. Rev. 2021, 87, 1–22. [Google Scholar] [CrossRef]
- Burducea, M.; Dincheva, I.; Dirvariu, L.; Oprea, E.; Zheljazkov, V.D.; Barbacariu, C.-A. Wheat and Barley Grass Juice Addition to a Plant-Based Feed Improved Growth and Flesh Quality of Common Carp (Cyprinus carpio). Animals 2022, 12, 1046. [Google Scholar] [CrossRef]
- Burducea, M.; Lobiuc, A.; Dirvariu, L.; Oprea, E.; Olaru, S.M.; Teliban, G.-C.; Stoleru, V.; Poghirc, V.A.; Cara, I.G.; Filip, M.; et al. Assessment of the Fertilization Capacity of the Aquaculture Sediment for Wheat Grass as Sustainable Alternative Use. Plants 2022, 11, 634. [Google Scholar] [CrossRef]
- Burducea, M.; Lobiuc, A.; Asandulesa, M.; Zaltariov, M.-F.; Burducea, I.; Popescu, S.M.; Zheljazkov, V.D. Effects of Sewage Sludge Amendments on the Growth and Physiology of Sweet Basil. Agronomy 2019, 9, 548. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Phys. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
- Burducea, I.; Ionescu, C.; Straticiuc, M.; Craciun, L.S.; Racolta, P.M.; Jipa, A.L. Characterization of Indium Nitride and Zinc Oxide Thin Films by AFM and RBS. Rom. Journ. Phys. 2013, 58, 345–353. [Google Scholar]
- Mereuta, P.; Constantinescu, B.; Cristea-Stan, D.; Serbanescu, D. SEM-EDS as Investigation Tool for Archaeological Artifacts—The Case of Nephrite Adornments. Rom. Rep. Phys. 2019, 71, 802. [Google Scholar]
- Opris, V.; Petruneac, M.; Focșăneanu, M.; Sîrbu, R.; Boroneant, A.; Golea, M.; Bonsall, C. Early Neolithic pottery at Schela Cladovei. A comparative study of archaeological and experimental vessels from the perspective of computed tomography. In Recreating Artefacts and Ancient Skills: From Experiment to Interpretation; Mărgărit, M., Boroneanț, A., Eds.; Cetatea de Scaun: Targoviste, Romania, 2022. [Google Scholar]
- Burducea, I.; Straticiuc, M.; Ghita, D.G.; Mosu, D.V.; Calinescu, C.I.; Podaru, N.C.; Mous, D.J.W.; Ursu, I.; Zamfir, N.V. A new ion beam facility based on a 3 MV Tandetron™ at IFIN-HH, Romania. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 359, 12–19. [Google Scholar] [CrossRef]
- Teliban, G.-C.; Burducea, M.; Mihalache, G.; Zheljazkov, V.D.; Dincheva, I.; Badjakov, I.; Popa, L.-D.; Bodale, I.; Vlăduț, N.-V.; Cojocaru, A.; et al. Morphological, Physiological and Quality Performances of Basil Cultivars under Different Fertilization Types. Agronomy 2022, 12, 3219. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Topala, I.; Grigoras, C.; Pohoata, V.; Popa, G. Stimulation of wound healing by helium atmospheric pressure plasma treatment. J. Phys. D Appl. Phys. 2011, 44, 105204. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Pohoata, V.; Topala, I. Atmospheric pressure plasma jet—Living tissue interface: Electrical, optical, and spectral characterization. J. App. Phys. 2013, 113, 183302. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Topala, I.; Pohoata, V.; Mihaila, I.; Agheorghiesei, C.; Dumitrascu, N. Atmospheric pressure plasma jets in inert gases: Electrical, optical and mass spectrometry diagnosis. Rom. Rep. Phys. 2017, 69, 407. [Google Scholar]
- Nastuta, A.V.; Popa, G. Surface oxidation and enhanced hydrophilization of polyamide fiber surface after He/Ar atmospheric pressure plasma exposure. Rom. Rep. Phys. 2019, 71, 413. [Google Scholar]
- Molina, R.; Lalueza, A.; Lopez-Santos, C.; Ghobeira, R.; Cools, P.; Morent, R.; Geyter, N.; Gonzalez-Elipe, A.R. Physicochemical surface analysis and germination at different irrigation conditions of DBD plasma-treated wheat seeds. Plasma Process. Polym. 2020, 18, 2000086. [Google Scholar] [CrossRef]
- Lineback, D.R.; Cashman, W.E.; Hoseney, R.C.; Ward, A.B. Note on measuring thickness of wheat bran by scanning electron microscopy. Cereal Chem. 1978, 55, 415–419. [Google Scholar]
- Starič, P.; Mravlje, J.; Mozetič, M.; Zaplotnik, R.; Šetina Batič, B.; Junkar, I.; Vogel Mikuš, K. The Influence of Glow and Afterglow Cold Plasma Treatment on Biochemistry, Morphology, and Physiology of Wheat Seeds. Int. J. Mol. Sci. 2022, 23, 7369. [Google Scholar] [CrossRef] [PubMed]
- Saberi, M.; Ghomi, H.; Andreasen, C. Eco-friendly approach to improve traits of winter wheat by combining cold plasma treatments and carbonization of subtropical biomass waste. Sci. Rep. 2022, 12, 11218. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Advantages and Limitations of Surface Analysis Techniques on Plasma-Treated Arabidopsis thaliana Seeds. Front. Mater. 2021, 8, 123. [Google Scholar] [CrossRef]
- Cui, D.J.; Yin, Y.; Wang, J.Q.; Wang, Z.W.; Ding, H.B.; Ma, R.N.; Jiao, Z. Research on the Physio-Biochemical Mechanism of Non-Thermal Plasma-Regulated Seed Germination and Early Seedling Development in Arabidopsis. Front. Plant Sci. 2019, 10, 1322. [Google Scholar] [CrossRef]
- Molina, R.; López-Santos, C.; Gómez-Ramírez, A.; Vílchez, A.; Espinós, J.P.; González-Elipe, A.R. Influence of irrigation conditions in the germination of plasma treated Nasturtium seeds. Sci. Rep. 2018, 8, 16442. [Google Scholar] [CrossRef]
- Srakaew, K.; Chingsungnoen, A.; Sutthisa, W.; Lakhonchai, A.; Poolcharuansin, P.; Chunpeng, P.; Rojviriya, C.; Thumanu, K.; Tunmee, S. Development of a Multihole Atmospheric Plasma Jet for Growth Rate Enhancement of Broccoli Seeds. Processes 2021, 9, 1134. [Google Scholar] [CrossRef]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current Advancements in the Molecular Mechanism of Plasma Treatment for Seed Germination and Plant Growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Lin, L.; Zvansky, M.; Kohanzadeh, L.; Taban, S.; Chriqui, S.; Keidar, M. Improving Seed Germination by Cold Atmospheric Plasma. Plasma 2022, 5, 98–110. [Google Scholar] [CrossRef]
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.-C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Sohan, S.R.; Sajib, S.A.; Hossain, F.; Miah, M.; Maruf, M.H.; Khalid-Bin-Ferdaus, K.; Kabir, A.H.; Talukder, M.R.; Rashid, M.; et al. The Effect of Low-Pressure Dielectric Barrier Discharge (LPDBD) Plasma in Boosting Germination, Growth, and Nutritional Properties in Wheat. Plasma Chem. Plasma Process. 2022, 42, 339–362. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Cold Plasma Processing on Fruits and Fruit Juices: A Review on the Effects of Plasma on Nutritional Quality. Processes 2021, 9, 2098. [Google Scholar] [CrossRef]
- Ji, W.; Li, M.; Yang, T.; Li, H.; Li, W.; Wang, J.; Ma, M. Effect of cold plasma on physical–biochemical properties and nutritional components of soybean sprouts. Food Res. Int. 2022, 161, 111766. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]






| Samples | Rq (nm) |
|---|---|
| DC | 116.8 |
| DT | 54.0 |
| OC | 90.0 |
| OT | 41.8 |
| Samples | C (at. %) | O (at. %) | Na (at. %) | S (at. %) | Cl (at. %) | K (at. %) | Ca (at. %) |
|---|---|---|---|---|---|---|---|
| DC | 66.38 ± 0.39 | 32.78 ± 0.31 | 0.13 ± 0.02 | 0.12 ± 0.02 | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.14 ± 0.02 |
| DT | 62.2 ± 0.35 | 37.22 ± 0.31 | - | - | - | 0.58 ± 0.02 | - |
| OC | 67.36 ± 0.42 | 31.42 ± 0.33 | 0.07 ± 0.03 | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.97 ± 0.03 | - |
| OT | 64.73 ± 0.36 | 34.16 ± 0.29 | 0.10 ± 0.02 | 0.08 ± 0.02 | 0.05 ± 0.01 | 0.67 ± 0.04 | 0.21 ± 0.05 |
| Samples | Thickness (μm) |
|---|---|
| DC | 83.33 ± 3.33 |
| DT | 76.67 ± 8.82 |
| OC | 73.33 ± 14.53 |
| OT | 63.33 ± 3.33 |
| Anova p-value | 0.485 |
| Samples | Fe/Zn | Mn/Zn | Ca/Zn |
|---|---|---|---|
| DC | 1.61 | 3.61 | 67.74 |
| DT | 2.45 | 3.65 | 78.34 |
| OC | 1.78 | 4.57 | 93.12 |
| OT | 2.78 | 4.64 | 109.85 |
| Samples | Germination (%) | Root Length (cm) | Plant Height (cm) | Total Length (cm) | Biomass (g) |
|---|---|---|---|---|---|
| DC | 90.22 ± 0.28 | 11.63 ± 1.40 b | 11.14 ± 1.16 | 22.77 ± 2.19 b | 0.22 ± 0.01 ab |
| DT | 90.67 ± 0.17 | 14.59 ± 1.22 a | 12.47 ± 0.77 | 27.06 ± 1.84 a | 0.24 ± 0.02 a |
| OC | 90.67 ± 0.17 | 14.38 ± 1.01 a | 12.09 ± 0.63 | 26.47 ± 1.49 a | 0.20 ± 0.02 b |
| OT | 90.22 ± 0.15 | 13.73 ± 0.98 a | 11.68 ± 0.88 | 25.41 ± 1.55 ab | 0.19 ± 0.01 b |
| Anova p-value | 0.18 | 0.00 | 0.10 | 0.00 | 0.00 |
| Samples | Chlorophyll a (µg g−1) | Chlorophyll b (µg g−1) | Carotenoids (µg g−1) | Proteins (mg g−1) |
|---|---|---|---|---|
| DC | 996.92 ± 6.38 c | 375.44 ± 3.45 bc | 146.29 ± 1.51 b | 4.98 ± 0.00 |
| DT | 1196.93 ± 60 a | 423.70 ± 3.13 a | 228.11 ± 5.05 a | 4.98 ± 0.00 |
| OC | 937.46 ± 7.46 d | 366.30 ± 5.03 c | 123.32 ± 0.90 c | 4.98 ± 0.00 |
| OT | 1095.11 ± 0.68 b | 386.77 ± 2.68 b | 224.03 ± 0.50 a | 4.98 ± 0.00 |
| Anova p-value | 0.00 | 0.00 | 0.00 | 0.19 |
| Sample | L * | a * | b * |
|---|---|---|---|
| DC | 31.37 ± 0.64 | −11.66 ± 0.23 | 22.56 ± 0.65 |
| DT | 31.23 ± 0.36 | −11.48 ± 0.21 | 22.19 ± 0.27 |
| OC | 30.29 ± 0.4 | −10.93 ± 0.13 | 22.97 ± 0.52 |
| OT | 31.13 ± 0.29 | −11.52 ± 0.03 | 24.06 ± 0.56 |
| Anova p-value | 0.36 | 0.06 | 0.13 |
| Sample | Flavonoids (mg QE g−1 fw) | Polyphenols (mg GAE g−1 fw) | DPPH (%) |
|---|---|---|---|
| DC | 7.20 ± 0.77 b | 3.59 ± 0.02 c | 92.17 ± 2.04 |
| DT | 11.80 ± 0.40 a | 4.78 ± 0.13 a | 85.60 ± 1.46 |
| OC | 8.95 ± 0.70 b | 4.14 ± 0.04 b | 88.83 ± 1.41 |
| OT | 7.70 ± 0.08 b | 3.61 ± 0.09 c | 88.74 ± 0.78 |
| Anova p-value | 0.00 | 0.00 | 0.08 |
| Sample | Humidity (%) | Protein (%) | Ash (%) | NDF (%) | ADF (%) | Fiber (%) | Energy (%) |
|---|---|---|---|---|---|---|---|
| DC | 87.10 ± 1.09 | 20.70 ± 0.38 | 9.70 ± 0.12 bc | 32.00 ± 0.58 | 44.00 ± 0.33 | 23.50 ± 0.09 a | 10.99 ± 0.09 |
| DT | 84.10 ± 0.35 | 20.50 ± 0.26 | 12.40 ± 0.45 a | 29.00 ± 1.45 | 44.00 ± 0.00 | 20.80 ± 0.35 c | 10.82 ± 0.07 |
| OC | 84.80 ± 0.49 | 18.30 ± 1.13 | 8.70 ± 0.32 c | 37.00 ± 2.33 | 45.00 ± 0.58 | 21.80 ± 0.12 b | 10.67 ± 0.08 |
| OT | 84.00 ± 0.15 | 21.10 ± 0.66 | 10.90 ± 0.03 ab | 27.00 ± 0.33 | 45.00 ± 0.00 | 22.50 ± 0.22 b | 10.76 ± 0.05 |
| Anova p-value | 0.49 | 0.73 | 0.00 | 0.12 | 0.19 | 0.00 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burducea, I.; Burducea, C.; Mereuta, P.-E.; Sirbu, S.-R.; Iancu, D.-A.; Istrati, M.-B.; Straticiuc, M.; Lungoci, C.; Stoleru, V.; Teliban, G.-C.; et al. Helium Atmospheric Pressure Plasma Jet Effects on Two Cultivars of Triticum aestivum L. Foods 2023, 12, 208. https://doi.org/10.3390/foods12010208
Burducea I, Burducea C, Mereuta P-E, Sirbu S-R, Iancu D-A, Istrati M-B, Straticiuc M, Lungoci C, Stoleru V, Teliban G-C, et al. Helium Atmospheric Pressure Plasma Jet Effects on Two Cultivars of Triticum aestivum L. Foods. 2023; 12(1):208. https://doi.org/10.3390/foods12010208
Chicago/Turabian StyleBurducea, Ion, Cristina Burducea, Paul-Emil Mereuta, Stefan-Robert Sirbu, Decebal-Alexandru Iancu, Melania-Beatrice Istrati, Mihai Straticiuc, Constantin Lungoci, Vasile Stoleru, Gabriel-Ciprian Teliban, and et al. 2023. "Helium Atmospheric Pressure Plasma Jet Effects on Two Cultivars of Triticum aestivum L." Foods 12, no. 1: 208. https://doi.org/10.3390/foods12010208
APA StyleBurducea, I., Burducea, C., Mereuta, P.-E., Sirbu, S.-R., Iancu, D.-A., Istrati, M.-B., Straticiuc, M., Lungoci, C., Stoleru, V., Teliban, G.-C., Robu, T., Burducea, M., & Nastuta, A. V. (2023). Helium Atmospheric Pressure Plasma Jet Effects on Two Cultivars of Triticum aestivum L. Foods, 12(1), 208. https://doi.org/10.3390/foods12010208









