Understanding the Effects of Self-Induced Anaerobic Fermentation on Coffee Beans Quality: Microbiological, Metabolic, and Sensory Studies
Abstract
1. Introduction
2. Materials and Methods
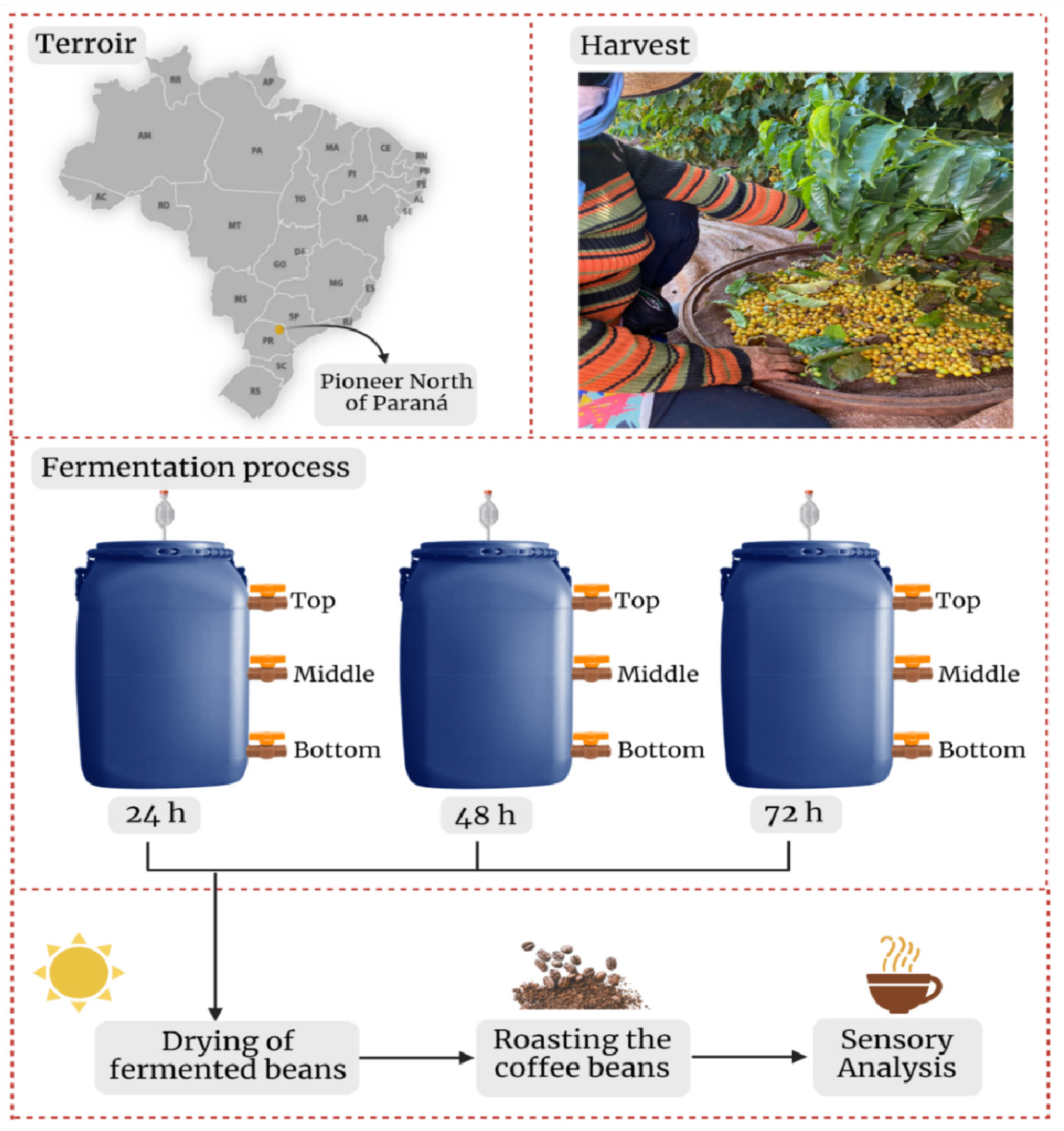
2.1. Coffee Fermentation Process
2.2. Determination of Microbial Diversity by High-Throughput Sequencing
2.3. Analysis of Sugar Consumption and Organic Acid Production in the Liquid Fermentation Fraction
2.4. GC/MS Analysis of the Fermentation Liquid Fraction
2.5. GC/MS Analysis of Green and Roasted Coffee Beans
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Alpha and Beta Diversity Analysis
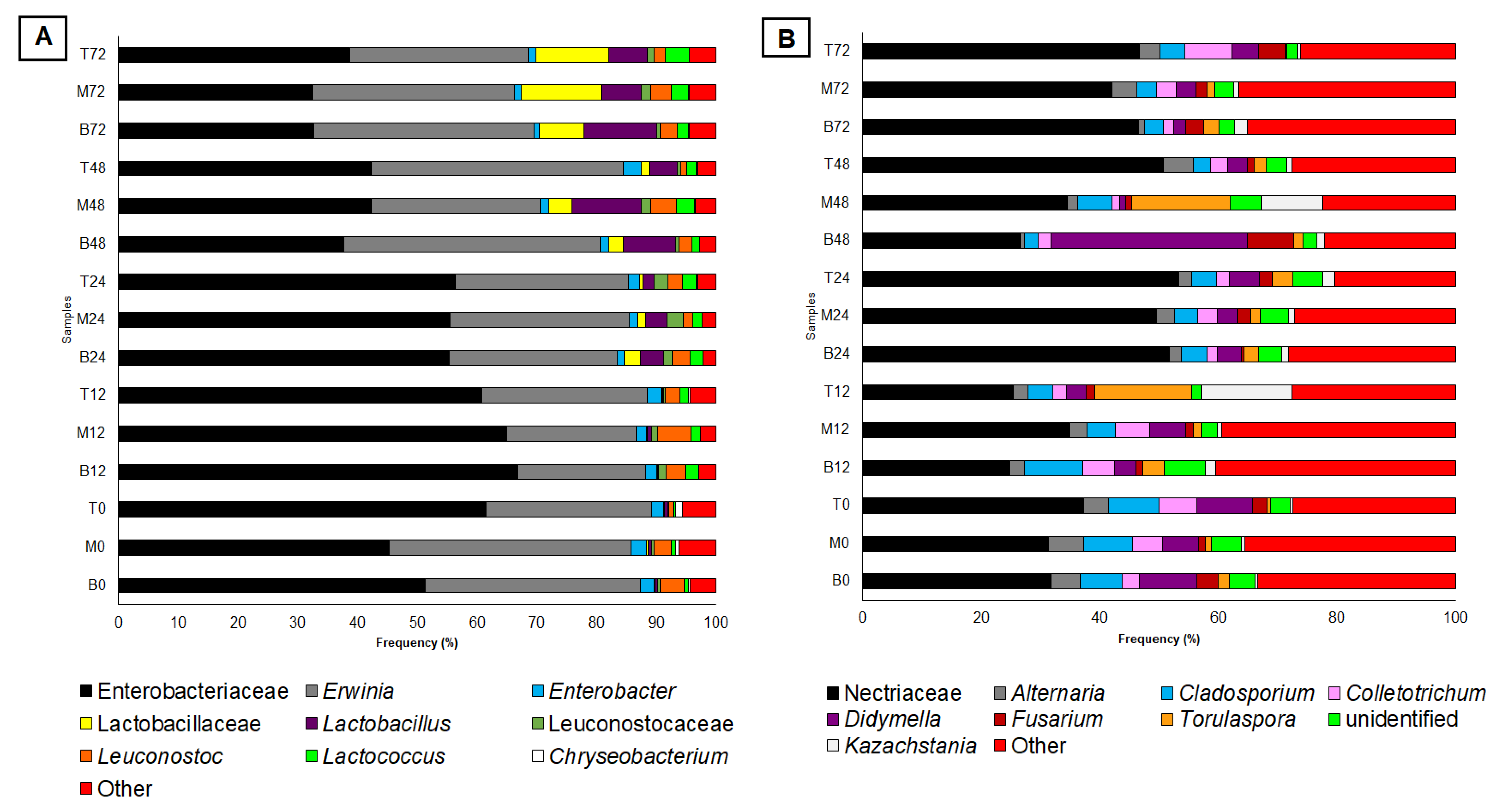
3.2. Microbial Diversity and Dynamics of Fermentation
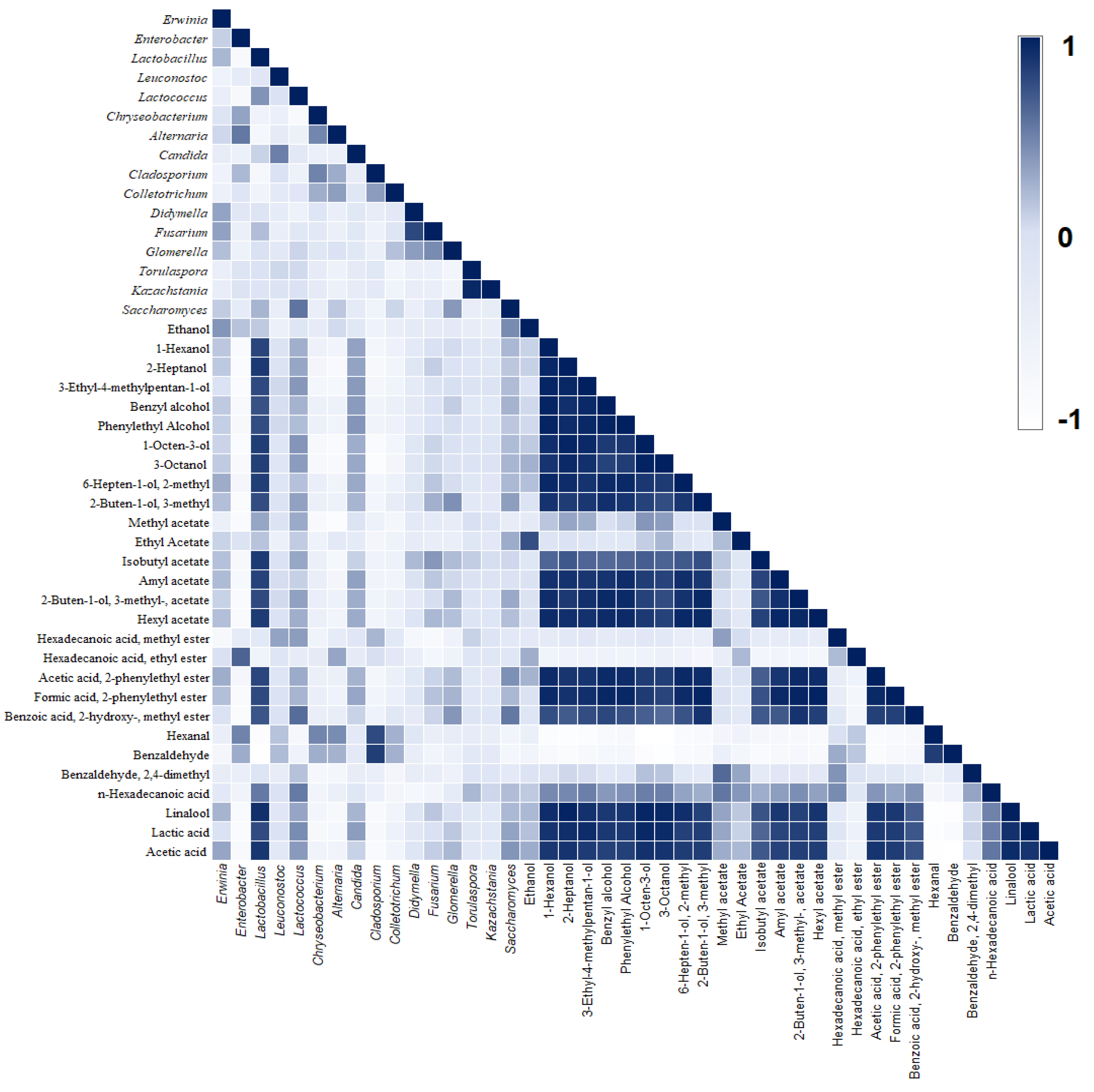
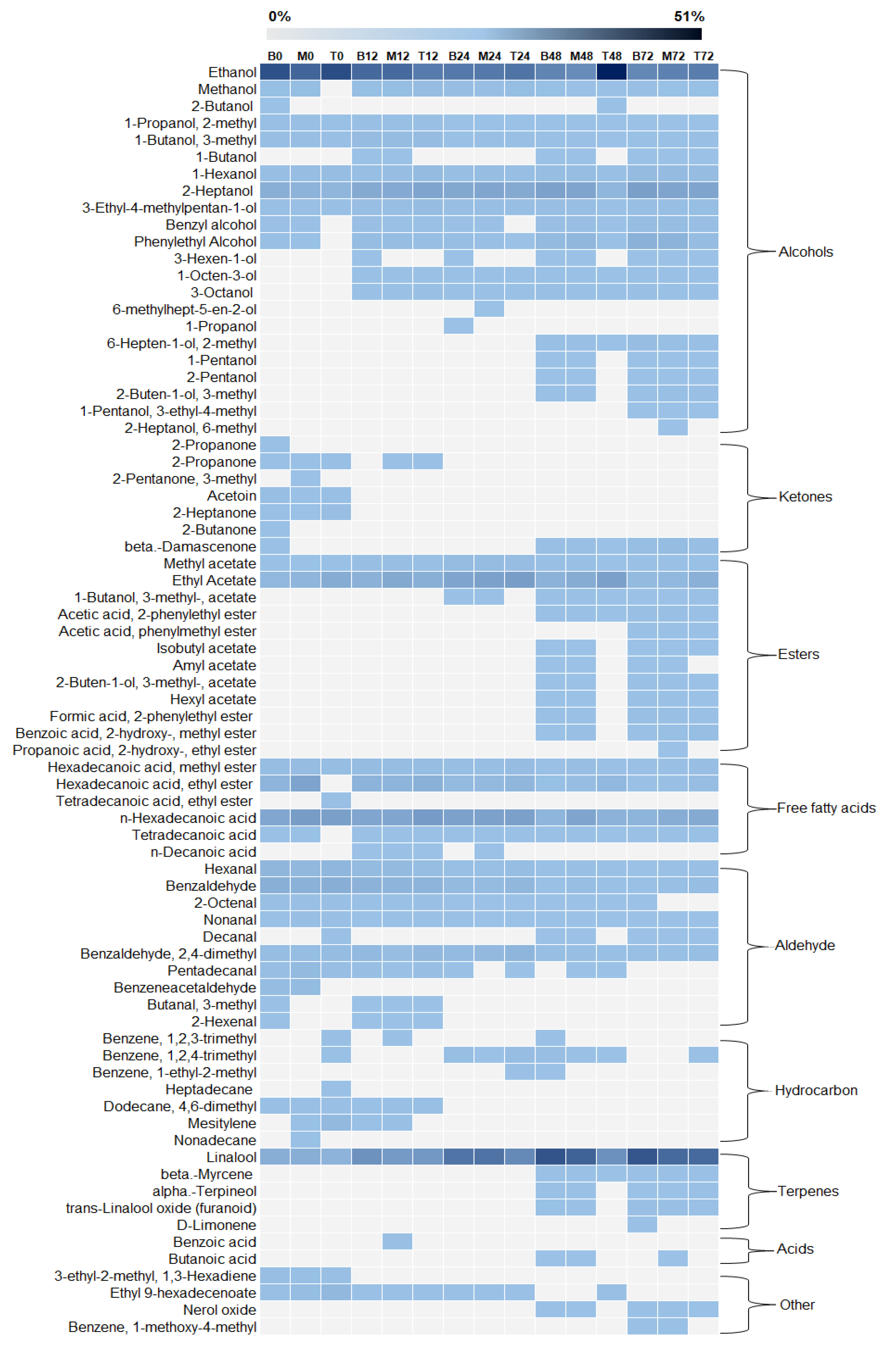
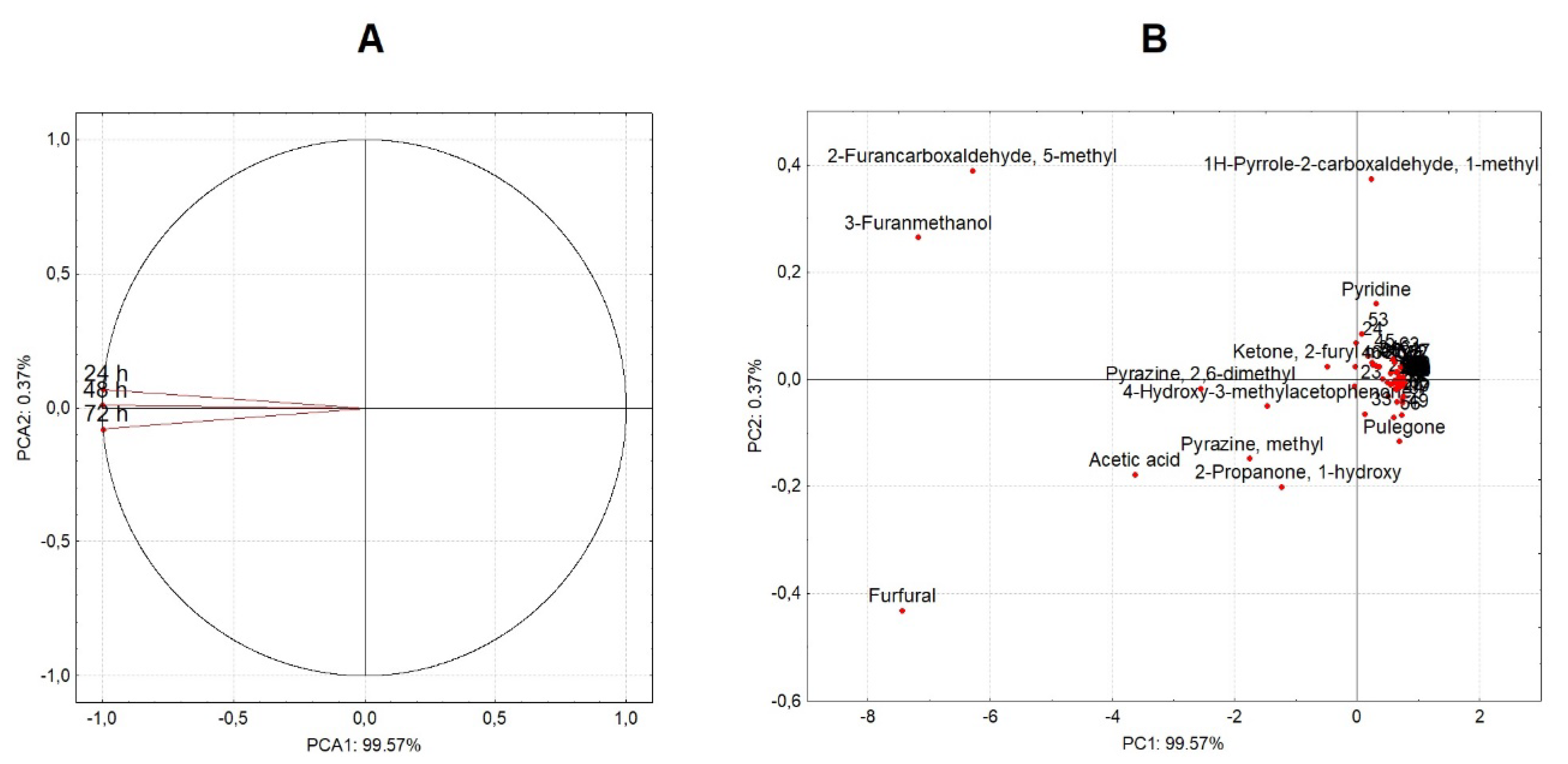
3.3. Profile of Sugars Consumption, Organic Acids Production, and Volatile Compounds
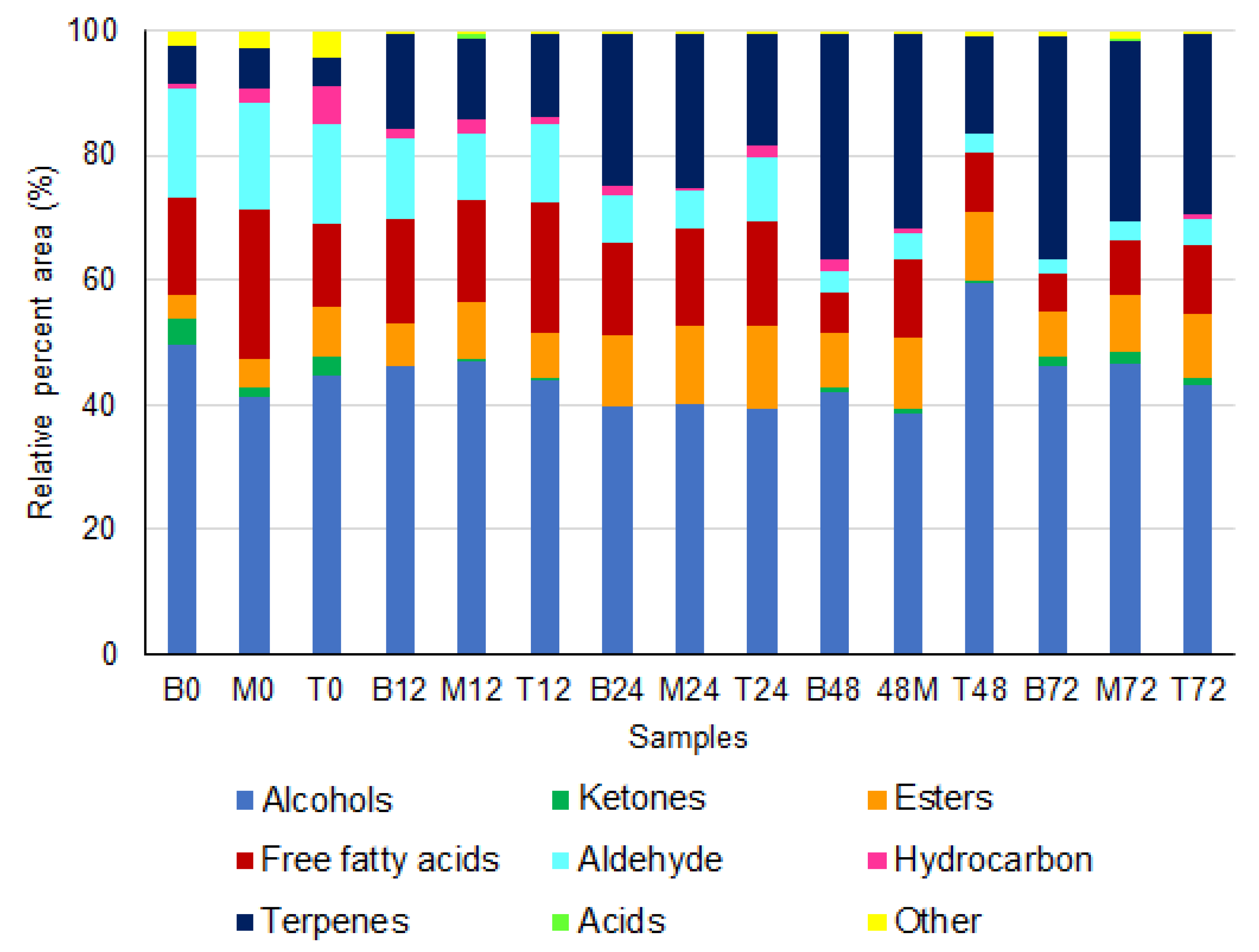
3.4. Volatile Composition of Green and Roasted Coffee Beans
3.4.1. Green Coffee Beans
3.4.2. Roasted Beans
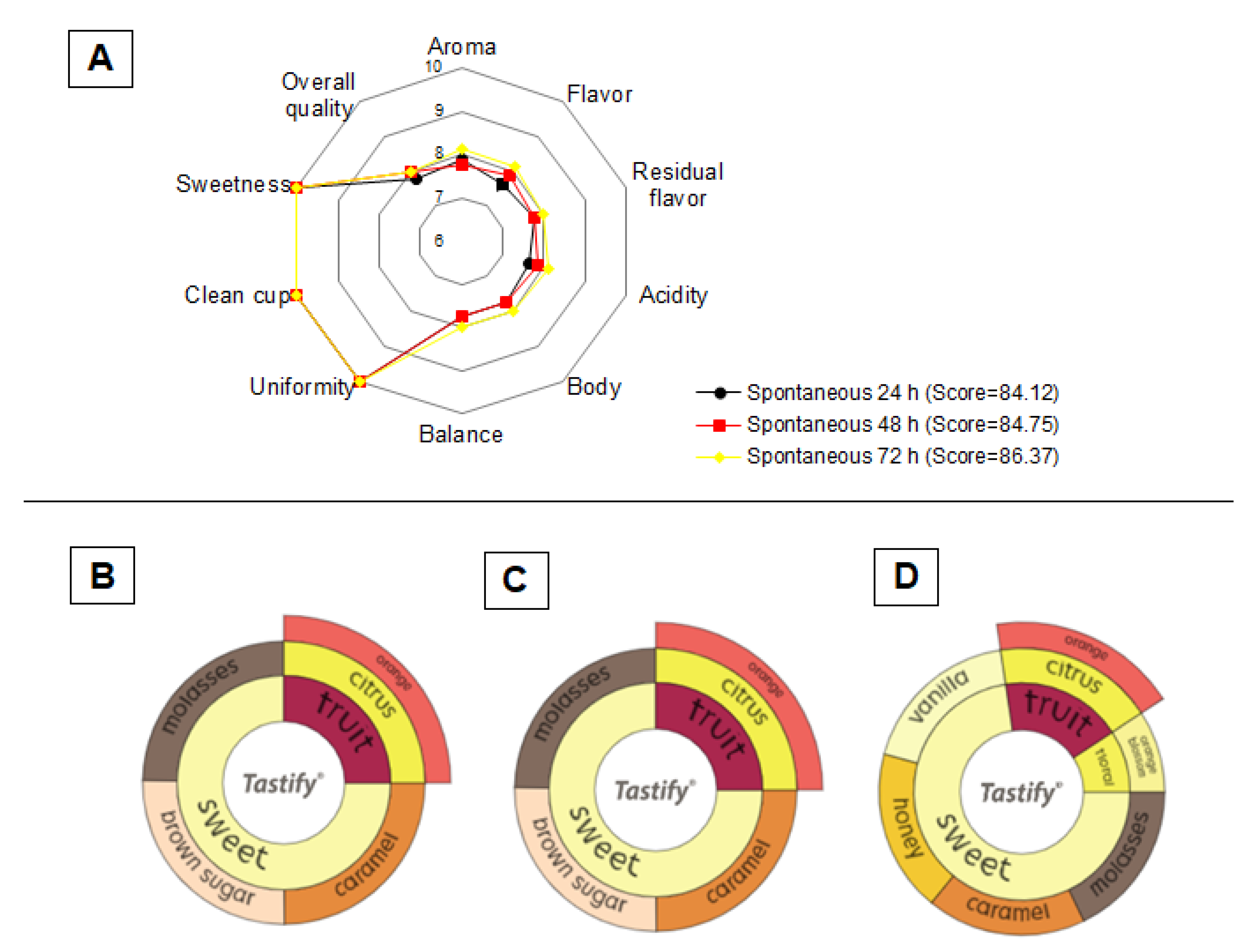
3.5. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barbosa, I.D.P.; De Oliveira, A.C.B.; Rosado, R.D.S.; Sakiyama, N.S.; Cruz, C.D.; Pereira, A.A. Sensory analysis of arabica coffee: Cultivars of rust resistance with potential for the specialty coffee market. Euphytica 2020, 216, 165. [Google Scholar] [CrossRef]
- Teles, C.R.A.; Behrens, J.H. The Waves of Coffee and the Emergence of the New Brazilian Consumer. In Coffee Consumption and Industry Strategies in Brazil: A Volume in the Consumer Science and Strategic Marketing Series; Elsevier: Amsterdam, The Netherlands, 2019; pp. 257–274. ISBN 9780128147221. [Google Scholar]
- Ferreira, J.; Ferreira, C. Challenges and opportunities of new retail horizons in emerging markets: The case of a rising coffee culture in China. Bus. Horizons 2018, 61, 783–796. [Google Scholar] [CrossRef]
- Gomes, W.D.S.; Pereira, L.L.; Filete, C.A.; Moreira, T.R.; Guarçoni, R.C.; Oliveira, E.C.D.S.; Moreli, A.P.; Guimarães, C.V.; Simmer, M.M.B.; Júnior, V.L.; et al. Changes in the Chemical and Sensory Profile of Coffea canephora var. Conilon Promoted by Carbonic Maceration. Agronomy 2022, 12, 2265. [Google Scholar] [CrossRef]
- Simmer, M.M.B.; Soares da Silva, M.D.C.; Pereira, L.L.; Moreira, T.R.; Guarçoni, R.C.; Veloso, T.G.R.; da Silva, I.M.R.; Entringer, T.L.; Kasuya, M.C.M.; da Luz, J.M.R.; et al. Edaphoclimatic conditions and the soil and fruit microbiota influence on the chemical and sensory quality of the coffee beverage. Eur. Food Res. Technol. 2022, 248, 2941–2953. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Júnior, A.I.M.; Vásquez, Z.S.; Medeiros, A.B.; Vandenberghe, L.P.; Soccol, C.R. Exploring the impacts of postharvest processing on the aroma formation of coffee beans—A review. Food Chem. 2018, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Scholz, M.B.; Kitzberger, C.S.G.; Prudencio, S.H. The typicity of coffees from different terroirs determined by groups of physico-chemical and sensory variables and multiple factor analysis. Food Res. Int. 2018, 114, 72–80. [Google Scholar] [CrossRef]
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- De Carvalho, A.M.; de Rezende, J.C.; Rezende, T.T.; Ferreira, A.D.; Rezende, R.M.; Mendes, A.N.G.; Carvalho, G.R. Relationship between the sensory attributes and the quality of coffee in different environments. Afr. J. Agric. Res. 2016, 11, 3607–3614. [Google Scholar] [CrossRef]
- Vale, A.S.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Sorto, R.D.; Goés-Neto, A.; Kato, R.; Soccol, C.R. Facility-specific ‘house’ microbiome ensures the maintenance of functional microbial communities into coffee beans fermentation: Implications for source tracking. Environ. Microbiol. Rep. 2021, 13, 470–481. [Google Scholar] [CrossRef]
- Salem, F.H.; Lebrun, M.; Mestres, C.; Sieczkowski, N.; Boulanger, R.; Collignan, A. Transfer kinetics of labeled aroma compounds from liquid media into coffee beans during simulated wet processing conditions. Food Chem. 2020, 322, 126779. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Coffee fermentation and flavor—An intricate and delicate relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Contreras, G.F.; Cai, Z.; Moccand, C.; Weckx, S.; De Vuyst, L. Influence of Various Processing Parameters on the Microbial Community Dynamics, Metabolomic Profiles, and Cup Quality During Wet Coffee Processing. Front. Microbiol. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Torres, J.; Falconi, C.; Moccand, C.; Weckx, S.; De Vuyst, L. Following Coffee Production from Cherries to Cup: Microbiological and Metabolomic Analysis of Wet Processing of Coffea arabica. Appl. Environ. Microbiol. 2019, 85, e02635-18. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Neto, D.P.; de Melo Pereira, G.V.; Finco, A.M.; Letti, L.A.; da Silva, B.J.; Vandenberghe, L.P.; Soccol, C.R. Efficient coffee beans mucilage layer removal using lactic acid fermentation in a stirred-tank bioreactor: Kinetic, metabolic and sensorial studies. Food Biosci. 2018, 26, 80–87. [Google Scholar] [CrossRef]
- De Carvalho Neto, D.P.; De Melo Pereira, G.V.; Finco, A.M.O.; Rodrigues, C.; De Carvalho, J.C.; Soccol, C.R. Microbiological, physicochemical and sensory studies of coffee beans fermentation conducted in a yeast bioreactor model. Food Biotechnol. 2020, 34, 172–192. [Google Scholar] [CrossRef]
- Magalhães Júnior, A.I.; de Carvalho Neto, D.P.; de Melo Pereira, G.V.; da Silva Vale, A.; Medina, J.D.C.; de Carvalho, J.C.; Soccol, C.R. A critical techno-economic analysis of coffee processing utilizing a modern fermentation system: Implications for specialty coffee production. Food Bioprod. Process. 2021, 125, 14–21. [Google Scholar] [CrossRef]
- da Mota, M.C.B.; Batista, N.N.; Dias, D.R.; Schwan, R.F. Impact of microbial self-induced anaerobiosis fermentation (SIAF) on coffee quality. Food Biosci. 2022, 47, 101640. [Google Scholar] [CrossRef]
- Martinez, S.J.; Bressani, A.P.P.; Simão, J.B.P.; Pylro, V.S.; Dias, D.R.; Schwan, R.F. Dominant microbial communities and biochemical profile of pulped natural fermented coffees growing in different altitudes. Food Res. Int. 2022, 159, 111605. [Google Scholar] [CrossRef]
- Pereira, T.S.; Batista, N.N.; Pimenta, L.P.S.; Martinez, S.J.; Ribeiro, L.S.; Naves, J.A.O.; Schwan, R.F. Self-induced anaerobiosis coffee fermentation: Impact on microbial communities, chemical composition and sensory quality of coffee. Food Microbiol. 2022, 103, 103962. [Google Scholar] [CrossRef]
- Cassimiro, D.M.d.J.; Batista, N.N.; Fonseca, H.C.; Naves, J.A.O.; Dias, D.R.; Schwan, R.F. Coinoculation of lactic acid bacteria and yeasts increases the quality of wet fermented Arabica coffee. Int. J. Food Microbiol. 2022, 369, 109627. [Google Scholar] [CrossRef]
- Martinez, S.J.; Simão, J.B.P.; Pylro, V.S.; Schwan, R.F. The Altitude of Coffee Cultivation Causes Shifts in the Microbial Community Assembly and Biochemical Compounds in Natural Induced Anaerobic Fermentations. Front. Microbiol. 2021, 12, 671395. [Google Scholar] [CrossRef] [PubMed]
- Pregolini, V.B.; de Melo Pereira, G.V.; da Silva Vale, A.; de Carvalho Neto, D.P.; Soccol, C.R. Influence of Environmental Microbiota on the Activity and Metabolism of Starter Cultures Used in Coffee Beans Fermentation. Fermentation 2021, 7, 278. [Google Scholar] [CrossRef]
- de Oliveira Junqueira, A.C.; de Melo Pereira, G.V.; Medina, J.D.C.; Alvear, M.C.R.; Rosero, R.; de Carvalho Neto, D.P.; Enriquez, H.A.G.; Soccol, C.R. First description of bacterial and fungal communities in Colombian coffee beans fermentation analysed using Illumina-based amplicon sequencing. Sci. Rep. 2019, 9, 8794. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.-H.; Lee, J.-H.; Kim, H.B.; Isaacson, R.E. Deciphering Diversity Indices for a Better Understanding of Microbial Communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological diversity, evolution and metabolism of microbial communities in the wet fermentation of Australian coffee beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; De Vuyst, L.; Zhang, S.J.; De Bruyn, F.; Verce, M.; Torres, J.; Callanan, M.; Moccand, C.; Weckx, S. Temporal shotgun metagenomics of an Ecuadorian coffee fermentation process highlights the predominance of lactic acid bacteria. Curr. Res. Biotechnol. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Neto, D.P.D.C.; De Melo, G.V.; Pereira; De Carvalho, J.C.; Soccol, V.T.; Soccol, C.R. High-Throughput rRNA Gene Sequencing Reveals High and Complex Bacterial Diversity Associated with Brazilian Coffee Bean Fermentation. Food Technol. Biotechnol. 2018, 56, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.; Slesarev, A.; Wolf, Y.; Sorokin, A.; Mirkin, B.; Koonin, E.; Pavlov, A.; Pavlova, N.; Karamychev, V.; Polouchine, N.; et al. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 15611–15616. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.-M.; Fornairon, C.; Barre, P. Determination of Oxygen Utilization Pathways in an Industrial Strain of Saccharomyces Cerevisiae during Enological Fermentation. J. Ferment. Bioeng. 1998, 86, 154–163. [Google Scholar] [CrossRef]
- Alexandre, H.; Guilloux-Benatier, M. Yeast Autolysis in Sparkling Wine—A Review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Vale, A.D.S.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rodrigues, C.; Pagnoncelli, M.G.B.; Soccol, C.R. Effect of Co-Inoculation with Pichia fermentans and Pediococcus acidilactici on Metabolite Produced During Fermentation and Volatile Composition of Coffee Beans. Fermentation 2019, 5, 67. [Google Scholar] [CrossRef]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the Impacts of Postharvest Processing on the Microbiota and Metabolite Profiles during Green Coffee Bean Production. Appl. Environ. Microbiol. 2017, 83, e02398-16. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.D.M.; Neto, E.; Soccol, V.T.; Medeiros, A.B.P.; Woiciechowski, A.L.; Soccol, C.R. Conducting starter culture-controlled fermentations of coffee beans during on-farm wet processing: Growth, metabolic analyses and sensorial effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef]
- Junqueira, A.C.D.O.; de Melo Pereira, G.V.; Viesser, J.A.; de Carvalho Neto, D.P.; Querne, L.B.P.; Soccol, C.R. Isolation and selection of fructose-consuming lactic acid bacteria associated with coffee bean fermentation. Food Biotechnol. 2022, 36, 58–75. [Google Scholar] [CrossRef]
- Pereira, G.V.D.M.; da Silva Vale, A.; de Carvalho Neto, D.P.; Muynarsk, E.S.; Soccol, V.T.; Soccol, C.R. Lactic Acid Bacteria: What Coffee Industry Should Know? Curr. Opin. Food Sci. 2020, 31, 1–8. [Google Scholar] [CrossRef]
- Gomes, R.J.; Borges, M.D.F.; Rosa, M.D.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of Acetic Acid Bacteria and Their Role in Vinegar and Fermented Beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef]
- Elferink, S.J.W.H.O.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef]
- Rivas, F.; Parra, A.; Martinez, A.; Garcia-Granados, A. Enzymatic glycosylation of terpenoids. Phytochem. Rev. 2013, 12, 327–339. [Google Scholar] [CrossRef]
- Mendes-Ferreira, A.; Barbosa, C.; Falco, V.; Leão, C.; Mendes-Faia, A. The production of hydrogen sulphide and other aroma compounds by wine strains of Saccharomyces cerevisiae in synthetic media with different nitrogen concentrations. J. Ind. Microbiol. Biotechnol. 2009, 36, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Joët, T.; Laffargue, A.; Descroix, F.; Doulbeau, S.; Bertrand, B.; de Kochko, A.; Dussert, S. Influence of environmental factors, wet processing and their interactions on the biochemical composition of green Arabica coffee beans. Food Chem. 2010, 118, 693–701. [Google Scholar] [CrossRef]
- Anese, M.; Suman, M. Mitigation strategies of furan and 5-hydroxymethylfurfural in food. Food Res. Int. 2013, 51, 257–264. [Google Scholar] [CrossRef]
- Bi, S.; Wang, A.; Lao, F.; Shen, Q.; Liao, X.; Zhang, P.; Wu, J. Effects of frying, roasting and boiling on aroma profiles of adzuki beans (Vigna angularis) and potential of adzuki bean and millet flours to improve flavor and sensory characteristics of biscuits. Food Chem. 2021, 339, 127878. [Google Scholar] [CrossRef] [PubMed]
- Pfamhauser, W. Volatiles Formed during Extrusion Cooking of Cereals. Flavour. Fragr. J. 1993, 8, 109–113. [Google Scholar] [CrossRef]
- Kwon, D.-J.; Jeong, H.-J.; Moon, H.; Kim, H.-N.; Cho, J.-H.; Lee, J.-E.; Hong, K.S.; Hong, Y.-S. Assessment of green coffee bean metabolites dependent on coffee quality using a 1H NMR-based metabolomics approach. Food Res. Int. 2015, 67, 175–182. [Google Scholar] [CrossRef]
- Chindapan, N.; Soydok, S.; Devahastin, S. Roasting Kinetics and Chemical Composition Changes of Robusta Coffee Beans During Hot Air and Superheated Steam Roasting. J. Food Sci. 2019, 84, 292–302. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Q.; Linforth, R.; Fisk, I.D.; Yang, N. Modifying Robusta coffee aroma by green bean chemical pre-treatment. Food Chem. 2019, 272, 251–257. [Google Scholar] [CrossRef]
- Nguyen, T.N.H.; Byun, S.Y. Combined changes of process conditions improved aromatic properties of Vietnamese Robusta. Biotechnol. Bioprocess Eng. 2013, 18, 248–256. [Google Scholar] [CrossRef]
| Sample | Bacteria Indices | Fungi Indices | ||||
|---|---|---|---|---|---|---|
| Chao | Shannon | Simpson | Chao | Shannon | Simpson | |
| B0 | 1686.7 | 1.56 | 0.68 | 149.4 | 2.79 | 0.87 |
| M0 | 1391 | 1.65 | 0.69 | 143.9 | 2.73 | 0.87 |
| T0 | 1526.9 | 1.52 | 0.64 | 179.5 | 2.59 | 0.83 |
| B12 | 1740.6 | 1.44 | 0.63 | 179.5 | 2.98 | 0.90 |
| M12 | 1114.1 | 1.40 | 0.61 | 110.2 | 2.67 | 0.85 |
| T12 | 1637.9 | 1.53 | 0.66 | 108.6 | 2.64 | 0.87 |
| B24 | 1424.5 | 1.56 | 0.67 | 137.4 | 2.19 | 0.71 |
| M24 | 1358.3 | 1.53 | 0.67 | 117.8 | 2.26 | 0.73 |
| T24 | 1774.6 | 1.59 | 0.68 | 111.5 | 2.19 | 0.70 |
| B48 | 1628 | 1.61 | 0.69 | 102.4 | 2.24 | 0.80 |
| M48 | 1444.5 | 1.89 | 0.77 | 83.8 | 2.40 | 0.83 |
| T48 | 1358.3 | 1.62 | 0.70 | 74.3 | 2.16 | 0.72 |
| B72 | 1142.7 | 1.84 | 0.76 | 85.2 | 2.00 | 0.75 |
| M72 | 1216 | 1.94 | 0.78 | 88.9 | 2.52 | 0.80 |
| T72 | 1293.3 | 1.93 | 0.78 | 89 | 2.19 | 0.75 |
| Time (h) | Glucose | Fructose | Lactic Acid | Acetic Acid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bottom | Middle | Top | Bottom | Middle | Top | Bottom | Middle | Top | Bottom | Middle | Top | |
| 0 | 9.12 ± 0.42 | 8.52 ± 1.05 | 3.06 ± 0.92 | 20.65 ± 0.90 | 16.23 ± 0.77 | 9.59 ± 0.72 | ND | ND | ND | ND | ND | ND |
| 6 | 8.03 ± 1.16 | 5.43 ± 1.31 | 4.4 ±0.82 | 20.37 ± 0.92 | 18.14 ± 0.98 | 13.41 ± 0.25 | 0.63 ± 0.09 | 0.41 ± 0.05 | 0.28 ± 0.08 | ND | ND | ND |
| 12 | 7.66 ± 0.87 | 5.57 ± 0.87 | 3.93 ± 0.77 | 20.61 ± 1.88 | 15.59 ± 0.41 | 10.92 ± 1.68 | 0.91 ± 0.19 | 0.78 ± 0.23 | 0.43 ± 0.02 | ND | ND | ND |
| 18 | 6.88 ± 0.23 | 4.49 ± 1.33 | 3.53 ± 0.27 | 19.25 ± 0.59 | 14.46 ± 1.91 | 10.98 ± 0.35 | 2.06 ± 0.38 | 1.60 ± 0.44 | 1.10 ± 0.07 | 0.25 ± 0.03 | 0.24 ± 0.06 | 0.14 ± 0.01 |
| 24 | 4.74 ± 0.94 | 3.63 ± 0.32 | 3.06 ± 0.61 | 16.36 ± 0.57 | 12.37 ± 0.70 | 8.52 ± 0.14 | 2.11 ± 0.23 | 1.66 ± 0.05 | 1.00 ± 0.02 | 0.38 ± 0.11 | 0.36 ± 0.03 | 0.19 ± 0.00 |
| 30 | 4.56 ± 0.25 | 2.77 ± 0.32 | 2.43 ± 0.03 | 16.10 ± 0.94 | 10.63 ± 1.04 | 8.98 ± 0.55 | 2.36 ± 0.22 | 2.15 ± 0.02 | 1.36 ± 0.16 | 0.54 ± 0.11 | 0.48 ± 0.06 | 0.24 ± 0.05 |
| 36 | 5.07 ± 0.70 | 2.58 ± 0.30 | 2.29 ± 0.28 | 17.96 ± 2.00 | 11.28 ± 1.12 | 8.19 ± 1.30 | 2.77 ± 0.28 | 2.26 ± 0.23 | 1.39 ± 0.25 | 0.59 ± 0.06 | 0.51 ± 0.03 | 0.26 ± 0.05 |
| 48 | 4.31 ± 0.51 | 2.04 ± 0.23 | 1.91 ± 0.14 | 16.23 ± 1.55 | 10.62 ± 0.66 | 9.23 ± 0.39 | 3.18 ± 0.27 | 2.83 ± 0.22 | 2.13 ± 013 | 0.67 ± 0.00 | 0.63 ± 0.06 | 0.50 ± 0.02 |
| 60 | 4.36 ± 0.06 | 1.23 ± 0.07 | 1.06 ± 0.04 | 17.76 ± 0.49 | 9.56 ± 0.20 | 5.67 ± 0.13 | 4.31 ± 0.23 | 3.24 ± 0.06 | 2.03 ± 0.02 | 0.88 ± 0.06 | 0.71 ± 0.01 | 0.40 ± 0.04 |
| 72 | 4.47 ± 0.04 | 1.04 ± 0.12 | 0.76 ± 0.01 | 18.67 ± 0.80 | 11.09 ± 0.46 | 5.34 ± 0.07 | 5.06 ± 0.02 | 4.70 ± 0.02 | 2.18 ± 0.01 | 1.00 ± 0.11 | 0.94 ± 0.00 | 0.40 ± 0.04 |
| Compounds | Green Beans | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Alcohols | |||
| Ethanol | 0.73 ± 0.03 A | 2.30 ± 0.27 B | 6.69 ± 0.34 C |
| 2-Propanol, 1-methoxy | 0.10 ± 0.01 | ND | ND |
| 1-Butanol, 3-methyl | 0.20 ± 0.02 A | 0.34 ± 0.01 B | 0.21 ± 0.02 A |
| 1-Pentanol | 0.28 ± 0.03 AB | 0.26 ± 0.02 A | 0.35 ± 0.02 B |
| 2-Hexanol, 5-methyl | 0.30 ± 0.28 | ND | ND |
| 2,3-Butanediol | 0.24 ± 0.01 AB | 0.20 ± 0.01 A | 0.25 ± 0.01 B |
| 3-Furanmethanol | 0.55 ± 0.02 | ND | ND |
| 1-Hexanol | 1.30 ± 0.02 A | 1.15 ± 0.09 B | 0.81 ± 0.02 C |
| 1-Propanol, 2 methyl | ND | ND | 0.03 ± 0.00 |
| Aldehydes | |||
| Butanal, 3-methyl | 0.19 ± 0.03 A | 0.16 ± 0.01 A | 0.15 ± 0.01 A |
| Butanal, 2-methyl | 0.11 ± 0.01 A | 0.10 ± 0.01 A | 0.07 ± 0.00 B |
| Pentanal | 0.31 ± 0.03 A | 0.27 ± 0.03 AB | 0.21 ± 0.03 A |
| Hexanal | 1.51 ± 0.16 A | 1.19 ± 0.28 AB | 0.92 ± 0.06 B |
| Heptanal | 0.16 ± 0.07 A | 0.12 ± 0.02 A | 0.09 ± 0.00 A |
| Nonanal | 0.53 ± 0.02 A | 0.46 ± 0.10 AB | 0.33 ± 0.05 B |
| Decanal | 0.16 ± 0.01 A | 0.15 ± 0.03 A | ND |
| Furans | |||
| Furan, 2-methyl | 0.12 ± 0.02 A | 0.10 ± 0.00 A | 0.22 ± 0.03 B |
| Furan, 2-pentyl | 0.16 ± 0.01 A | 0.22 ± 0.01 A | 0.19 ± 0.04 A |
| Esters | |||
| Ethyl Acetate | ND | ND | 0.21 ± 0.01 |
| Pentanoic acid, ethyl ester | ND | ND | 0.17 ± 0.02 |
| Acids | |||
| Butanoic acid, 3-methyl | 0.21 ± 0.02 A | 0.16 ± 0.01 A | 0.18 ± 0.03 A |
| Acetic acid | 0.20 ± 0.00 A | 0.62 ± 0.24 B | 0.47 ± 0.06 AB |
| Ketones | |||
| 2-Propanone, 1-hydroxy | 0.10 ± 0.00 | ND | ND |
| 5-Hepten-2-one, 6-methyl | 0.08 ± 0.00 A | 0.04 ± 0.00 B | 0.11 ± 0.00 C |
| 2-Propanone | ND | 0.85 ± 0.11 | ND |
| 2-Pentanone, 3-methyl | ND | 0.04 ± 0.00 A | 0.03 ± 0.01 A |
| Pyrazines | |||
| 2-Isobutyl-3-methoxypyrazine | 0.93 ± 0.01 A | 1.03 ± 0.12 A | 0.72 ± 0.05 B |
| Terpenes | |||
| D-Limonene | ND | 0.13 ± 0.02 | ND |
| Hydrocarbon | |||
| Nonane, 3-methyl-5-propyl | 0.12 ± 0.01 A | 0.11 ± 0.00 A | 0.07 ± 0.00 B |
| Compounds | Roasted Beans | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Furans | |||
| Furan, 2-methyl | 0.37 ± 0.06 A | 1.46 ± 0.24 B | 1.00 ± 0.11 C |
| Furan, 2,5-dimethyl | 0.06 ± 0.01 A | 0.12 ± 0.01 B | 0.12 ± 0.01 B |
| 2-Vinylfuran | 0.23 ± 0.07 A | 0.26 ± 0.02 A | 0.22 ± 0.04 A |
| 3(2H)-Furanone, dihydro-2-methyl | 1.87 ± 0.03 A | 2.72 ± 0.12 B | 2.63 ± 0.25 B |
| Furfuryl formate | 2.44 ± 0.12 A | 2.90 ± 0.02 B | 2.69 ± 0.12 AB |
| 2,5-Dimethylfuran-3,4(2H,5H)-dione | 2.39 ± 0.16 A | 3.24 ± 0.21 B | 3.93 ± 0.08 C |
| 5-Ethylfurfural | ND | ND | 0.30 ± 0.01 |
| Furfural | 77.06 ± 2.54 A | 101.21 ± 1.78 B | 110.10 ± 2.09 C |
| 2-Furancarboxaldehyde, 5-methyl | 75.75 ± 2.74 A | 87.10 ± 3.56 B | 82.83 ± 3.49 AB |
| Alcohols | |||
| Ethanol | ND | 0.22 ± 0.02 A | 1.12 ± 0.05 B |
| 3-Furanmethanol | 84.08 ± 3.09 A | 96.17 ± 3.65 B | 96.76 ± 1.58 B |
| Propanol, 2 methyl | ND | 0.63 ± 0.05 | ND |
| Ketones | |||
| 2-Propanone, 1-hydroxy | 17.44 ± 0.53 A | 25.24 ± 2.32 B | 28.24 ± 2.26 B |
| 2,3-Pentanedione | 2.08 ± 0.12 A | 2.99 ± 0.16 B | 3.03 ± 0.23 B |
| Acetoin | 0.67 ± 0.03 A | 1.31 ± 0.05 B | 1.28 ± 0.04 B |
| 2-Propanone | 1.64 ± 0.21 A | 1.73 ± 0.13 A | 1.64 ± 0.05 A |
| 1-Hydroxy-2-butanone | 1.02 ± 0.04 A | 1.47 ± 0.12 B | 1.59 ± 0.12 B |
| 3-Pentanone, 2-methyl | 0.69 ± 0.02 | ND | ND |
| Ketone, 2-furyl methyl | 12.77 ± 0.49 A | 15.92 ± 0.43 B | 15.33 ± 0.32 B |
| 2,5-Hexanedione | 0.43 ± 0.02 A | 0.55 ± 0.01 A | 0.46 ± 0.09 A |
| 2-Butanone, 1-hydroxy-, acetate | 1.43 ± 0.15 A | 1.59 ± 0.05 A | 2.26 ± 1.34 A |
| 1-Propanone, 1-(2-furanyl) | 0.55 ± 0.06 A | 0.79 ± 0.03 A | 0.68 ± 0.04 A |
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 0.90 ± 0.06 A | 1.07 ± 0.03 A | 0.97 ± 0.03 A |
| 2-Acetyl-3-methylpyrazine | 8.47 ± 0.43 A | 9.15 ± 0.60 A | 10.83 ± 0.32 A |
| 2-Propanone, 1-hydroxy-, acetate | 8.68 ± 0.35 A | 10.03 ± 0.33 A | 8.82 ± 0.30 A |
| 2-Acetyl-5-methylfuran | 4.38 ± 0.54 A | 5.16 ± 0.39 A | 4.77 ± 0.07 A |
| Resorcinol, 2-acetyl | 3.42 ± 0.56 A | 4.95 ± 0.50 A | 4.36 ± 0.04 A |
| 4-Hydroxy-3-methylacetophenone | 21.92 ± 4.30 A | 27.58 ± 2.84 A | 29.02 ± 0.20 A |
| Pyrazines | |||
| 1,3-Diazine | 1.71 ± 0.13 A | 2.07 ± 0.04 AB | 2.27 ± 0.21 B |
| Pyrazine, methyl | 24.12 ± 0.28 A | 29.88 ± 0.74 B | 34.60 ± 1.45 C |
| Pyrazine, 2,6-dimethyl | 34.55 ± 1.30 A | 38.35 ± 0.14 A | 43.27 ± 2.48 B |
| Pyrazine, ethyl | 2.91 ± 0.19 A | 3.31 ± 0.08 AB | 3.75 ± 0.33 B |
| Pyrazine, 2,3-dimethyl | 0.99 ± 0.08 A | 1.13 ± 0.05 AB | 1.26 ± 0.10 B |
| Pyrazine, ethenyl | 0.84 ± 0.13 A | 1.10 ± 0.11 B | 1.20 ± 0.00 B |
| Pyrazine, 2-ethyl-6-methyl | 5.67 ± 0.27 AB | 4.54 ± 0.18 A | 6.02 ± 0.75 B |
| Pyrazine, trimethyl | 6.27 ± 0.17 A | 6.74 ± 0.15 A | 9.59 ± 0.52 B |
| Pyrazine, 3-ethyl-2,5-dimethyl | 6.35 ± 0.28 A | 5.40 ± 0.16 B | 6.70 ± 0.31 A |
| 1-(6-Methyl-2-pyrazinyl)-1-ethanone | 1.81 ± 0.17 A | 1.77 ± 0.07 A | 2.26 ± 0.03 B |
| Pyrazine, 2-methyl-5-(1-propenyl)-, (E) | 0.99 ± 0.17 A | 1.16 ± 0.11 A | 1.14 ± 0.04 A |
| Pyrazine, 2-methyl-6-propenyl | ND | ND | 1.23 ± 0.02 |
| Pyrazine, 2,3-diethyl-5-methyl | ND | ND | 0.23 ± 0.00 |
| Acids | |||
| Butanoic acid, 3-methyl | 1.06 ± 0.17 A | 1.95 ± 0.09 B | 1.96 ± 0.31 B |
| Butanoic acid, 2-methyl | ND | ND | 0.18 ± 0.04 |
| Acetic acid | 42.44 ± 4.45 A | 53.49 ± 3.10 B | 58.60 ± 0.73 B |
| Formic acid | 0.62 ± 0.13 A | 1.14 ± 0.05 B | 1.24 ± 0.08 B |
| Propanoic acid | 0.92 ± 0.07 A | 1.24 ± 0.05 B | 1.22 ± 0.08 B |
| 2-Butenoic acid, 3-methyl | 0.58 ± 0.02 A | 1.76 ± 0.07 B | 2.05 ± 0.09 C |
| Pyrroles | |||
| 1H-Pyrrole-2-carboxaldehyde | 6.35 ± 0.58 A | 7.67 ± 0.45 B | 6.65 ± 0.26 AB |
| Ketone, methyl pyrrol-2-yl | 8.34 ± 0.61 A | 9.91 ± 0.71 B | 9.71 ± 0.17 AB |
| Ethanone, 1-(1-methyl-1H-pyrrol-2-yl) | 1.51 ± 0.13 A | 1.57 ± 0.03 A | 1.43 ± 0.27 A |
| 1H-Pyrrole-2-carboxaldehyde, 1-methyl | 8.90 ± 0.07 A | 9.09 ± 0.71 A | ND |
| 1H-Pyrrole, 1-(2-furanylmethyl) | 1.80 ± 0.13 A | 1.86 ± 0.06 A | 1.76 ± 0.04 A |
| Pyrrole | ND | ND | 1.93 ± 0.14 |
| Esters | |||
| Methyl acetate | 0.31 ± 0.07 A | 0.29 ± 0.01 A | 0.27 ± 0.02 A |
| Propanoic acid, 2-oxo-, methyl ester | 0.20 ± 0.03 A | 0.20 ± 0.03 A | 0.21 ± 0.03 A |
| Acetic acid, 4-methylphenyl ester | 0.38 ± 0.06 A | 1.90 ± 0.08 B | ND |
| Furfuryl acetate | 7.97 ± 0.26 A | 8.77 ± 0.12 B | 7.38 ± 0.27 C |
| 2-Furancarboxylic acid, methyl ester | 1.56 ± 0.17 A | 2.04 ± 0.20 B | 2.04 ± 0.04 B |
| Pyrans | |||
| 4H-Pyran-4-one, 3-hydroxy-2-methyl | 5.20 ± 0.86 A | 6.84 ± 0.58 B | 5.77 ± 0.17 AB |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl | 1.05 ± 0.08 A | 1.88 ± 0.15 B | 3.46 ± 0.18 C |
| Pyridines | |||
| Pyridine | 4.91 ± 0.34 A | 8.63 ± 0.45 B | 2.37 ± 0.03 C |
| Pyridine, 2-methyl | 0.17 ± 0.07 AB | 0.17 ± 0.01 A | 0.28 ± 0.00 B |
| Aldehydes | |||
| Propanal, 2-methyl | 0.46 ± 0.03 A | 0.63 ± 0.05 B | 0.75 ± 0.05 B |
| Butanal, 3-methyl | 0.40 ± 0.04 A | 0.64 ± 0.01 B | 0.72 ± 0.03 B |
| Benzeneacetaldehyde | 1.88 ± 0.06 A | 2.34 ± 0.07 B | 2.18 ± 0.04 C |
| Other | |||
| Cyclopent-4-ene-1,3-dione | 1.30 ± 0.09 A | 1.74 ± 0.07 B | 1.72 ± 0.22 B |
| 1,2-Cyclopentanedione, 3-methyl | 2.93 ± 0.93 A | 2.23 ± 0.10 A | 1.95 ± 0.08 A |
| 4-Methylthiazole | ND | ND | 0.15 ± 0.03 |
| 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy | ND | ND | 0.90 ± 0.04 |
| Terpenes | |||
| Pulegone | ND | ND | 3.37 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Vale, A.; Balla, G.; Rodrigues, L.R.S.; de Carvalho Neto, D.P.; Soccol, C.R.; de Melo Pereira, G.V. Understanding the Effects of Self-Induced Anaerobic Fermentation on Coffee Beans Quality: Microbiological, Metabolic, and Sensory Studies. Foods 2023, 12, 37. https://doi.org/10.3390/foods12010037
da Silva Vale A, Balla G, Rodrigues LRS, de Carvalho Neto DP, Soccol CR, de Melo Pereira GV. Understanding the Effects of Self-Induced Anaerobic Fermentation on Coffee Beans Quality: Microbiological, Metabolic, and Sensory Studies. Foods. 2023; 12(1):37. https://doi.org/10.3390/foods12010037
Chicago/Turabian Styleda Silva Vale, Alexander, Gabriel Balla, Luiz Roberto Saldanha Rodrigues, Dão Pedro de Carvalho Neto, Carlos Ricardo Soccol, and Gilberto Vinícius de Melo Pereira. 2023. "Understanding the Effects of Self-Induced Anaerobic Fermentation on Coffee Beans Quality: Microbiological, Metabolic, and Sensory Studies" Foods 12, no. 1: 37. https://doi.org/10.3390/foods12010037
APA Styleda Silva Vale, A., Balla, G., Rodrigues, L. R. S., de Carvalho Neto, D. P., Soccol, C. R., & de Melo Pereira, G. V. (2023). Understanding the Effects of Self-Induced Anaerobic Fermentation on Coffee Beans Quality: Microbiological, Metabolic, and Sensory Studies. Foods, 12(1), 37. https://doi.org/10.3390/foods12010037







