Structural Property, Immunoreactivity and Gastric Digestion Characteristics of Glycated Parvalbumin from Mandarin Fish (Siniperca chuaisi) during Microwave-Assisted Maillard Reaction
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Orthogonal Designed Experiments of Microwave-Assisted Maillard Reaction
2.2.1. Sample Preparation
2.2.2. Orthogonal Designed Experiment
2.2.3. Preparation of Crude Extract
2.2.4. Determination of Browning Index of Maillard Reaction
2.3. Purification of Parvalbumin
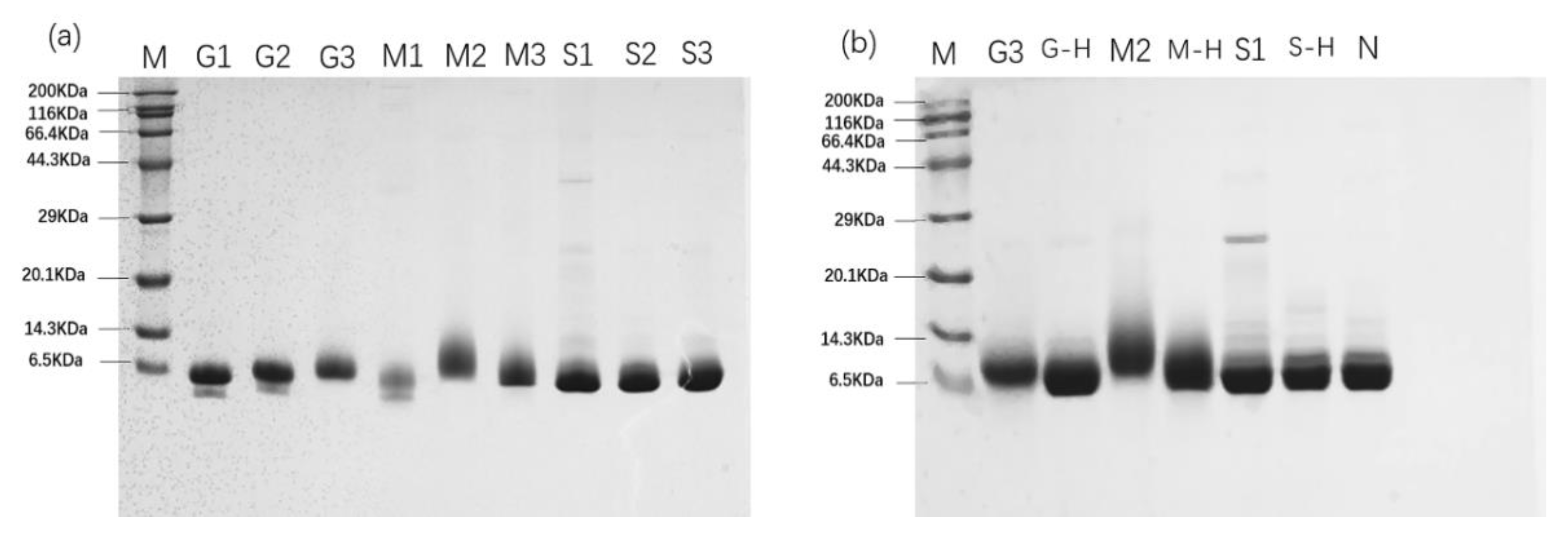
2.4. SDS-PAGE (Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis) Analysis
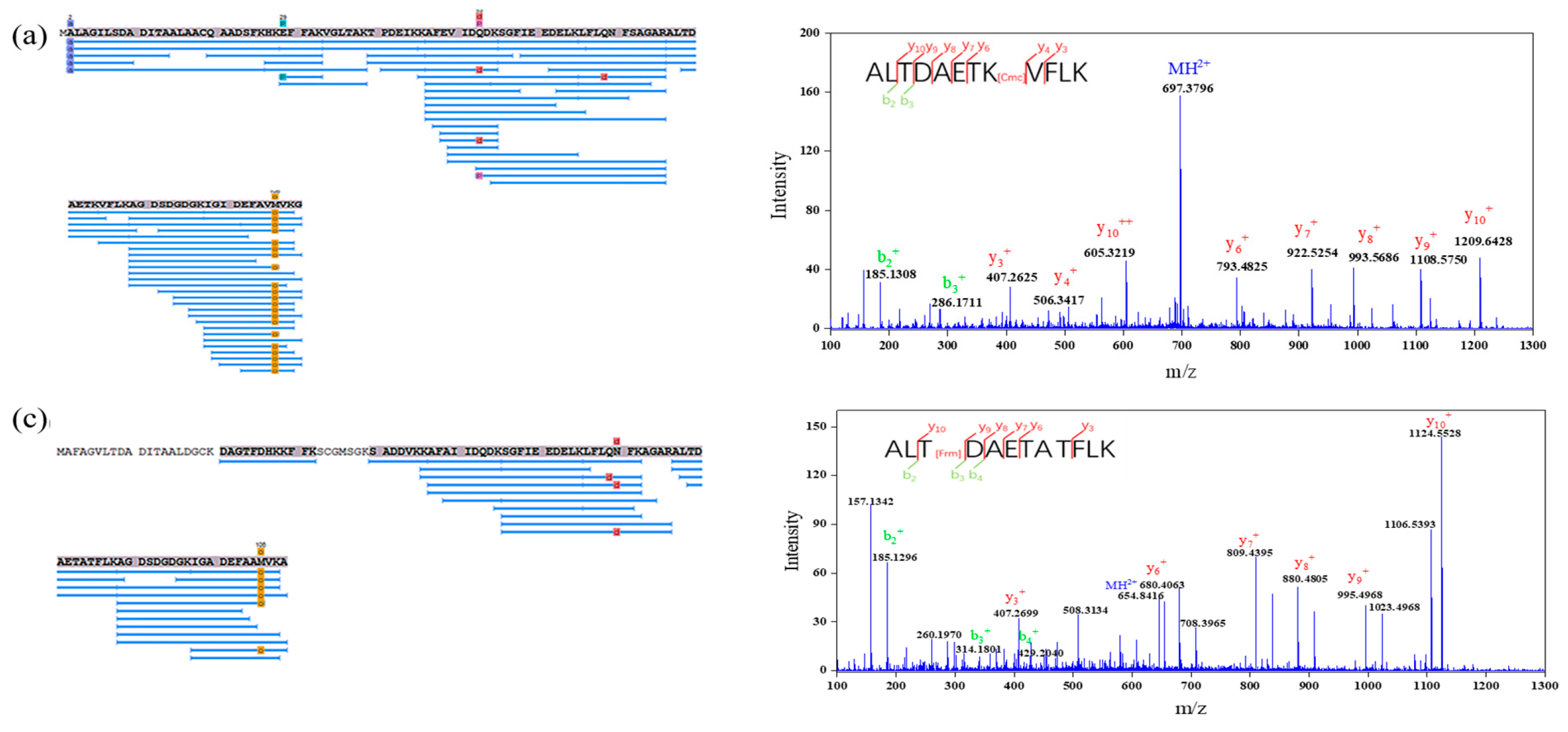
2.5. Nano-LC-MS/MS Analysis of Glycated Parvalbumin Peptides
2.6. Immunization of Rabbit by Mandarin Fish Parvalbumin and Preparation of Antiserum
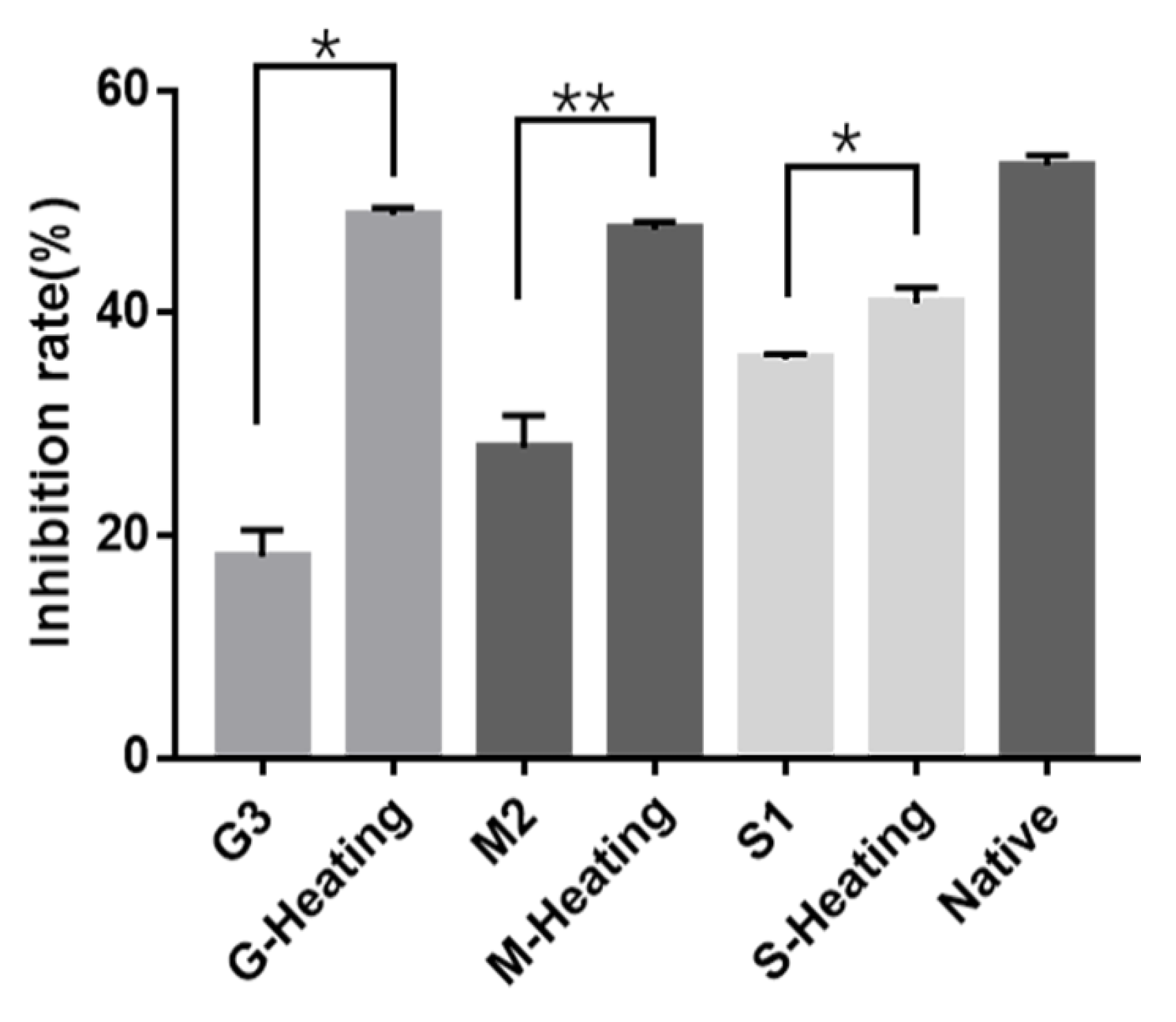
2.7. Indirect Competitive Enzyme Linked Immunosorbent Assay (ic-ELISA)
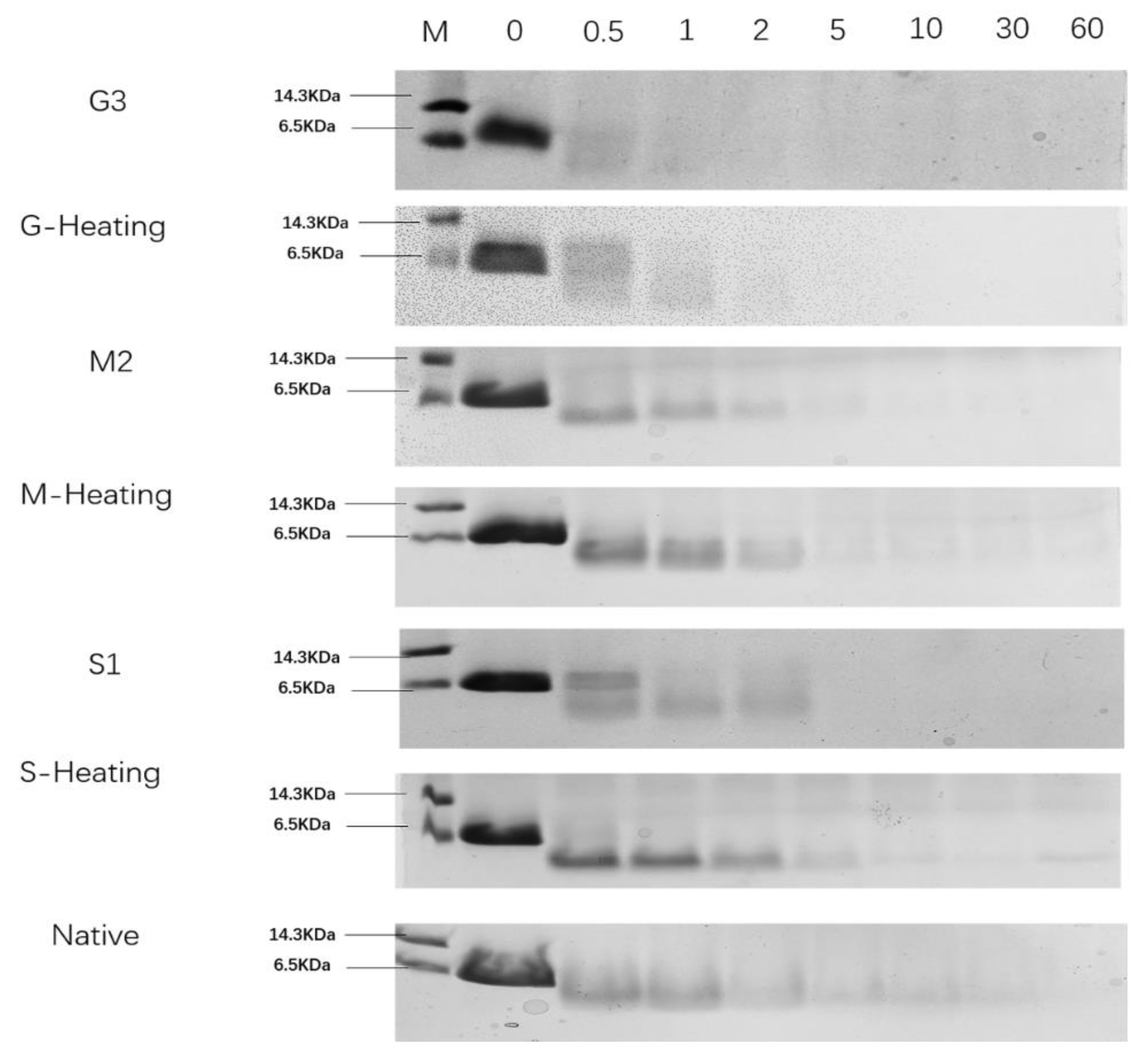
2.8. In Vitro Gastric Digestion
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Maillard Reaction
3.2. SDS-PAGE Analysis of Parvalbumin
3.3. Identification of Glycation Sites in Parvalbumin
3.4. The Influence of Glycation on Parvalbumin Immunological Properties
3.5. In Vitro Gastric Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stervander, M.; A Cresko, W. A highly contiguous nuclear genome assembly of the mandarinfish Synchiropus splendidus (Syngnathiformes: Callionymidae). G3 Genes|Genomes|Genetics 2021, 11, jkab306. [Google Scholar] [CrossRef]
- Jeebhay, M.F.; Robins, T.G.; Lehrer, S.B.; Lopata, A.L. Occupational seafood allergy: A review. Occup. Environ. Med. 2001, 58, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Sharp, M.F.; Lopata, A.L. Fish Allergy: In Review. Clin. Rev. Allergy Immunol. 2013, 46, 258–271. [Google Scholar] [CrossRef]
- Kretsinger, R.H.; Nockolds, C.E. Carp Muscle Calcium-binding Protein. J. Biol. Chem. 1973, 248, 3313–3326. [Google Scholar] [CrossRef]
- Bragança, M.; Bartolomé, B.; Coimbra, A.; Carneiro-Leão, L.; Amaral, L. Fish allergy: Unusual patterns of parvalbumin allergenicity. Ann. Allergy Asthma Immunol. 2022, 128, 607–609. [Google Scholar] [CrossRef]
- Cai, Q.F.; Liu, G.M.; Li, T.; Hara, K.; Wang, X.C.; Su, W.J.; Cao, M.J. Purification and characterization of parvalbumins, the major allergens in red stingray (Dasyatis akajei). J. Agric. Food Chem. 2010, 58, 12964–12969. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, J.; Li, C.; Zhu, S.; Hao, Y.; Cheng, Y.; Wu, H. Morphological and skeletal comparison and ecological adaptability of Mandarin fish Siniperca chuatsi and big-eye Mandarin fish Siniperca kneri. Aquac. Fish. 2021, 6, 455–464. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.Y.; Xue, W.T. Identification of IgE-binding protein from mandarin fish and antigenicity reduced by proteinases hydrolysis. J. Food Sci. Technol. 2012, 82, 4100–4104. [Google Scholar] [CrossRef] [Green Version]
- Kuehn, A.; Hilger, C.; Graf, T.; Hentges, F. Protein and DNA-based assays as complementary tools for fish allergen detection. Allergol. Sel. 2017, 1, 120. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Goktepe, I.; Ahmedna, M. The potential of papain and alcalase enzymes and process optimizations to reduce allergenic gliadins in wheat flour. Food Chem. 2016, 196, 1338–1345. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Chen, W.; Zhou, P. Conformation stability, in vitro digestibility and allergenicity of tropomyosin from shrimp (Exopalaemon modestus) as affected by high intensity ultrasound. Food Chem. 2018, 245, 997–1009. [Google Scholar] [CrossRef]
- Costa, J.; Amaral, J.S.; Fernandes, T.J.; Batista, A.; Oliveira, M.B.P.; Mafra, I. DNA extraction from plant food supplements: Influence of different pharmaceutical excipients. Mol. Cell. Probes 2015, 29, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Suri, K.; Singh, B.; Kaur, A. Impact of microwave roasting on physicochemical properties, maillard reaction products, antioxidant activity and oxidative stability of nigella seed (Nigella sativa L.) oil. Food Chem. 2022, 368, R713–R715. [Google Scholar] [CrossRef]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a potential functional food ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- van de Lagemaat, J.; Silván, J.M.; Moreno, F.J.; Olano, A.; Del Castillo, M.D. In vitro glycation and antigenicity of soy proteins. Food Res. Int. 2007, 40, 153–160. [Google Scholar] [CrossRef]
- Fu, L.; Wang, C.; Wang, J.; Ni, S.; Wang, Y. Maillard reaction with ribose, galacto-oligosaccharide or chitosan-oligosaccharide reduced the allergenicity of shrimp tropomyosin by inducing conformational changes. Food Chem. 2019, 274, 789–795. [Google Scholar] [CrossRef]
- Han, X.Y.; Yang, H.; Rao, S.T.; Liu, G.Y.; Hu, M.J.; Zeng, B.C.; Cao, M.J.; Liu, G.M. The maillard reaction reduced the sensitization of tropomyosin and arginine kinase from scylla paramamosain, simultaneously. J. Agric. Food Chem. 2018, 66, 2934–2943. [Google Scholar] [CrossRef]
- Jiang, W.; He, X.; Yang, H.; Xiang, X.; Hu, S.; Li, S.; Liu, Y. Histamine reduction by Maillard reaction with glucose. Food Control. 2017, 82, 136–144. [Google Scholar] [CrossRef]
- Cucu, T.; De Meulenaer, B.; Bridts, C.; Devreese, B.; Ebo, D. Impact of thermal processing and the Maillard reaction on the basophil activation of hazelnut allergic patients. Food Chem. Toxicol. 2012, 50, 1722–1728. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Kim, S.-W.; Kim, Y.; Lee, S.-H.; Jeon, H.; Lee, K.-W. Optimization of Maillard reaction with ribose for enhancing anti-allergy effect of fish protein hydrolysates using response surface methodology. Food Chem. 2015, 176, 420–425. [Google Scholar] [CrossRef]
- Teodorowicz, M.; Fiedorowicz, E.; Kostyra, H.; Wichers, H.; Kostyra, E. Effect of Maillard reaction on biochemical properties of peanut 7S globulin (Ara h 1) and its interaction with a human colon cancer cell line (Caco-2). Eur. J. Nutr. 2013, 52, 1927–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beale, J.E.; Jeebhay, M.F.; Lopata, A.L. Characterisation of purified parvalbumin from five fish species and nucleotide sequencing of this major allergen from Pacific pilchard, Sardinops sagax. Mol. Immunol. 2009, 46, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Offengenden, M.; Fentabil, M.A.; Wu, J. N-glycosylation of ovomucin from hen egg white. Glycoconj. J. 2011, 28, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xin, L.; Shan, B.; Chen, W.; Xie, M.; Yuen, D.; Zhang, W.; Zhang, Z.; Lajoie, G.A.; Ma, B. PEAKS DB: De Novo Sequencing Assisted Database Search for Sensitive and Accurate Peptide Identification. Mol. Cell. Proteomics 2012, 11, M111.010587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibahara, Y.; Uesaka, Y.; Wang, J.; Yamada, S.; Shiomi, K. A sensitive enzyme-linked immunosorbent assay for the determination of fish protein in processed foods. Food Chem. 2013, 136, 675–681. [Google Scholar] [CrossRef]
- Tukiran, N.A.; Ismail, A.; Mustafa, S.; Hamid, M. Determination of porcine gelatin in edible bird’s nest by competitive indirect ELISA based on anti-peptide polyclonal antibody. Food Control 2016, 59, 561–566. [Google Scholar] [CrossRef]
- Bu, D.; Zhuang, H.; Zhou, X.; Yang, G. Biotin–streptavidin enzyme-linked immunosorbent assay for detecting Tetrabromobisphenol A in electronic waste. Talanta 2014, 120, 40–46. [Google Scholar] [CrossRef]
- Thomas, K.; Aalbers, M.; Bannon, G.; Bartels, M.; Dearman, R.; Esdaile, D.; Fu, T.; Glatt, C.; Hadfield, N.; Hatzos, C.; et al. A multi-laboratory evaluation of a common in vitro pepsin digestion assay protocol used in assessing the safety of novel proteins. Regul. Toxicol. Pharmacol. 2004, 39, 87–98. [Google Scholar] [CrossRef]
- Brands, C.M.; van Boekel, M.A. Kinetic modeling of reactions in heated monosaccharide− casein systems. J. Agric. Food Chem. 2002, 50, 6725–6739. [Google Scholar] [CrossRef]
- Larhed, M.; Hallberg, A. Microwave-assisted high-speed chemistry: A new technique in drug discovery. Drug Discov. Today 2001, 6, 406–416. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Xue, Y.; Ruan, S.; Zhou, A.; Huang, S.; Ma, H. The Preparation and Identification of Characteristic Flavour Compounds of Maillard Reaction Products of Protein Hydrolysate from Grass Carp (Ctenopharyngodon idella) Bone. J. Food Qual. 2021, 2021, 8394152. [Google Scholar] [CrossRef]
- Sun, L.R.; Lin, H.; Li, Z.X.; Zhao, J.X.; Lin, H.; Luo, C.; Tian, S.L. Purification and characterization of parvalbumin isotypes from Japanese flounder (Paralichthys olivaceus). Shipin Kexue/Food Sci. 2019, 40, 308–314. [Google Scholar]
- Soboleva, A.; Vikhnina, M.; Grishina, T.; Frolov, A. Probing Protein Glycation by Chromatography and Mass Spectrometry: Analysis of Glycation Adducts. Int. J. Mol. Sci. 2017, 18, 2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheijen, J.L.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- Hegele, J.; Buetler, T.; Delatour, T. Comparative LC–MS/MS profiling of free and protein-bound early and advanced glycation-induced lysine modifications in dairy products. Analytica Chimica Acta 2008, 617, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zheng, J.; Ge, K.; Zhang, H.; Fang, B.; Jiang, L.; Guo, H.; Ding, Q.; Ren, F. Glycation of α-lactalbumin with different size saccharides: Effect on protein structure and antigenicity. Int. Dairy J. 2014, 34, 220–228. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Tchiakpe, L.S.; Dalle Ore, F. Effects of pH on Caramelization and Maillard Reaction Kinetics in Fructose-Lysine Model Systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Delaroque, N.; Müller, D.G.; Botheb, G.; Pohlb, T.; Knippersa, R.; Bolandc, W. The Complete DNA Sequence of the Ectocarpus siliculosus Virus EsV-1 Genome. Virology 2001, 287, 112–132. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, S.; Apold, J. Immunochemical Analysis of Cod Fish Allergen M: Locations of the Immunoglobulin Binding Sites as Demonstrated by the Native and Synthetic Peptides. Allergy 1983, 38, 449–459. [Google Scholar] [CrossRef]
- Untersmayr, E.; Szalai, K.; Riemer, A.B.; Hemmer, W.; Swoboda, I.; Hantusch, B.; Schöll, I.; Spitzauer, S.; Scheiner, O.; Jarisch, R.; et al. Mimotopes identify conformational epitopes on parvalbumin, the major fish allergen. Mol. Immunol. 2006, 43, 1454–1461. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Ichimura, A.; Kobayashi, Y.; Shiomi, K. IgE-binding epitopes of various fish parvalbumins exist in a stereoscopic conformation maintained by Ca2+ binding. Allergol. Int. 2016, 65, 345–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparini, A.; Benedé, S.; Tedeschi, T.; Sforza, S.; Recio, I.; Miralles, B. In vitro simulated semi-dynamic gastrointestinal digestion: Evaluation of the effects of processing on whey proteins digestibility and allergenicity. Food Funct. 2022, 13, 1593–1602. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Zhang, Y.; Huang, M.; Xu, X.; Ho, H.; Huang, H.; Sun, J. Improving physicochemical properties of myofibrillar proteins from wooden breast of broiler by diverse glycation strategies. Food Chemistry 2022, 382, 132328. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Genetically Modified Organisms (GMO); Naegeli, H.; Bresson, J.L.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; Firbank, L.G.; Guerche, P.; Hejatko, J.; Moreno, F.J.; et al. Statement on in vitro protein digestibility tests in allergenicity and protein safety assessment of genetically modified plants. EFSA J. 2021, 19, e06350. [Google Scholar] [PubMed]
- Zhao, Y.J.; Cai, Q.F.; Jin, T.C.; Zhang, L.J.; Fei, D.X.; Liu, G.M.; Cao, M.J. Effect of Maillard reaction on the structural and immunological properties of recombinant silver carp parvalbumin. LWT 2017, 75, 25–33. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Saini, C.S.; Sharma, H.K. Synergistic effect of microwave heating and hydrothermal treatment on cyanogenic glycosides and bioactive compounds of plum (Prunus domestica L.) kernels: An analytical approach. Curr. Res. Food Sci. 2021, 5, 65–72. [Google Scholar] [CrossRef]

| Experimental Group | Group Name | Factors | Browning Index | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Orthogonal experiment group a | G1 | 1 | 1 | 1 | 0.150 |
| G2 | 1 | 2 | 2 | 0.220 | |
| G3 | 1 | 3 | 3 | 0.345 ** | |
| M1 | 2 | 1 | 2 | 0.105 | |
| M2 | 2 | 2 | 3 | 0.198 ** | |
| M3 | 2 | 3 | 1 | 0.070 | |
| S1 | 3 | 1 | 3 | 0.145 ** | |
| S2 | 3 | 2 | 1 | 0.056 | |
| S3 | 3 | 3 | 2 | 0.065 | |
| Source of variation | SS | df | MS | F | p |
| Type of sugar (A) | 0.037 | 2 | 0.018 | 23.777 | <0.05 |
| concentration (B) | 0.001 | 2 | 0.001 | 0.832 | >0.05 |
| heating time (C) | 0.030 | 2 | 0.015 | 19.396 | <0.05 |
| Error | 0.001 | 2 | 0.001 | ||
| Total variation | 0.075 | 8 | |||
| Control group b | G-Heating | 1 | 3 | 3 | 0.062 ** |
| M-Heating | 2 | 3 | 3 | 0.060 ** | |
| S-Heating | 3 | 3 | 3 | 0.051 ** | |
| Sample | Protein | Peptide Location | Native Peptide Sequence | Modified Peptide Sequence | Theoretical Mass | Observed Mass |
|---|---|---|---|---|---|---|
| G3 | PV-I | 34–45 | VGLTAKTPDEIK | VGLTAK[G]TPDEIK | 1270.71 | 1432.76 |
| VGLTAKTPDEIK[Arg] | 1270.71 | 1426.81 | ||||
| G3 | PV-I | 46–55 | KAFEVIDQDK | K[Cmc]AFEVIDQDK | 1191.61 | 1248.61 |
| K[Frm]AFEVIDQDK | 1191.61 | 1219.61 | ||||
| G3 | PV-I | 47–55 | AFEVIDQDK | AFEVIDQDK[G] | 1063.52 | 1224.58 |
| G3 | PV-I | 77–88 | ALTDAETKVFLK | ALTDAETK[G]VFLK | 1334.74 | 1496.79 |
| ALTDAETK[Cmc]VFLK | 1334.74 | 1392.74 | ||||
| G3 | PV-I | 89–108 | AGDSDGDGKIGIDEFAVM[O]VK | AGDSDGDGK[G]IGID EFAVM[O]VK | 2038.95 | 2201.00 |
| G3 | PV-II | 77–88 | ALTDAETATFLK | ALTDAETA[Frm]TFLK | 1279.67 | 1307.67 |
| ALTDA[Frm]ETATFLK | 1279.67 | 1307.67 | ||||
| M2 | PV-I | 34–45 | VGLTAKTPDEIK | VGLTAK[Frm]TPDEIK | 1270.71 | 1298.71 |
| M2 | PV-I | 77–88 | ALTDAETKVFLK | ALTDAETK[Frm]VFLK | 1334.74 | 1362.74 |
| ALTDAETK[Cmc]VFLK | 1334.74 | 1392.74 | ||||
| ALTDAETK[*]VFLK | 1334.74 | 1431.77 | ||||
| ALTDAETKVFLK[Frm] | 1334.74 | 1362.74 | ||||
| ALTDAETKVFLK[Cmc] | 1334.74 | 1392.74 | ||||
| M2 | PV-II | 77–88 | ALTDAETATFLK | ALTD[Frm]AETATFLK | 1279.67 | 1307.67 |
| ALT[Frm]DAETATFLK | 1279.67 | 1307.67 |
Disclaimer/Publisher•s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, J.; Qiao, D.; Huang, X.; Hu, H.; Li, W.; Liang, X.; Zhang, F.; Lu, Y.; Zhang, H. Structural Property, Immunoreactivity and Gastric Digestion Characteristics of Glycated Parvalbumin from Mandarin Fish (Siniperca chuaisi) during Microwave-Assisted Maillard Reaction. Foods 2023, 12, 52. https://doi.org/10.3390/foods12010052
Tai J, Qiao D, Huang X, Hu H, Li W, Liang X, Zhang F, Lu Y, Zhang H. Structural Property, Immunoreactivity and Gastric Digestion Characteristics of Glycated Parvalbumin from Mandarin Fish (Siniperca chuaisi) during Microwave-Assisted Maillard Reaction. Foods. 2023; 12(1):52. https://doi.org/10.3390/foods12010052
Chicago/Turabian StyleTai, Jingjing, Dan Qiao, Xue Huang, Huang Hu, Wanzheng Li, Xinle Liang, Fuming Zhang, Yanbin Lu, and Hong Zhang. 2023. "Structural Property, Immunoreactivity and Gastric Digestion Characteristics of Glycated Parvalbumin from Mandarin Fish (Siniperca chuaisi) during Microwave-Assisted Maillard Reaction" Foods 12, no. 1: 52. https://doi.org/10.3390/foods12010052
APA StyleTai, J., Qiao, D., Huang, X., Hu, H., Li, W., Liang, X., Zhang, F., Lu, Y., & Zhang, H. (2023). Structural Property, Immunoreactivity and Gastric Digestion Characteristics of Glycated Parvalbumin from Mandarin Fish (Siniperca chuaisi) during Microwave-Assisted Maillard Reaction. Foods, 12(1), 52. https://doi.org/10.3390/foods12010052








