Effects of Heat-Moisture Treatment on the Digestibility and Physicochemical Properties of Waxy and Normal Potato Starches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Modification with HMT
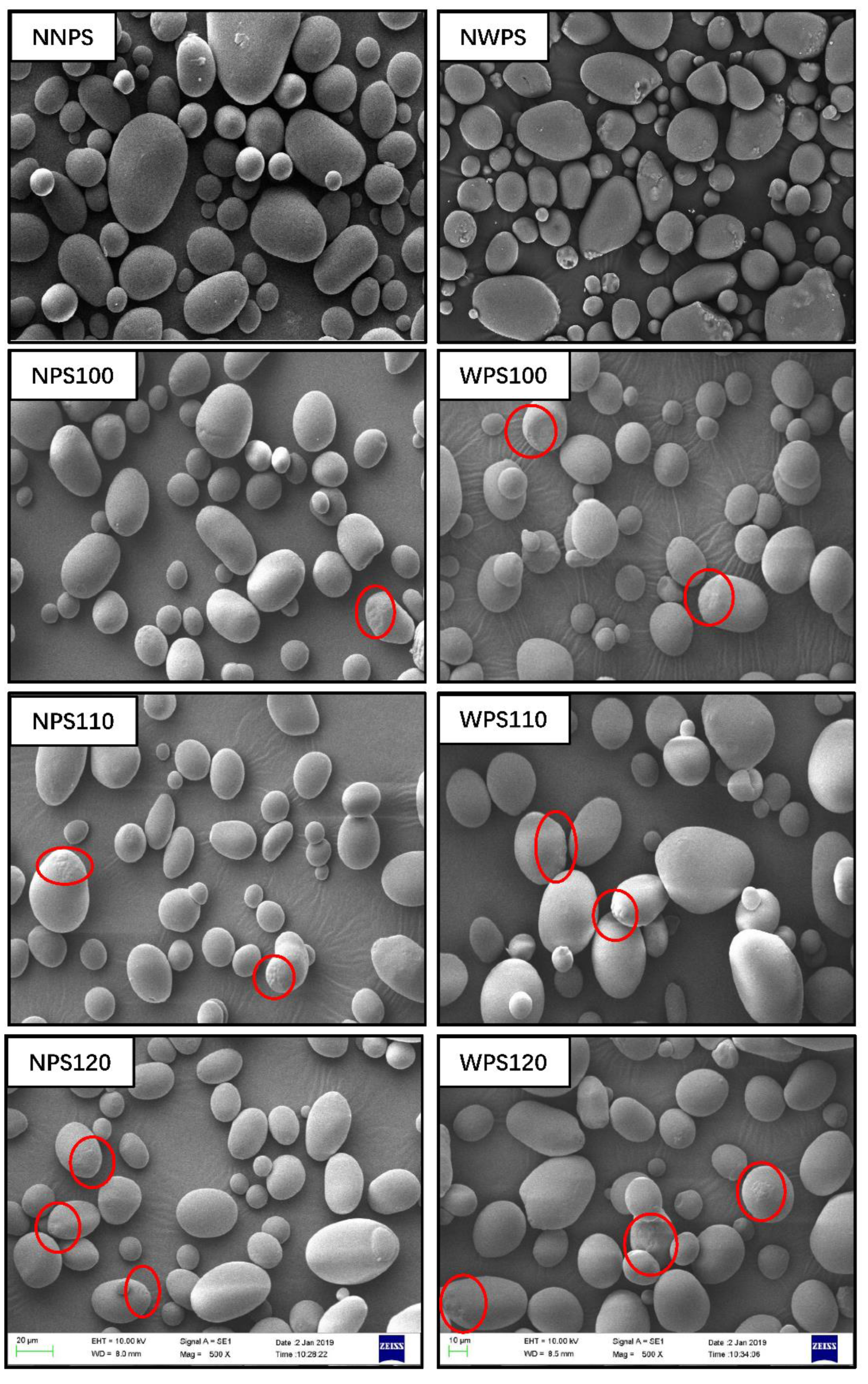
2.3. Scanning Electron Microscopy (SEM)
2.4. Polarized Light Microscopy
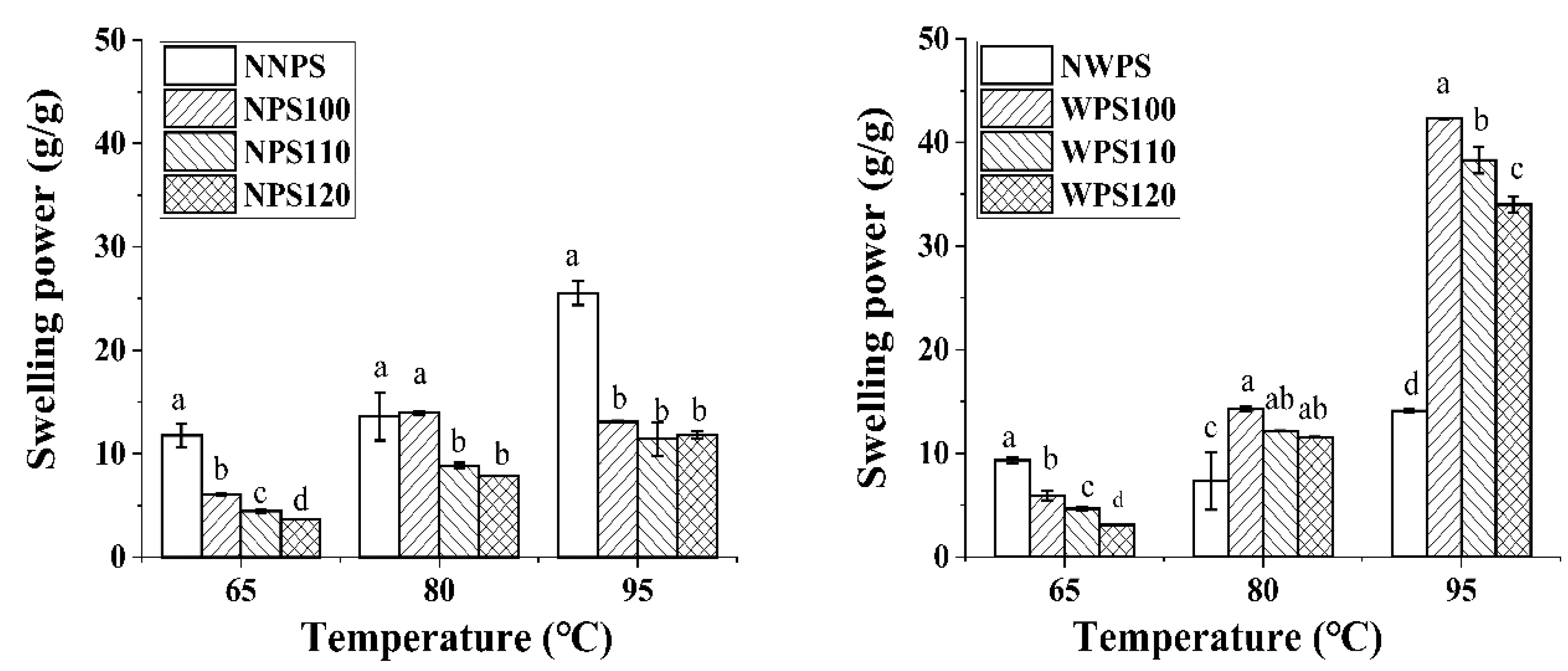
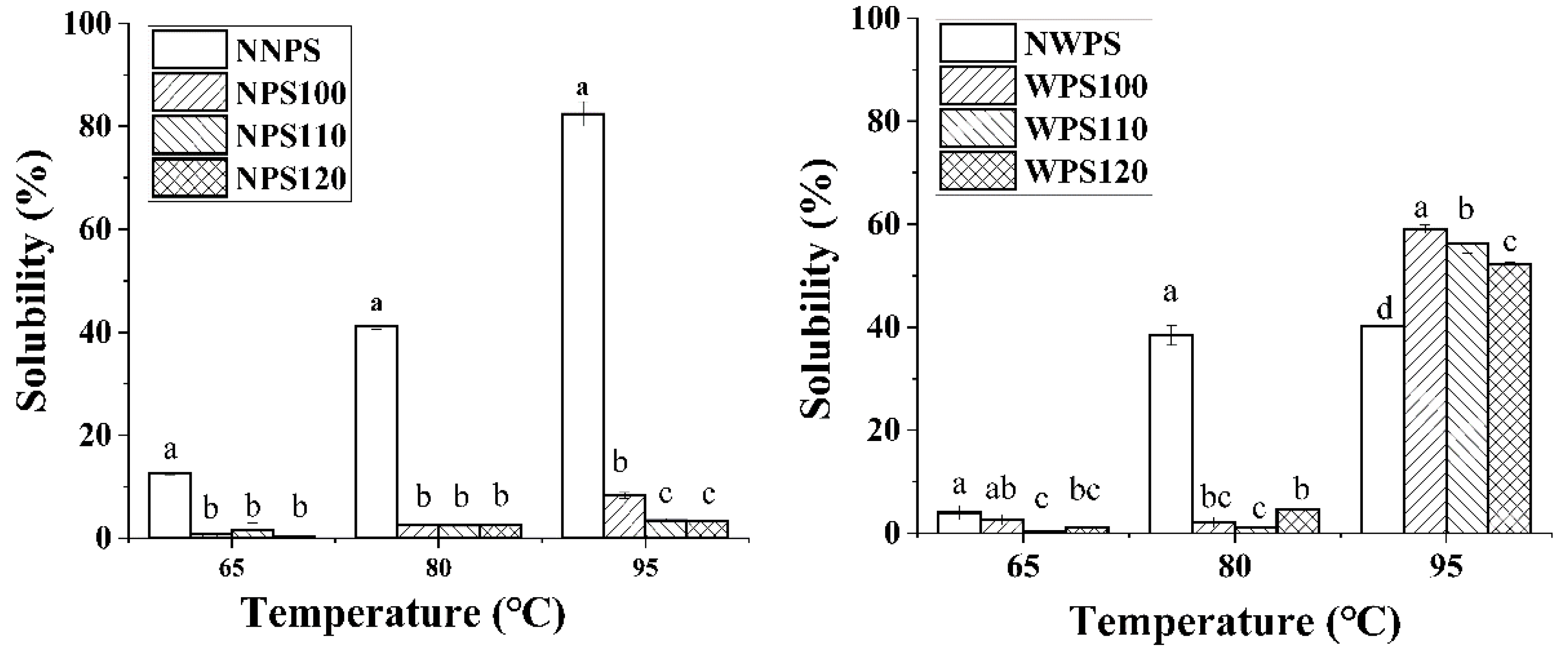
2.5. Solubility and Swelling Power
2.6. Amylose Content
2.7. Differential Scanning Calorimetry (DSC)
2.8. Pasting Properties
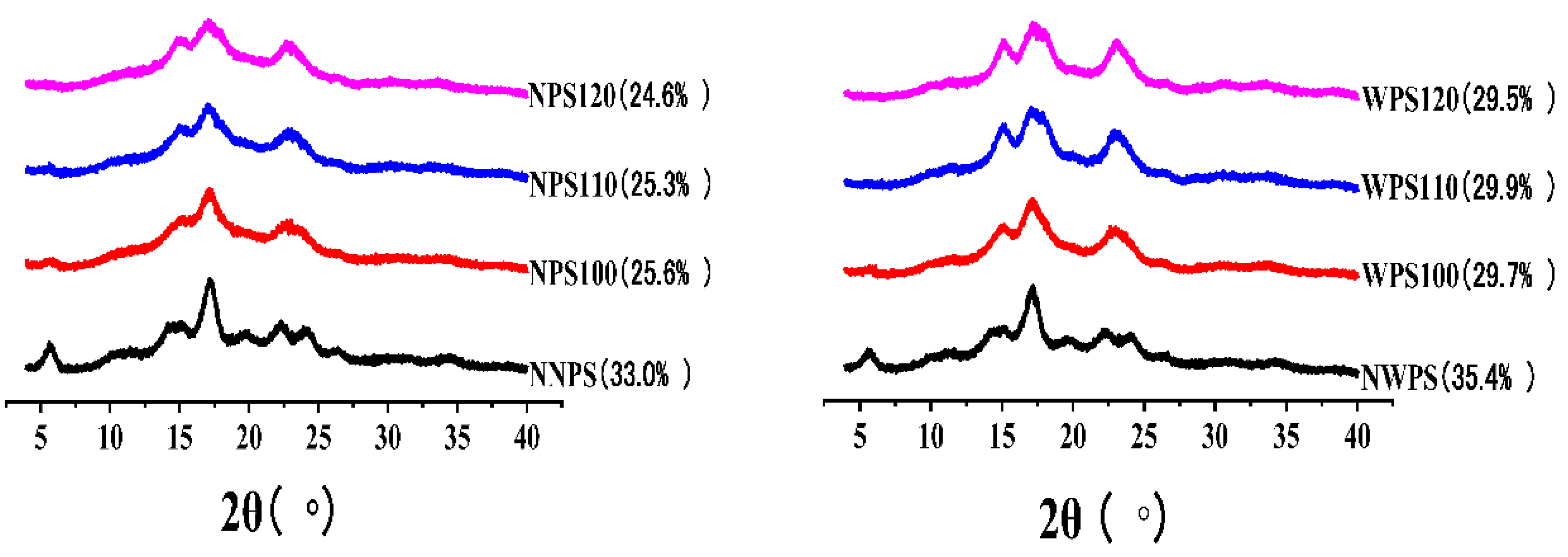
2.9. X-ray Diffraction
2.10. In-Vitro Digestion
2.11. Statistical Analysis
3. Results and Discussion
3.1. Morphology of the Starch Granules
3.2. Swelling Power
3.3. Solubility
3.4. Amylose Content
3.5. Thermal Properties
3.6. Pasting Properties
3.7. XRD Pattern
3.8. The Impact of HMT on Starch Digestibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohan, V.; Khunti, K.; Chan, S.P.; Filho, F.F.; Tran, N.Q.; Ramaiya, K.; Joshi, S.; Mithal, A.; Mbaye, M.N.; Nicodemus, N.A., Jr.; et al. Management of Type 2 Diabetes in Developing Countries: Balancing Optimal Glycaemic Control and Outcomes with Affordability and Accessibility to Treatment. Diabetes Ther. 2020, 11, 15–35. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhang, Y.; Kong, L.; Tan, L. In Vitro Starch Digestibility and In Vivo Glycemic Response of Starch Inclusion Complexes Produced With Different Methods and Hydrothermal Treatments. Curr. Dev. Nutr. 2022, 6, 442. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Mousavi Khaneghah, A.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Nep, E.I.; Ngwuluka, N.C.; Kemas, C.U.; Ochekpe, N.A. Rheological and structural properties of modified starches from the young shoots of Borassus aethiopium. Food Hydrocoll. 2016, 60, 265–270. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, H.; Wang, Y.; Zeng, S.; Zheng, B. Structural characteristics and crystalline properties of lotus seed resistant starch and its prebiotic effects. Food Chem. 2014, 155, 311–318. [Google Scholar] [CrossRef]
- Sindhu, R.; Devi, A.; Khatkar, B.S. Morphology, structure and functionality of acetylated, oxidized and heat moisture treated amaranth starches. Food Hydrocoll. 2021, 118, 106800. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Liu, C.; Zheng, X. Heat-moisture modified blue wheat starch: Physicochemical properties modulated by its multi-scale structure. Food Chem. 2022, 386, 132771. [Google Scholar] [CrossRef]
- Piecyk, M.; Domian, K. Effects of heat–moisture treatment conditions on the physicochemical properties and digestibility of field bean starch (Vicia faba var. minor). Int. J. Biol. Macromol. 2021, 182, 425–433. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.; Wang, L. Insight into the biphasic transition of heat-moisture treated waxy maize starch through controlled gelatinization. Food Chem. 2021, 341, 128214. [Google Scholar] [CrossRef]
- Xie, X.; Qi, L.; Xu, C.; Shen, Y.; Wang, H.; Zhang, H. Understanding how the cooking methods affected structures and digestibility of native and heat-moisture treated rice starches. J. Cereal Sci. 2020, 95, 103085. [Google Scholar] [CrossRef]
- Van Hung, P.; Binh, V.T.; Nhi, P.H.Y.; Phi, N.T.L. Effect of heat-moisture treatment of unpolished red rice on its starch properties and in vitro and in vivo digestibility. Int. J. Biol. Macromol. 2020, 154, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kazerski, R.T.d.S.; Biduski, B.; Weber, F.H.; Plata-Oviedo, M.S.V.; Gutkoski, L.C.; Bertolin, T.E. Substitution of chemically modified corn starch with heat-moisture treated cassava starch in Brazilian pão de queijo. Int. J. Gastron. Food Sci. 2022, 28, 100541. [Google Scholar] [CrossRef]
- Xie, Y.; Li, M.-N.; Chen, H.-Q.; Zhang, B. Effects of the combination of repeated heat-moisture treatment and compound enzymes hydrolysis on the structural and physicochemical properties of porous wheat starch. Food Chem. 2019, 274, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Trung, P.T.B.; Ngoc, L.B.B.; Hoa, P.N.; Tien, N.N.T.; Hung, P.V. Impact of heat-moisture and annealing treatments on physicochemical properties and digestibility of starches from different colored sweet potato varieties. Int. J. Biol. Macromol. 2017, 105, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Suriya, M.; Reddy, C.K.; Haripriya, S. Functional and thermal behaviors of heat-moisture treated elephant foot yam starch. Int. J. Biol. Macromol. 2019, 137, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Varatharajan, V.; Hoover, R.; Liu, Q.; Seetharaman, K. The impact of heat-moisture treatment on the molecular structure and physicochemical properties of normal and waxy potato starches. Carbohydr. Polym. 2010, 81, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Jiranuntakul, W.; Puttanlek, C.; Rungsardthong, V.; Puncha-arnon, S.; Uttapap, D. Microstructural and physicochemical properties of heat-moisture treated waxy and normal starches. J. Food Eng. 2011, 104, 246–258. [Google Scholar] [CrossRef]
- Liu, K.; Hao, Y.; Chen, Y.; Gao, Q. Effects of dry heat treatment on the structure and physicochemical properties of waxy potato starch. Int. J. Biol. Macromol. 2019, 132, 1044–1050. [Google Scholar] [CrossRef]
- Yassaroh, Y.; Nurhaini, F.F.; Woortman, A.J.J.; Loos, K. Physicochemical properties of heat-moisture treated, sodium stearate complexed starch: The effect of sodium stearate concentration. Carbohydr. Polym. 2021, 269, 118263. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M..; Cummings, J.H. Classification and Measurement of Nutritionally Important Starch Fractions. Eur. J. Clin. Nutr. 1992, 46, s33–s50. [Google Scholar]
- Liu, C.; Hong, J.; Zheng, X. Effect of heat-moisture treatment on morphological, structural and functional characteristics of ball-milled wheat starches. Starch Stärke 2017, 69, 1500141. [Google Scholar] [CrossRef]
- Liu, C.; Song, M.; Liu, L.; Hong, J.; Guan, E.; Bian, K.; Zheng, X. Effect of heat-moisture treatment on the structure and physicochemical properties of ball mill damaged starches from different botanical sources. Int. J. Biol. Macromol. 2020, 156, 403–410. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Li, X.; Chen, L.; Zhang, B. Multi-scale structure, pasting and digestibility of heat moisture treated red adzuki bean starch. Int. J. Biol. Macromol. 2017, 102, 162–169. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Chen, L.; Li, X.; Wang, J.; Xie, F. Insights into the multi-scale structure and digestibility of heat-moisture treated rice starch. Food Chem. 2018, 242, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q. Starch chain interactions within the amorphous and crystalline domains of pulse starches during heat-moisture treatment at different temperatures and their impact on physicochemical properties. Food Chem. 2014, 143, 175–184. [Google Scholar] [CrossRef]
- Li, S.; Ward, R.; Gao, Q. Effect of heat-moisture treatment on the formation and physicochemical properties of resistant starch from mung bean (Phaseolus radiatus) starch. Food Hydrocoll. 2011, 25, 1702–1709. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, H.; Wei, T.; Shen, J.; Wang, M. Differences in physicochemical properties and in vitro digestibility between tartary buckwheat flour and starch modified by heat-moisture treatment. LWT Food Sci. Technol. 2017, 86, 285–292. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, S. Influence of heat-moisture treatment (HMT) on physicochemical and functional properties of starches from different Indian oat (Avena sativa L.) cultivars. Int. J. Biol. Macromol. 2019, 122, 312–319. [Google Scholar] [CrossRef]
- Tarahi, M.; Shahidi, F.; Hedayati, S. Physicochemical, Pasting, and Thermal Properties of Native Corn Starch-Mung Bean Protein Isolate Composites. Gels 2022, 8, 693. [Google Scholar] [CrossRef]
- Liu, H.; Lv, M.M.; Wang, L.J.; Li, Y.L.; Fan, H.H.; Wang, M. Comparative study: How annealing and heat-moisture treatment affect the digestibility, textural, and physicochemical properties of maize starch. Starch Starke 2016, 68, 1158–1168. [Google Scholar] [CrossRef]
- Goel, C.; Semwal, A.D.; Khan, A.; Kumar, S.; Sharma, G.K. Physical modification of starch: Changes in glycemic index, starch fractions, physicochemical and functional properties of heat-moisture treated buckwheat starch. J. Food Sci. Technol. Mysore 2020, 57, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, L.; Zheng, X. Recent advances in heat-moisture modified cereal starch: Structure, functionality and its applications in starchy food systems. Food Chem. 2021, 344, 128700. [Google Scholar] [CrossRef] [PubMed]
- Zavareze, E.d.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Han, L.; Wei, Q.; Cao, S.; Yu, Y.; Cao, X.; Chen, W. The assisting effects of ultrasound on the multiscale characteristics of heat-moisture treated starch from Agriophyllum squarrosum seeds. Int. J. Biol. Macromol. 2021, 187, 471–480. [Google Scholar] [CrossRef]
- Manuel, R.H.a.H. The Effect of Heat–Moisture Treatment on the Structure and Physicochemical Properties of Normal Maize, Waxy Maize, Dull Waxy Maize and Amylomaize V Starches. J. Cereal Sci. 1996, 23, 153–162. [Google Scholar]
- Chung, H.J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Singh, H.; Chang, Y.-H.; Lin, J.H.; Sodhi, N.S.; Singh, N. Influence of heat–moisture treatment and annealing on functional properties of sorghum starch. Food Res. Int. 2011, 44, 2949–2954. [Google Scholar] [CrossRef]
- Na, J.H.; Kim, H.R.; Kim, Y.; Lee, J.S.; Park, H.J.; Moon, T.W.; Lee, C.J. Structural characteristics of low-digestible sweet potato starch prepared by heat-moisture treatment. Int. J. Biol. Macromol. 2020, 151, 1049–1057. [Google Scholar] [CrossRef]
- Xia, H.; Li, Y.; Gao, Q. Preparation and properties of RS4 citrate sweet potato starch by heat-moisture treatment. Food Hydrocoll. 2016, 55, 172–178. [Google Scholar] [CrossRef]
- Lazaridou, A.; Marinopoulou, A.; Biliaderis, C.G. Impact of flour particle size and hydrothermal treatment on dough rheology and quality of barley rusks. Food Hydrocoll. 2019, 87, 561–569. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, B.J.; Chen, L.; Li, X.X.; Zheng, B. Hierarchical structure and physicochemical properties of highland barley starch following heat moisture treatment. Food Chem. 2019, 271, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Yassaroh, Y.; Woortman, A.J.J.; Loos, K. A new way to improve physicochemical properties of potato starch. Carbohydr. Polym. 2019, 204, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-F.; Liu, W.; Whaley, J.K.; Shi, Y.-C. Structure, properties, and potential applications of waxy tapioca starches—A review. Trends Food Sci. Technol. 2019, 83, 225–234. [Google Scholar] [CrossRef]
- Zeng, F.; Ma, F.; Kong, F.; Gao, Q.; Yu, S. Physicochemical properties and digestibility of hydrothermally treated waxy rice starch. Food Chem. 2015, 172, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Oyeyinka, S.A.; Oyeyinka, A.T. A review on isolation, composition, physicochemical properties and modification of Bambara groundnut starch. Food Hydrocoll. 2018, 75, 62–71. [Google Scholar] [CrossRef]
- Tan, X.; Li, X.; Chen, L.; Xie, F.; Li, L.; Huang, J. Effect of heat-moisture treatment on multi-scale structures and physicochemical properties of breadfruit starch. Carbohydr. Polym. 2017, 161, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, D.H.; Kim, J.Y. Effect of heat-moisture treatment under mildly acidic condition on fragmentation of waxy maize starch granules into nanoparticles. Food Hydrocoll. 2017, 63, 59–66. [Google Scholar] [CrossRef]
- Lin, C.L.; Lin, J.H.; Lin, J.J.; Chang, Y.H. Progressive alterations in crystalline structure of starches during heat-moisture treatment with varying iterations and holding times. Int. J. Biol. Macromol. 2019, 135, 472–480. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Q.; Xu, X.; Qi, L.; Dong, Z.; Luo, Z.; Lu, X.; Peng, X. Structural changes of waxy and normal maize starches modified by heat moisture treatment and their relationship with starch digestibility. Carbohydr. Polym. 2017, 177, 232–240. [Google Scholar] [CrossRef]
- Han, M.J.; Bao, W.J.; Wu, Y.W.; Ouyang, J. Insights into the effects of caffeic acid and amylose on in vitro digestibility of maize starch-caffeic acid complex. Int. J. Biol. Macromol. 2020, 162, 922–930. [Google Scholar] [CrossRef]
- Yang, X.; Chi, C.; Liu, X.; Zhang, Y.; Zhang, H.; Wang, H. Understanding the structural and digestion changes of starch in heat-moisture treated polished rice grains with varying amylose content. Int. J. Biol. Macromol. 2019, 139, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Du, X.; Chen, P.; Guo, L.; Xu, Y.; Zhou, X. Morphologies and gelatinization behaviours of high-amylose maize starches during heat treatment. Carbohydr. Polym. 2017, 157, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Dhital, S.; Zhang, M.-N.; Wang, J.; Chen, Z.-G. Structural, gelatinization, and rheological properties of heat-moisture treated potato starch with added salt and its application in potato starch noodles. Food Hydrocoll. 2022, 131, 107802. [Google Scholar] [CrossRef]
- Kim, H.R.; Choi, S.J.; Choi, H.D.; Park, C.S.; Moon, T.W. Amylosucrase-modified waxy potato starches recrystallized with amylose: The role of amylopectin chain length in formation of low-digestible fractions. Food Chem. 2020, 318, 126490. [Google Scholar] [CrossRef] [PubMed]



| Sample | Amylose Content (%) | Sample | Amylose Content (%) |
|---|---|---|---|
| NNPS | 40.68 ± 0.23 a | NWPS | 3.40 ± 0.38 a |
| NPS100 | 40.63 ± 0.31 a | WPS100 | 3.02 ± 0.30 a |
| NPS110 | 39.49 ± 0.10 a | WPS110 | 3.02 ± 0.00 a |
| NPS120 | 40.68 ± 1.31 a | WPS120 | 2.70 ± 0.46 a |
| Sample | To (°C) | Tp (°C) | Tc (°C) | Tc–To (°C) | ΔH (J/g) |
|---|---|---|---|---|---|
| NNPS | 64.37 ± 1.67 d | 68.90 ± 2.09 c | 76.57 ± 2.34 c | 12.20 ± 0.67 a | 15.74 ± 0.67 a |
| NPS100 | 76.31 ± 0.21 c | 84.43 ± 0.00 b | 94.46 ± 0.64 b | 18.15 ± 0.86 a | 9.29 ± 1.18 b |
| NPS110 | 82.16 ± 0.64 b | 87.95 ± 0.80 ab | 96.12 ± 0.11 b | 14.14 ± 0.27 a | 7.73 ± 1.87 b |
| NPS120 | 91.25 ± 1.26 a | 99.67 ± 5.34 a | 110.41 ± 5.42 a | 19.16 ± 4.16 a | 6.75 ± 1.27 b |
| WPS | 68.06 ± 0.08 b | 73.27 ± 0.00 a | 79.90 ± 0.07 b | 11.85 ± 0.15 b | 14.68 ± 0.60 a |
| WPS100 | 69.61 ± 0.23 b | 82.97 ± 0.11 a | 88.21 ± 0.08 ab | 18.61 ± 0.15 a | 8.52 ± 1.53 b |
| WPS110 | 71.58 ± 0.80 a | 82.03 ± 7.40 a | 93.90 ± 1.14 a | 22.32 ± 0.34 a | 9.40 ± 0.45 b |
| WPS120 | 74.44 ± 1.94 a | 82.58 ± 1.03 a | 92.78 ± 4.65 a | 18.34 ± 2.71 a | 8.31 ± 2.13 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, G.; Liu, K.; Gao, Q. Effects of Heat-Moisture Treatment on the Digestibility and Physicochemical Properties of Waxy and Normal Potato Starches. Foods 2023, 12, 68. https://doi.org/10.3390/foods12010068
Fang G, Liu K, Gao Q. Effects of Heat-Moisture Treatment on the Digestibility and Physicochemical Properties of Waxy and Normal Potato Starches. Foods. 2023; 12(1):68. https://doi.org/10.3390/foods12010068
Chicago/Turabian StyleFang, Guihong, Ke Liu, and Qunyu Gao. 2023. "Effects of Heat-Moisture Treatment on the Digestibility and Physicochemical Properties of Waxy and Normal Potato Starches" Foods 12, no. 1: 68. https://doi.org/10.3390/foods12010068




