Tofu Whey Wastewater as a Beneficial Supplement to Poultry Farming: Improving Production Performance and Protecting against Salmonella Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Chemical Composition Analysis
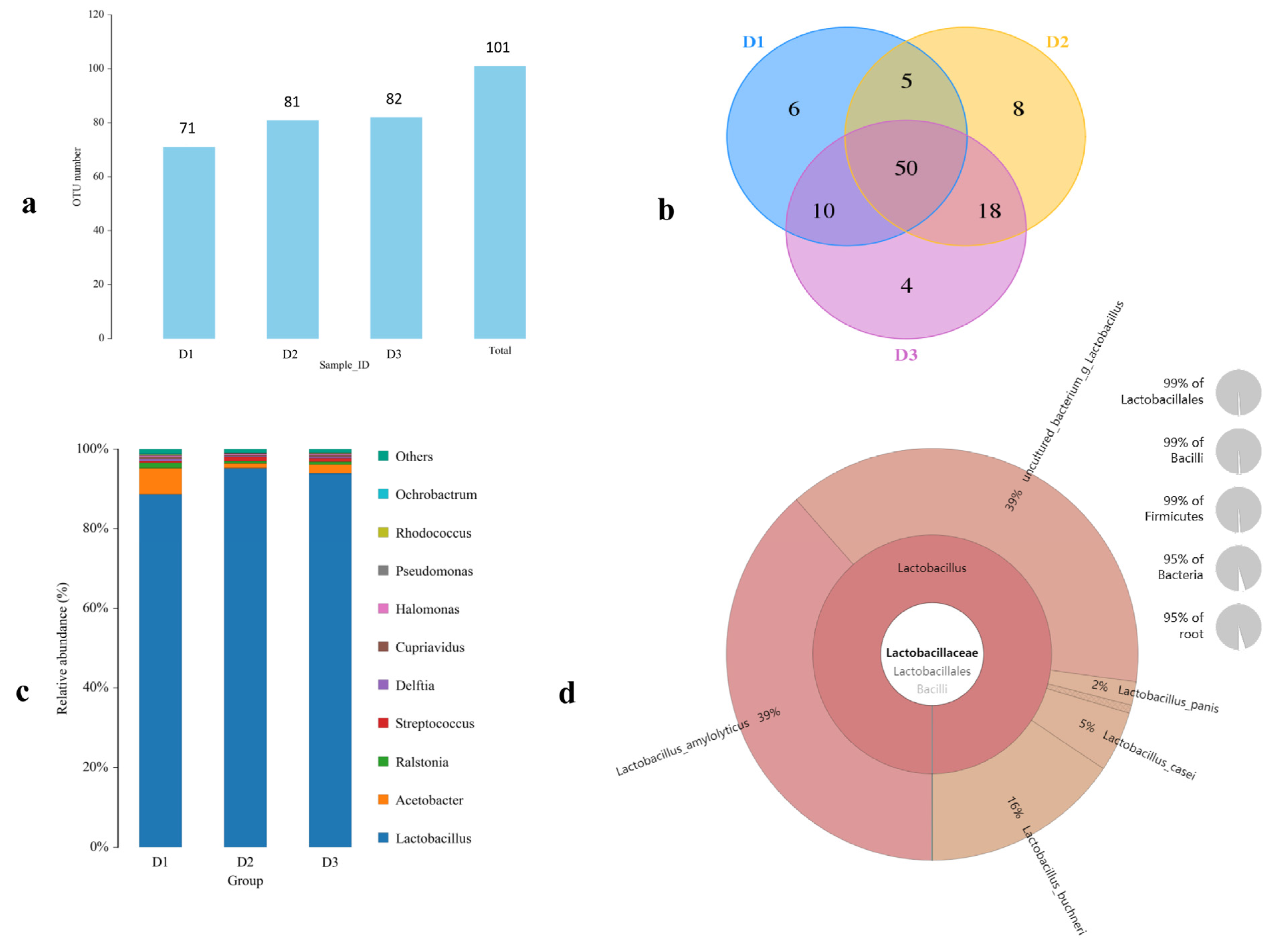
2.2. Microbiota Analysis of TWW
2.3. Chickens and Experiments Design
2.3.1. Effects of TWW Supplementation on Chicken Performance, Organ Index, Antioxidant Capacity, and Immunity
2.3.2. Effects of TWW Supplementation on the Resistance of Chickens Infected with S. enteritidis
2.4. Determination of Organ Index
2.5. Determination of Biochemical Indicators
2.6. Determination of Immune Parameters
2.7. Determination of S. enteritidis in Feces Samples
2.8. Histological Analyses of Intestinal Tissue
2.9. Immunofluorescence of ZO-1 in Jejunum Tissues
2.10. Quantitative Real-Time PCR Analysis
2.11. Gut Microbiota Analysis
2.12. Data Analysis and Statistics
3. Results
3.1. The Chemical Parameters and Microbial Composition of TWW
3.2. Effects of TWW Supplementation on Chicken Performance, Organ Index, Antioxidant Capacity, and Immunity
3.2.1. Effects of TWW Supplementation on Chicken Performance
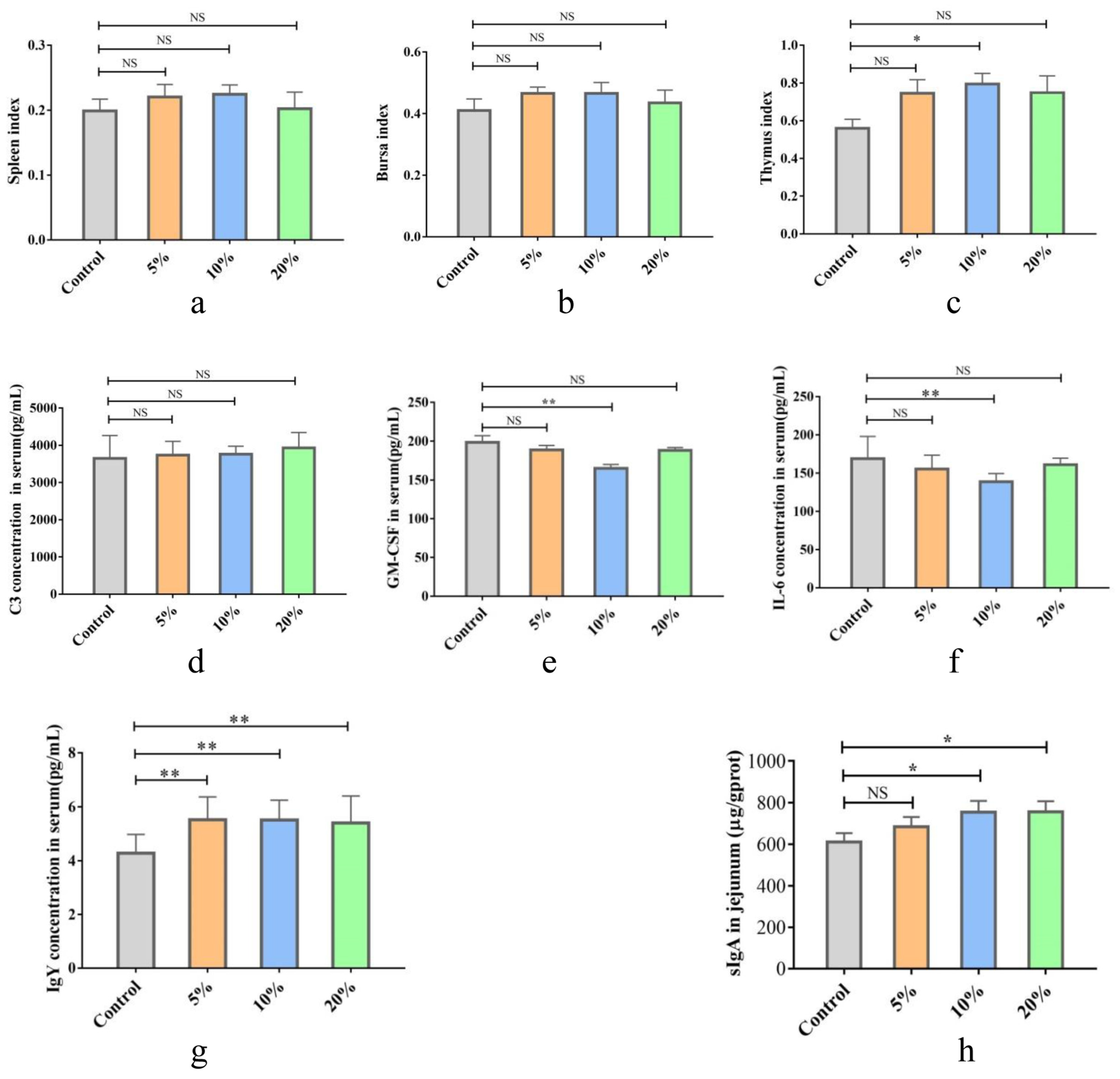
3.2.2. Effects of TWW Supplementation on Chicken Organ Index
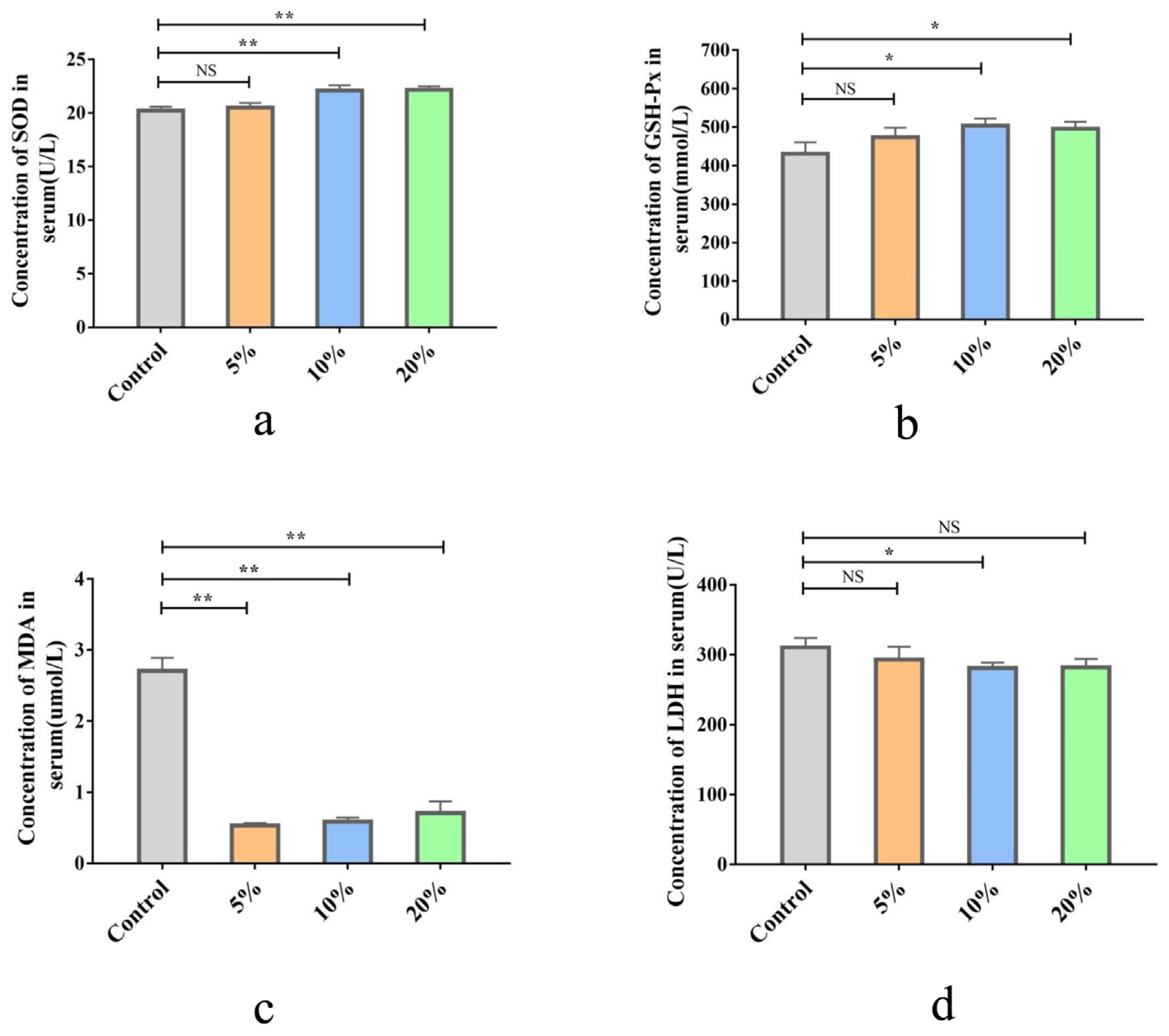
3.2.3. Effects of TWW Supplementation on Antioxidant Capacity
3.2.4. Effects of TWW Supplementation on Chicken Immunity
3.3. Effects of TWW Supplementation on Chicken Infected with S. enteritidis
3.3.1. Effects of TWW Supplementation on the Pathological Changes of Chicks Infected with S. enteritidis
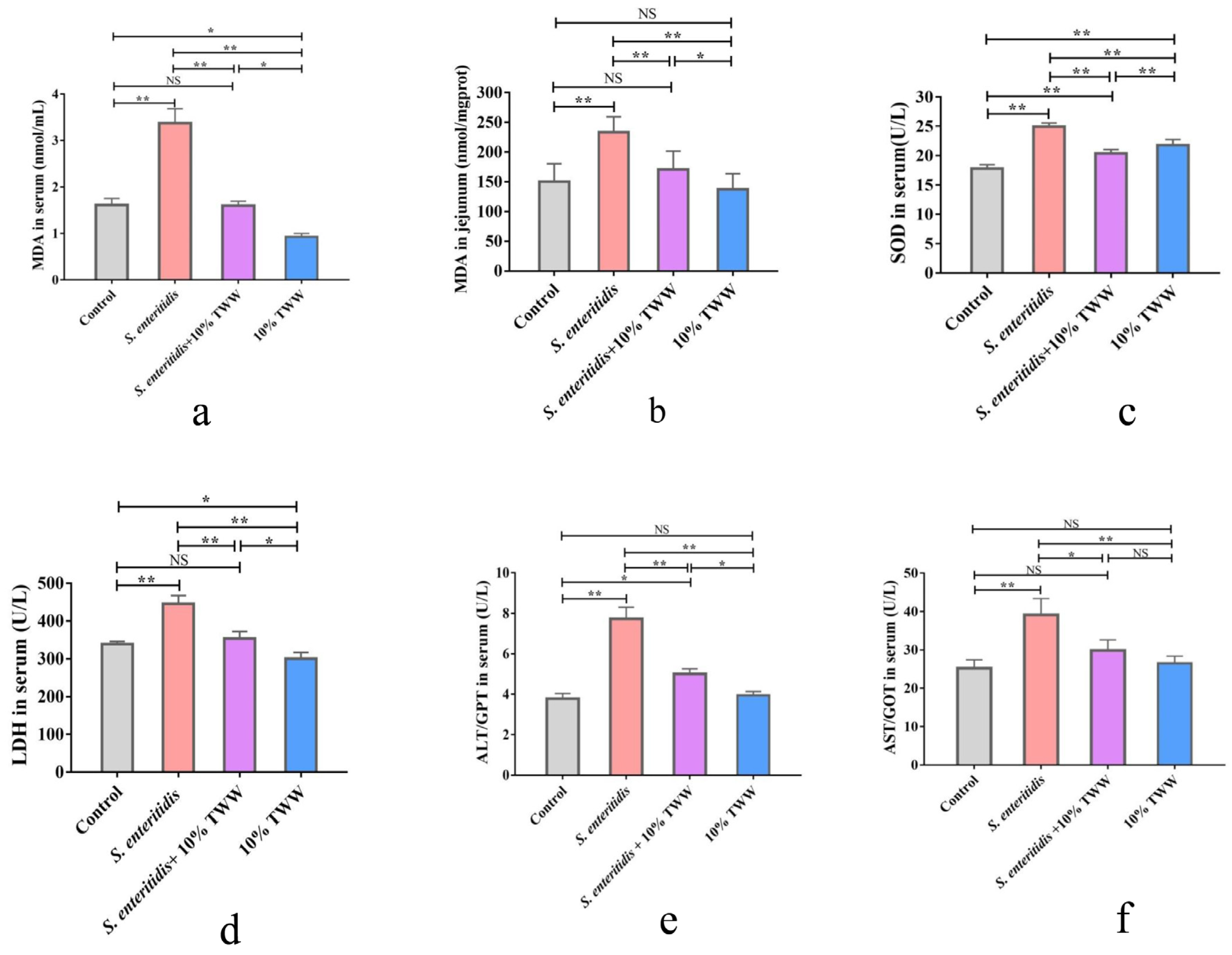
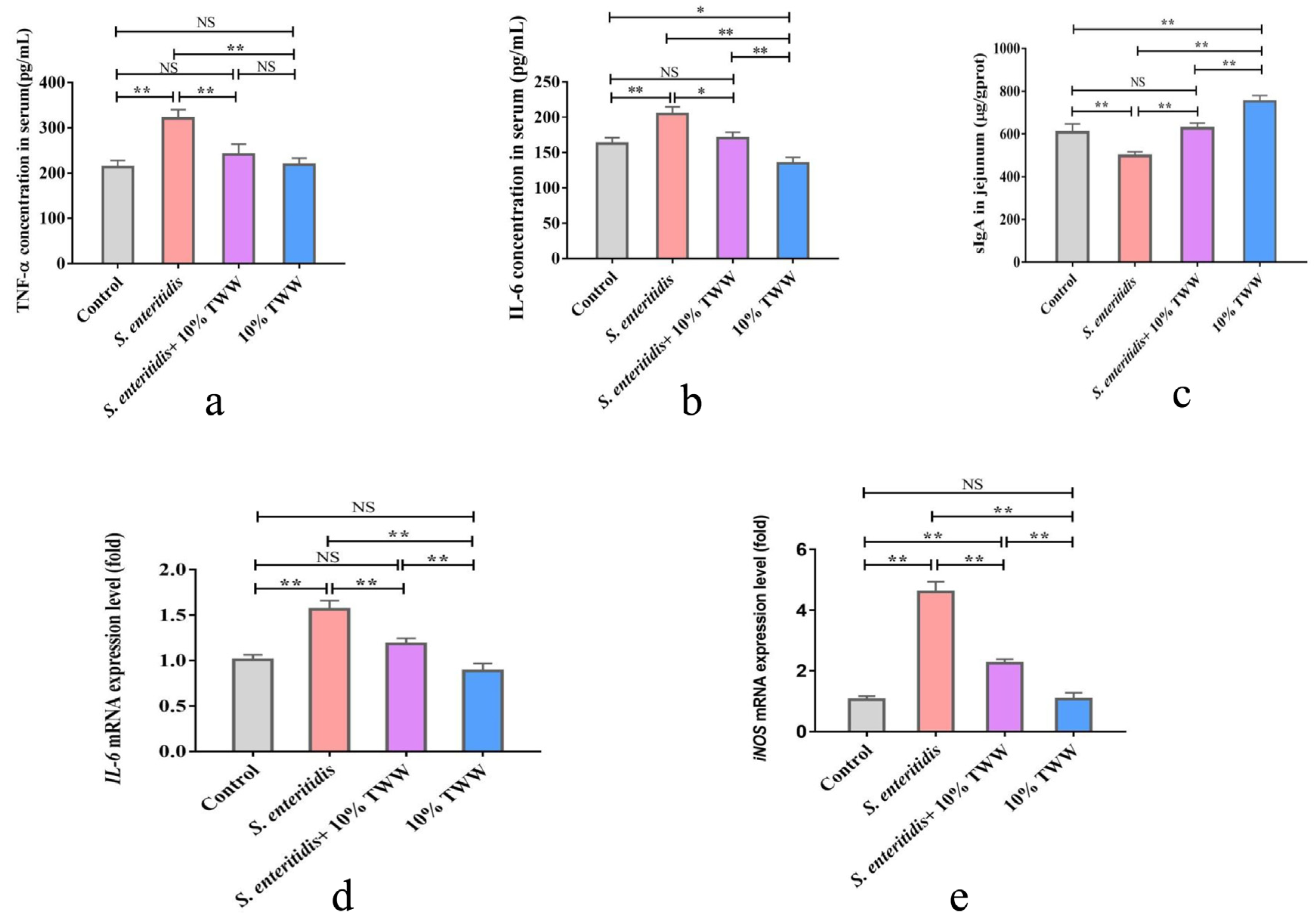
3.3.2. Effects of TWW Supplementation on the Oxidative Stress Index in S. enteritidis-Infected Chickens
3.3.3. Effects of TWW Supplementation on the Immune Function in S. enteritidis-Infected Chickens
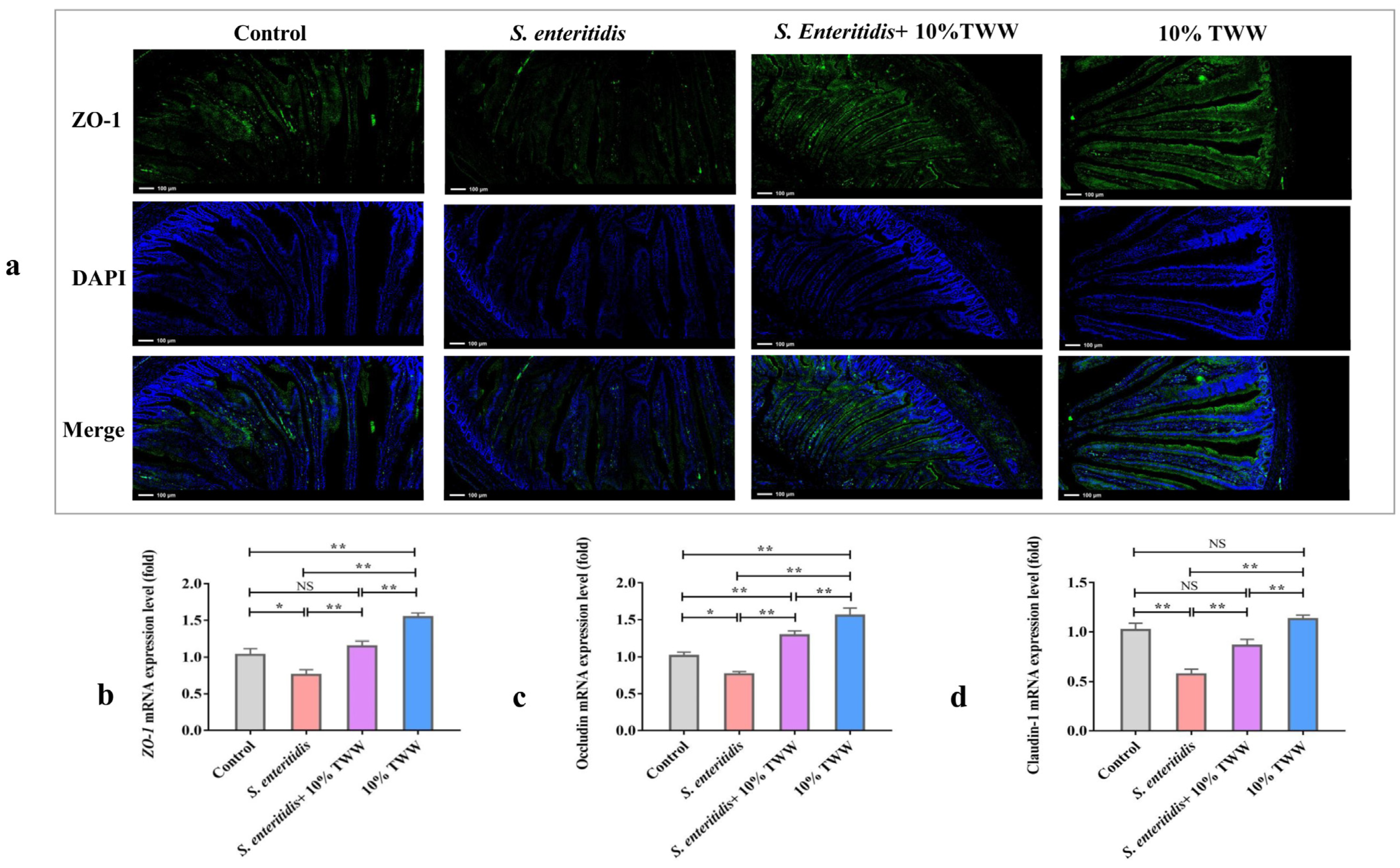
3.3.4. Effect of TWW Supplementation on the Epithelial Tight Junction Protein Expression of Jejunum
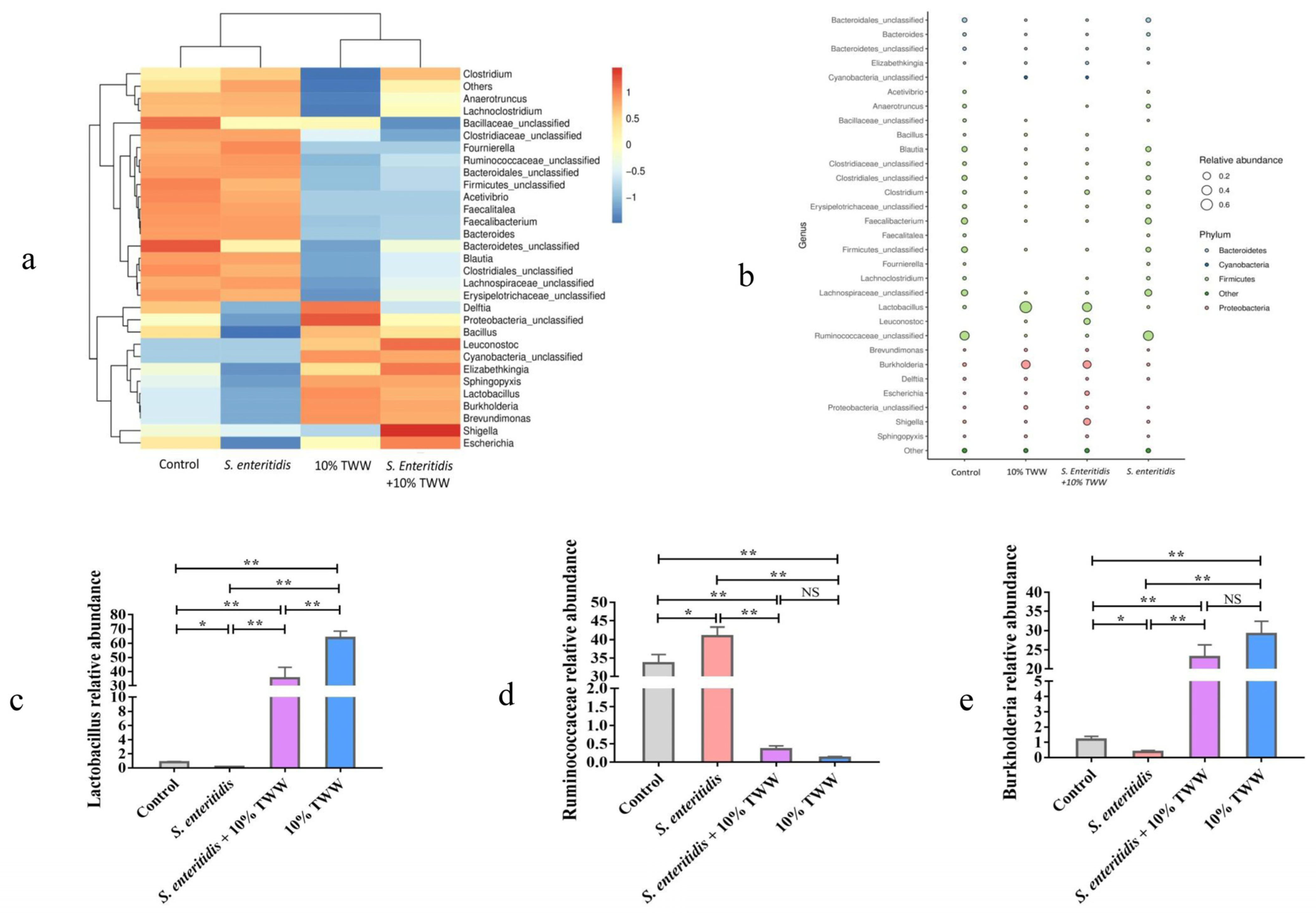
3.3.5. Effect of TWW Supplementation on the Gut Microbiota
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Corzo-Martínez, M.; García-Campos, G.; Montilla, A.; Moreno, F.J. Tofu Whey Permeate Is an Efficient Source To Enzymatically Produce Prebiotic Fructooligosaccharides and Novel Fructosylated α-Galactosides. J. Agric. Food Chem. 2016, 64, 4346–4352. [Google Scholar] [CrossRef]
- Chua, J.-Y.; Liu, S.-Q. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Technol. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Nieto-Veloza, A.; Zhong, Q.; Kim, W.S.; D’Souza, D.; Krishnan, H.B.; Dia, V.P. Utilization of tofu processing wastewater as a source of the bioactive peptide lunasin. Food Chem. 2021, 362, 130220. [Google Scholar] [CrossRef]
- Wang, S.K.; Tian, Y.T.; Dai, Y.R.; Wang, D.; Liu, K.C.; Cui, Y.H. Development of an alternative medium via completely replaces the medium components by mixed wastewater and crude glycerol for efficient production of docosahexaenoic acid by Schizochytrium sp. Chemosphere 2022, 291 Pt 1, 132868. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, X.; Shen, Y.; Yao, H.; Wang, P.; Ye, K.; Wang, X.; Gu, Q. Enhancing the vitamin B12 production and growth of Propionibacterium freudenreichii in tofu wastewater via a light-induced vitamin B12 riboswitch. Appl. Microbiol. Biotechnol. 2015, 99, 10481–10488. [Google Scholar] [CrossRef]
- Li, T.; Zhan, C.; Guo, G.; Liu, Z.; Hao, N.; Ouyang, P. Tofu processing wastewater as a low-cost substrate for high activity nattokinase production using Bacillus subtilis. BMC Biotechnol. 2021, 21, 57. [Google Scholar] [CrossRef]
- Wang, S.K.; Wang, X.; Miao, J.; Tian, Y.T. Tofu whey wastewater is a promising basal medium for microalgae culture. Bioresour. Technol. 2018, 253, 79–84. [Google Scholar] [CrossRef]
- Fei, Y.; Li, L.; Chen, L.; Zheng, Y.; Yu, B. High-throughput sequencing and culture-based approaches to analyze microbial diversity associated with chemical changes in naturally fermented tofu whey, a traditional Chinese tofu-coagulant. Food Microbiol. 2018, 76, 69–77. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Hao, L.; Zhang, G.; Jin, Z.; Li, C.; Yang, Y.; Rao, J.; Chen, B. Traditional fermented soybean products: Processing, flavor formation, nutritional and biological activities. Crit. Rev. Food Sci. Nutr. 2022, 62, 1971–1989. [Google Scholar] [CrossRef]
- Fei, Y.; Jiao, W.; Wang, Y.; Liang, J.; Liu, G.; Li, L. Cloning and expression of a novel α-galactosidase from Lactobacillus amylolyticus L6 with hydrolytic and transgalactosyl properties. PLoS ONE 2020, 15, e0235687. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.U.; Jahid, I.K. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019, 19, 253. [Google Scholar] [CrossRef]
- Tabashsum, Z.; Peng, M.; Alvarado-Martinez, Z.; Aditya, A.; Bhatti, J.; Romo, P.B.; Young, A.; Biswas, D. Competitive reduction of poultry-borne enteric bacterial pathogens in chicken gut with bioactive Lactobacillus casei. Sci. Rep. 2020, 10, 16259. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, Z.; Yu, L.; Jiang, K.; Xia, J.; Mi, Y.; Zhang, C.; Li, J. Lactobacillus salivarius and Lactobacillus agilis feeding regulates intestinal stem cells activity by modulating crypt niche in hens. Appl. Microbiol Biotechnol. 2021, 105, 8823–8835. [Google Scholar] [CrossRef]
- Kim, I.S.; Yang, W.S.; Kim, C.H. Beneficial Effects of Soybean-Derived Bioactive Peptides. Int. J. Mol. Sci. 2021, 22, 8570. [Google Scholar] [CrossRef]
- Chelliah, R.; Wei, S.; Daliri, E.B.; Elahi, F.; Yeon, S.J.; Tyagi, A.; Liu, S.; Madar, I.H.; Sultan, G.; Oh, D.H. The Role of Bioactive Peptides in Diabetes and Obesity. Foods 2021, 10, 2220. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Indiano-Romacho, P.; Mora-Gutiérrez, I.; Pérez-Rodríguez, L.; Ortega Moreno, L.; Marin, A.C.; Baldán-Martín, M.; Moreno-Monteagudo, J.A.; Santander, C.; Chaparro, M.; et al. Lunasin Peptide is a Modulator of the Immune Response in the Human Gastrointestinal Tract. Mol. Nutr. Food Res. 2021, 65, e2001034. [Google Scholar] [CrossRef]
- Hai, D.; Yin, X.; Lu, Z.; Lv, F.; Zhao, H.; Bie, X. Occurrence, drug resistance, and virulence genes of Salmonella isolated from chicken and eggs. Food Control 2020, 113, 107109. [Google Scholar] [CrossRef]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef]
- Efsa. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2011. EFSA J. 2011, 11, 19–73. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Chen, J.; Chen, L.; Qi, X.; Zhang, R. Epidemiology of Foodborne Disease Outbreaks Caused by Nontyphoidal Salmonella in Zhejiang Province, China, 2010-2019. Foodborne Pathog. Dis. 2021, 18, 880–886. [Google Scholar] [CrossRef]
- Song, Y.; Wang, F.; Liu, Y.; Song, Y.; Zhang, L.; Zhang, F.; Gu, X.; Sun, S. Occurrence and Characterization of Salmonella Isolated From Chicken Breeder Flocks in Nine Chinese Provinces. Front. Vet. Sci. 2020, 7, 479. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, X.; Jiang, Z.; Qi, Y.; Ed-Dra, A.; Yue, M. Epidemiological Investigation and Antimicrobial Resistance Profiles of Salmonella Isolated From Breeder Chicken Hatcheries in Henan, China. Front. Cell Infect. Microbiol. 2020, 10, 497. [Google Scholar] [CrossRef]
- Lyu, N.; Feng, Y.; Pan, Y.; Huang, H.; Liu, Y.; Xue, C.; Zhu, B.; Hu, Y. Genomic Characterization of Salmonella enterica Isolates From Retail Meat in Beijing, China. Front. Microbiol. 2021, 12, 636332. [Google Scholar] [CrossRef]
- Shen, X.; Yin, L.; Ma, H.; Pan, X.; Zhang, D.; Zhao, R.; Dai, Y.; Hou, H.; Hu, X. Comprehensive genomic analysis and characterization of a new ST 174 type Klebsiella variicola strain isolated from chicken embryos. Infect. Genet. Evol. 2021, 90, 104768. [Google Scholar] [CrossRef]
- Okoń, A.; Szymański, P.; Zielińska, D.; Szydłowska, A.; Siekierko, U.; Kołożyn-Krajewska, D.; Dolatowski, Z.J. The Influence of Acid Whey on the Lipid Composition and Oxidative Stability of Organic Uncured Fermented Bacon after Production and during Chilling Storage. Antioxidants 2021, 10, 1711. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, Q.; Zhang, H.; Yu, Y.; Li, X.; Zhang, Z.; Zhang, L. Naturally Fermented Acid Slurry of Soy Whey: High-Throughput Sequencing-Based Characterization of Microbial Flora and Mechanism of Tofu Coagulation. Front. Microbiol. 2019, 10, 1088. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Li, L. A new style of fermented tofu by Lactobacillus casei combined with salt coagulant. 3 Biotech. 2020, 10, 81. [Google Scholar] [CrossRef]
- Fei, Y.; Li, L.; Zheng, Y.; Liu, D.; Zhou, Q.; Fu, L. Characterization of Lactobacillus amylolyticus L6 as potential probiotics based on genome sequence and corresponding phenotypes. LWT 2018, 90, 460–468. [Google Scholar] [CrossRef]
- Ahmed, Z.; Vohra, M.S.; Khan, M.N.; Ahmed, A.; Khan, T.A. Antimicrobial role of Lactobacillus species as potential probiotics against enteropathogenic bacteria in chickens. J. Infect. Dev. Ctries. 2019, 13, 130–136. [Google Scholar] [CrossRef]
- Kowalska, J.D.; Nowak, A.; Śliżewska, K.; Stańczyk, M.; Łukasiak, M.; Dastych, J. Anti-Salmonella Potential of New Lactobacillus Strains with the Application in the Poultry Industry. Pol. J. Microbiol. 2020, 69, 5–18. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Yu, Y.; Zhang, R.; Wu, Y.; Yue, M.; Yang, C. Effects of Bacillus Coagulans on growth performance, antioxidant capacity, immunity function, and gut health in broilers. Poult. Sci. 2021, 100, 101168. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, H.; Han, P.; Li, Y.; Sun, Y.; Yuan, J.; Wang, Y.; Ni, A.; Zong, Y.; Bian, S.; et al. Effects of feed systems on growth performance, carcass characteristics, organ index, and serum biochemical parameters of pigeon. Poult. Sci. 2022, 101, 102224. [Google Scholar] [CrossRef]
- Chen, S.; Yan, C.; Xiao, J.; Liu, W.; Li, Z.; Liu, H.; Liu, J.; Zhang, X.; Ou, M.; Chen, Z.; et al. Domestication and Feed Restriction Programming Organ Index, Dopamine, and Hippocampal Transcriptome Profile in Chickens. Front. Vet. Sci. 2021, 8, 701850. [Google Scholar] [CrossRef]
- Liu, Y.S.; Zhang, Y.Y.; Li, J.L.; Wang, X.F.; Xing, T.; Zhu, X.D.; Zhang, L.; Gao, F. Growth performance, carcass traits and digestive function of broiler chickens fed diets with graded levels of corn resistant starch. Br. Poult. Sci. 2020, 61, 146–155. [Google Scholar] [CrossRef]
- Jiménez-Moreno, E.; González-Alvarado, J.M.; González-Serrano, A.; Lázaro, R.; Mateos, G.G. Effect of dietary fiber and fat on performance and digestive traits of broilers from one to twenty-one days of age. Poult. Sci. 2009, 88, 2562–2574. [Google Scholar] [CrossRef]
- Iji, P.A.; Khumalo, K.; Slippers, S.; Gous, R.M. Intestinal function and body growth of broiler chickens on diets based on maize dried at different temperatures and supplemented with a microbial enzyme. Reprod Nutr. Dev. 2003, 43, 77–90. [Google Scholar] [CrossRef]
- Elsenhans, B.; Caspary, W.F. Food viscosity as determinant for adaptive growth responses in rat intestine: Long-term feeding of different hydroxyethyl celluloses. Br. J. Nutr. 2000, 84, 39–48. Available online: https://pubmed.ncbi.nlm.nih.gov/10961159/ (accessed on 3 September 2022).
- Gast, R.K.; Jones, D.R.; Guraya, R.; Garcia, J.S.; Karcher, D.M. Research Note: Internal organ colonization by Salmonella Enteritidis in experimentally infected layer pullets reared at different stocking densities in indoor cage-free housing. Poult. Sci. 2022, 101, 102104. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Wu, H.; Wang, H.; Bian, H.; Zhu, Y.; Xu, W.; Liu, F.; Wang, D.; Fu, L. Antibacterial activity and action mode of chlorogenic acid against Salmonella Enteritidis, a foodborne pathogen in chilled fresh chicken. World J. Microbiol. Biotechnol. 2020, 36, 24. [Google Scholar] [CrossRef]
- Ansari-Lari, M.; Hosseinzadeh, S.; Manzari, M.; Khaledian, S. Survey of Salmonella in commercial broiler farms in Shiraz, southern Iran. Prev. Vet. Med. 2022, 198, 105550. [Google Scholar] [CrossRef]
- Monte, D.F.M.; Andrigheto, C.; Ribeiro, V.B.; Landgraf, M.; Destro, M.T. Highly clonal relationship among Salmonella Enteritidis isolates in a commercial chicken production chain, Brazil. Braz. J. Microbiol. 2020, 51, 2049–2056. [Google Scholar] [CrossRef]
- Khatun, M.F.; Khan, M.A.S.; Ahmed, M.F.; Rahman, M.M.; Rahman, S.R. Assessment of foodborne transmission of Salmonella enteritidis in hens and eggs in Bangladesh. Vet. Med. Sci. 2022, 8, 2032–2039. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Van Immerseel, F. Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol. Rev. 2009, 33, 718–738. [Google Scholar] [CrossRef]
- Nakphaichit, M.; Sobanbua, S.; Siemuang, S.; Vongsangnak, W.; Nakayama, J.; Nitisinprasert, S. Protective effect of Lactobacillus reuteri KUB-AC5 against Salmonella Enteritidis challenge in chickens. Benef. Microbes. 2019, 10, 43–54. [Google Scholar] [CrossRef]
- Gu, Y.F.; Chen, Y.P.; Jin, R.; Wang, C.; Wen, C.; Zhou, Y.M. Age-related changes in liver metabolism and antioxidant capacity of laying hens. Poult. Sci. 2021, 100, 101478. [Google Scholar] [CrossRef]
- Amevor, F.K.; Cui, Z.; Ning, Z.; Shu, G.; Du, X.; Jin, N.; Deng, X.; Xu, D.; Tian, Y.; Zhang, Y.; et al. Dietary quercetin and vitamin E supplementation modulates the reproductive performance and antioxidant capacity of aged male breeder chickens. Poult. Sci. 2022, 101, 101851. [Google Scholar] [CrossRef]
- Inoue, M.; Watanabe, N.; Utsumi, T.; Sasaki, J. Targeting SOD by gene and protein engineering and inhibition of free radical injury. Free Radic. Res. Commun. 1991, 12–13 Pt 1, 391–399. [Google Scholar] [CrossRef]
- Oztürk-Urek, R.; Bozkaya, L.A.; Tarhan, L. The effects of some antioxidant vitamin- and trace element-supplemented diets on activities of SOD, CAT, GSH-Px and LPO levels in chicken tissues. Cell Biochem. Funct. 2001, 19, 125–132. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. Available online: https://pubmed.ncbi.nlm.nih.gov/15765761/ (accessed on 25 April 2021).
- Tong, Y.; Yu, C.; Xie, Z.; Zhang, X.; Yang, Z.; Wang, T. Trans-anethole ameliorates lipopolysaccharide-induced acute liver inflammation in broilers via inhibiting NF-κB signaling pathway. Poult. Sci. 2022, 101, 101962. [Google Scholar] [CrossRef]
- Jesudhasan, P.R.; McReynolds, J.L.; Byrd, A.J.; He, H.; Genovese, K.J.; Droleskey, R.; Swaggerty, C.L.; Kogut, M.H.; Duke, S.; Nisbet, D.J.; et al. Electron-Beam-Inactivated Vaccine against Salmonella Enteritidis Colonization in Molting Hens. Avian Dis. 2015, 59, 165–170. [Google Scholar] [CrossRef]
- Nobs, S.P.; Zmora, N.; Elinav, E. Nutrition Regulates Innate Immunity in Health and Disease. Annu. Rev. Nutr. 2020, 40, 189–219. [Google Scholar] [CrossRef]
- Chen, Y.H.; He, J.G. Effects of environmental stress on shrimp innate immunity and white spot syndrome virus infection. Fish Shellfish Immunol. 2019, 84, 744–755. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Giorgetti, G.; Brandimarte, G.; Fabiocchi, F.; Ricci, S.; Flamini, P.; Sandri, G.; Trotta, M.C.; Elisei, W.; Penna, A.; Lecca, P.G.; et al. Interactions between Innate Immunity, Microbiota, and Probiotics. J. Immunol. Res. 2015, 2015, 501361. [Google Scholar] [CrossRef]
- Ragu, S.; Matos-Rodrigues, G.; Lopez, B.S. Replication Stress, DNA Damage, Inflammatory Cytokines and Innate Immune Response. Genes 2020, 11, 409. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, X.; Han, D.; Liu, Y.; Song, W.; Tong, T.; Ma, Y. Lactobacillus casei DBN023 protects against jejunal mucosal injury in chicks infected with Salmonella pullorum CMCC-533. Res. Vet. Sci. 2019, 127, 33–41. [Google Scholar] [CrossRef]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Z.; Xiao, C.; Zhu, Q.; Song, Z. Glycerol Monolaurate Ameliorated Intestinal Barrier and Immunity in Broilers by Regulating Intestinal Inflammation, Antioxidant Balance, and Intestinal Microbiota. Front. Immunol. 2021, 12, 713485. [Google Scholar] [CrossRef]
- Uerlings, J.; Song, Z.G.; Hu, X.Y.; Wang, S.K.; Lin, H.; Buyse, J.; Everaert, N. Heat exposure affects jejunal tight junction remodeling independently of adenosine monophosphate-activated protein kinase in 9-day-old broiler chicks. Poult. Sci. 2018, 97, 3681–3690. [Google Scholar] [CrossRef]
- Aljahdali, N.H.; Sanad, Y.M.; Han, J.; Foley, S.L. Current knowledge and perspectives of potential impacts of Salmonella enterica on the profile of the gut microbiota. BMC Microbiol. 2020, 20, 353. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics To Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Hai, D.; Lu, Z.; Huang, X.; Lv, F.; Bie, X. In Vitro Screening of Chicken-Derived Lactobacillus Strains that Effectively Inhibit Salmonella Colonization and Adhesion. Foods 2021, 10, 569. [Google Scholar] [CrossRef]
- El Hage, R.; El Hage, J.; Snini, S.P.; Ammoun, I.; Touma, J.; Rachid, R.; Mathieu, F.; Sabatier, J.M.; Abi Khattar, Z.; El Rayess, Y. The Detection of Potential Native Probiotics Lactobacillus spp. against Salmonella Enteritidis, Salmonella Infantis and Salmonella Kentucky ST198 of Lebanese Chicken Origin. Antibiotics 2022, 11, 1147. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef]
- Sobotik, E.B.; Ramirez, S.; Roth, N.; Tacconi, A.; Pender, C.; Murugesan, R.; Archer, G.S. Evaluating the effects of a dietary synbiotic or synbiotic plus enhanced organic acid on broiler performance and cecal and carcass Salmonella load. Poult. Sci. 2021, 100, 101508. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Yang, G. Effects of soybean oligosaccharide, stachyose, and raffinose on growth performance and cecal microbiota in broiler chickens. Anim. Sci. J. 2021, 92, e13668. [Google Scholar] [CrossRef]
- Manvatkar, P.N.; Kulkarni, R.C.; Awandkar, S.P.; Chavhan, S.G.; Durge, S.M.; Avhad, S.R.; Channa, G.R.; Kulkarni, M.B. Performance of broiler chicken on dietary supplementation of protected organic acids blend. Br. Poult. Sci. 2022, 63, 633–640. [Google Scholar] [CrossRef]
- Pham, V.H.; Abbas, W.; Huang, J.; He, Q.; Zhen, W.; Guo, Y.; Wang, Z. Effect of blending encapsulated essential oils and organic acids as an antibiotic growth promoter alternative on growth performance and intestinal health in broilers with necrotic enteritis. Poult. Sci. 2022, 101, 101563. [Google Scholar] [CrossRef]
- An, K.; Gao, W.; Li, P.; Li, L.; Xia, Z. Dietary Lactobacillus plantarum improves the growth performance and intestinal health of Pekin ducks. Poult. Sci. 2022, 101, 101844. [Google Scholar] [CrossRef]

| Gene Name b | Primer Sequence (5′to 3′) | Accession Number |
|---|---|---|
| invA | CCCGCTGCCGGTATTTGTTA | MK017942.1 |
| TCAGTCCTAACGACGACCCT | ||
| IL-6 | GATCCGGCAGATGGTGATAA | NM_204628.1 |
| AGGATGAGGTGCATGGTGAT | ||
| iNOS | CCTGGGTTTCAGAAGTGGC | NM_204961.2 |
| CCTGGAGGTCCTGGAAGAGT | ||
| ZO-1 | CAGTCTGTGCGAAGTAGAGT | XM_046925209.1 |
| GAGGTTTGACAACTGACGTG | ||
| Claudin-1 | CGGAGTGAAACATCCTACCC | NM_001013611.2 |
| GGCATTGTAGTGTCCTCTCC | ||
| Occludin | ACAAGCTCTTCCACATCAAG | NM_205128.1 |
| GGATGTGATTGAGTGTGTTT | ||
| β-actin | ACGTCGCACTGGATTTCGAG | NM_205518.2 |
| TGTCAGCAATGCCAGGGTAC |
| Parameter | Value | Genus Ratio (%) | Value |
|---|---|---|---|
| PH | 4.08 ± 0.26 | Lactobacillus | 92.50 ± 2.05 |
| Solid suspension (g/L) | 3.13 ± 0.46 | Acetobacter | 3.75 ± 1.36 |
| Total sugar (g/L) | 4.81 ± 0.36 | Burkholderiaceae | 1.75 ± 0.39 |
| Reducing sugar (g/L) | 1.52 ± 0.05 | Actinobacteria | 0.45 ± 0.10 |
| Total nitrogen (g/L) | 0.60 ± 0.02 | Other | 1.55 ± 0.61 |
| Total fat (g/L) | 0.74 ± 0.06 | Total viable count (109 CFU/Ml) | 3.00 ± 0.28 |
| Lactic acid (g/L) | 2.67 ± 0.18 | ||
| Acetic acid (g/L) | 1.87 ± 0.13 |
| Index | Groups | |||
|---|---|---|---|---|
| Control | 5% | 10% | 20% | |
| Total weight gain (g) | 191.09 ± 9.78 a | 199.69 ± 7.88 a | 212.23 ± 2.51 b | 197.19 ± 3.95 a |
| Average daily gain (g) | 9.55 ± 0.49 a | 9.98 ± 0.39 a | 10.66 ± 0.13 b | 9.86 ± 0.20 a |
| Total feed intake (g) | 584.58 ± 5.42 a | 595.35 ± 6.69 a | 622.57 ± 2.52 b | 594.98 ± 6.00 a |
| feed/gain (F/G) | 3.09 ± 0.18 a | 2.98 ± 0.10 a | 2.92 ± 0.04 a | 3.02 ± 0.07 a |
| Organ Index | Groups | |||
|---|---|---|---|---|
| Control | 5% | 10% | 20% | |
| Heart | 0.69 ± 0.05 a | 0.69 ± 0.11 ab | 0.78 ± 0.09 b | 0.74 ± 0.05 ab |
| Liver | 3.45 ± 0.42 a | 3.61 ± 0.21 a | 3.56 ± 0.49 a | 3.37 ± 0.29 a |
| Kidney | 1.14 ± 0.10 a | 1.33 ± 0.19 b | 1.30 ± 0.14 b | 1.31 ± 0.14 b |
| Proventriculus and Gizzard | 5.44 ± 1.06 a | 5.26 ± 1.13 ab | 4.37 ± 0.68 b | 4.81 ± 1.19 ab |
| Pancreas | 0.46 ± 0.08 a | 0.47 ± 0.06 a | 0.50 ± 0.07 a | 0.54 ± 0.13 a |
| Total intestinal | 6.70 ± 0.57 a | 7.17 ± 0.42 a | 7.26 ± 0.35 b | 7.24 ± 0.69 b |
| Cecum | 0.90 ± 0.07 a | 1.43 ± 0.11 b | 1.41 ± 0.04 b | 1.42 ± 0.12 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Xu, Y.; Yin, L.; Cheng, J.; Yin, D.; Zhao, R.; Dai, Y.; Hu, X.; Hou, H.; Qian, K.; et al. Tofu Whey Wastewater as a Beneficial Supplement to Poultry Farming: Improving Production Performance and Protecting against Salmonella Infection. Foods 2023, 12, 79. https://doi.org/10.3390/foods12010079
Shen X, Xu Y, Yin L, Cheng J, Yin D, Zhao R, Dai Y, Hu X, Hou H, Qian K, et al. Tofu Whey Wastewater as a Beneficial Supplement to Poultry Farming: Improving Production Performance and Protecting against Salmonella Infection. Foods. 2023; 12(1):79. https://doi.org/10.3390/foods12010079
Chicago/Turabian StyleShen, Xuehuai, Yayuan Xu, Lei Yin, Jianghua Cheng, Dongdong Yin, Ruihong Zhao, Yin Dai, Xiaomiao Hu, Hongyan Hou, Kun Qian, and et al. 2023. "Tofu Whey Wastewater as a Beneficial Supplement to Poultry Farming: Improving Production Performance and Protecting against Salmonella Infection" Foods 12, no. 1: 79. https://doi.org/10.3390/foods12010079
APA StyleShen, X., Xu, Y., Yin, L., Cheng, J., Yin, D., Zhao, R., Dai, Y., Hu, X., Hou, H., Qian, K., Pan, X., & Liu, Y. (2023). Tofu Whey Wastewater as a Beneficial Supplement to Poultry Farming: Improving Production Performance and Protecting against Salmonella Infection. Foods, 12(1), 79. https://doi.org/10.3390/foods12010079






