Comparison of Binding Properties of a Laccase-Treated Pea Protein–Sugar Beet Pectin Mixture with Methylcellulose in a Bacon-Type Meat Analogue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Binders
2.3. Probe Tack Test
2.4. Tensile Strength Test
2.5. Statistics
3. Results & Discussion
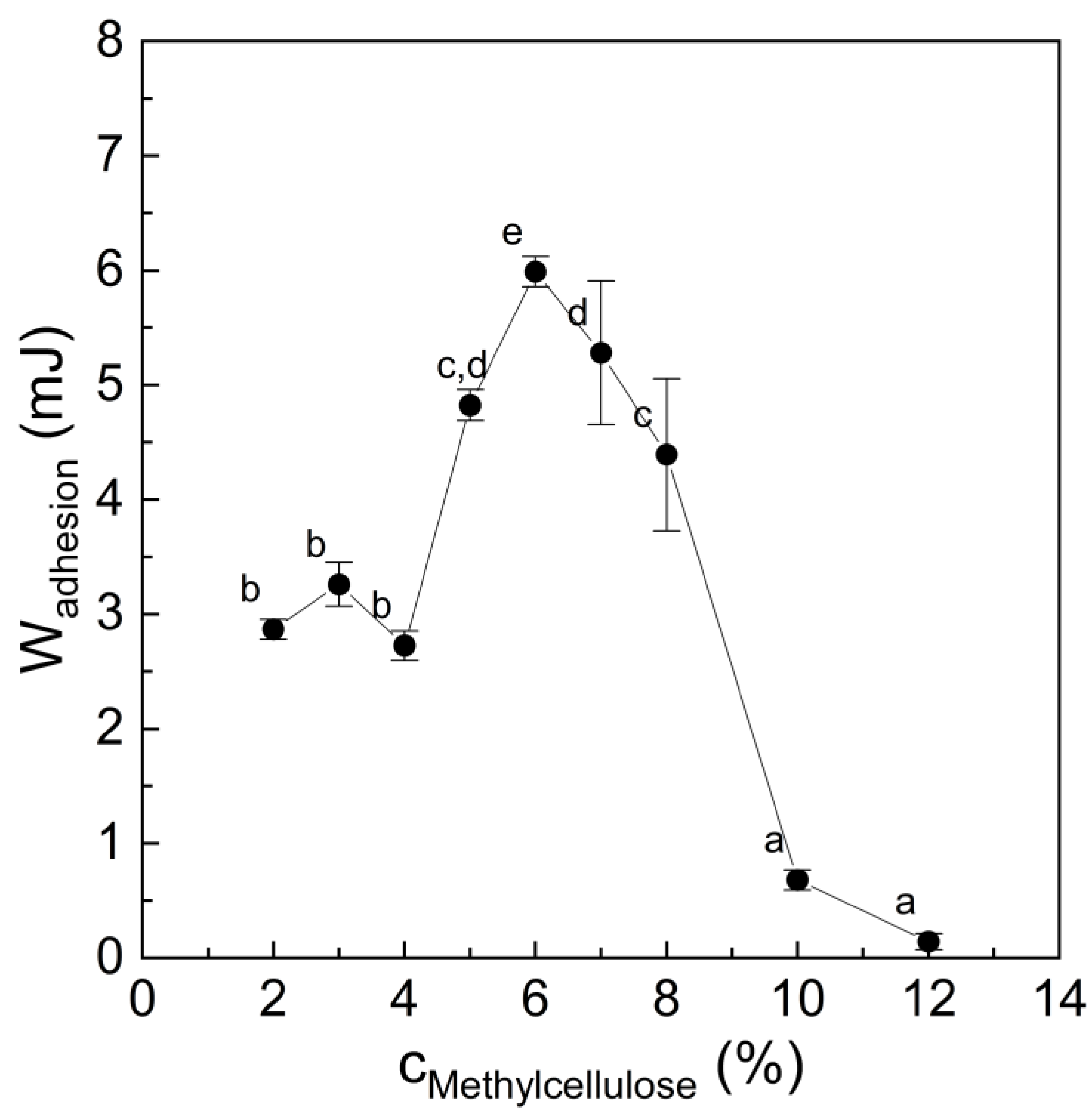
3.1. Stickiness of Methylcellulose Hydrogel
3.2. Binding Properties of the Binder Systems
3.3. Proposed Mechanisms
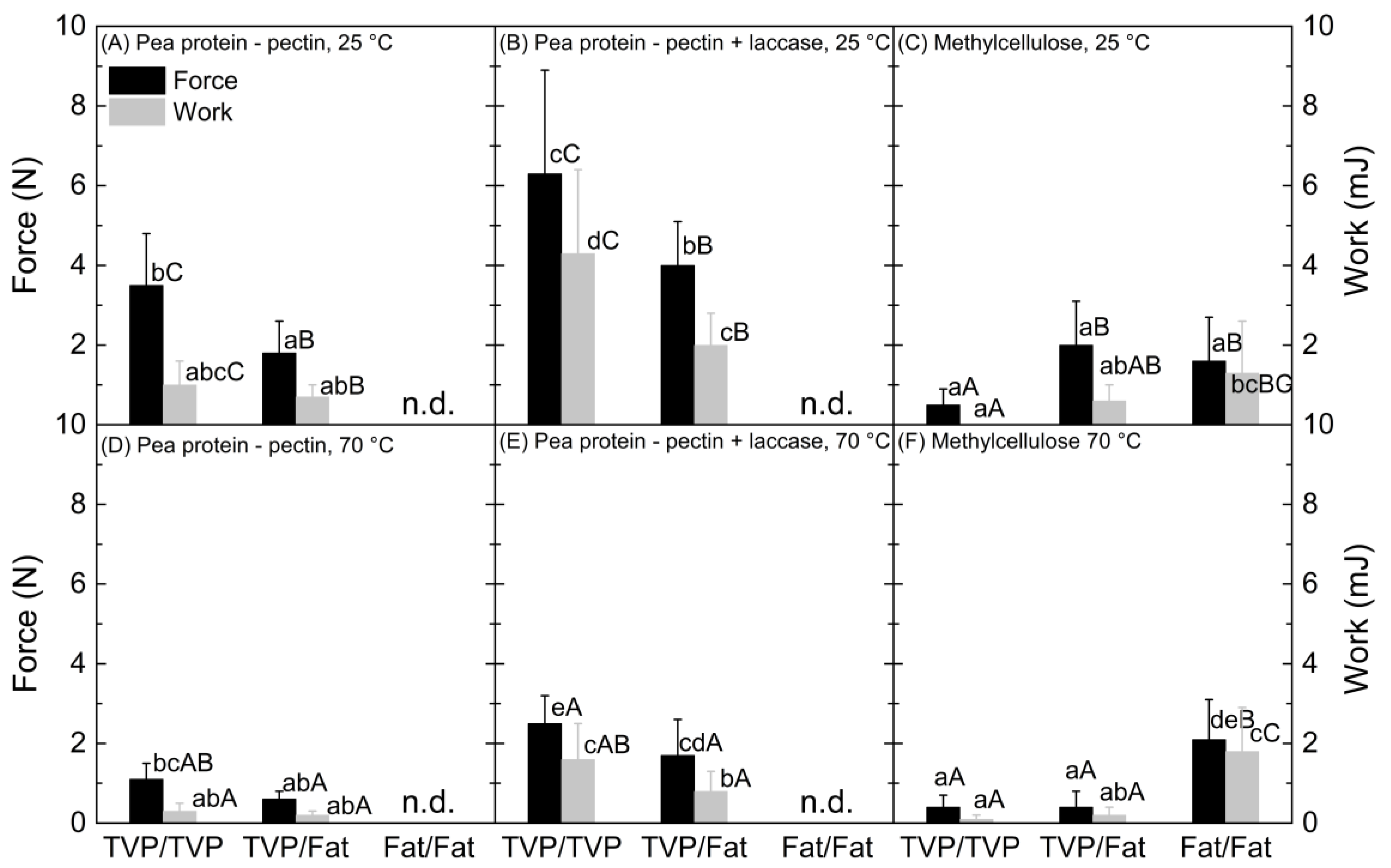
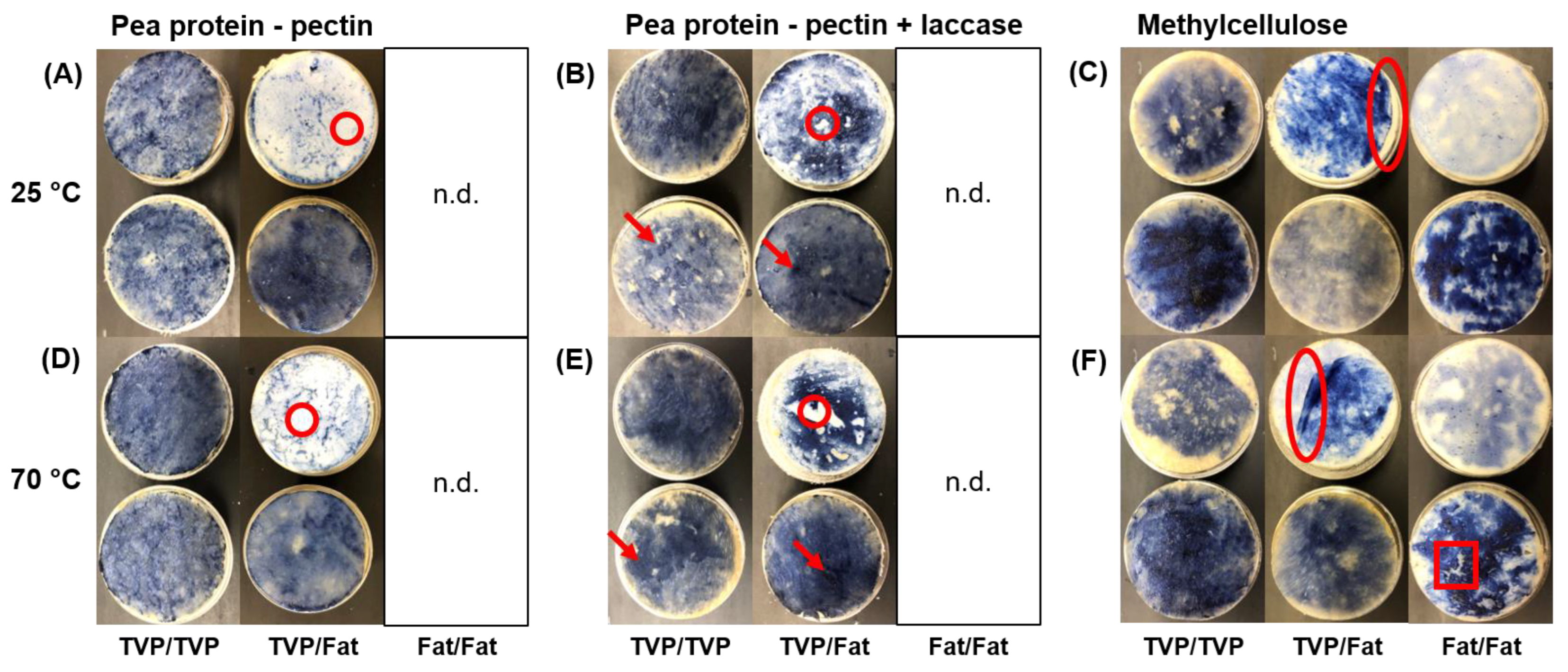
- Pea protein–sugar beet pectin mixtures had higher binding strength between TVP layers. TVPs that are produced by high-moisture extrusion, as was the case for the material used in this study, consist mainly of proteins with hydrophilic and hydrophobic binding sites [31]. The biopolymers pea protein and sugar beet pectin used in the binder mixture are amphiphilic [25,32], facilitating a thermodynamically-driven adhesion between the binder and the TVP layer [18]. On the other hand, the used fat mimetic is a gelled emulsion, with crosslinked soy protein acting as the continuous phase [24]. The soy protein is also amphiphilic; however, the heating step in the preparation of the bacon-type analogue (see Section 2.4) led to fat melting and some fat leaking out of the dispersed phase [24,33]. Consequently, the fat mimetic became more hydrophobic, thus restricting adhesion to the binder (Figure 3A,D), in turn decreasing binding strength. In addition, high-moisture extrusion of plant protein leads to a layered and fibrous structure with irregularities and cavities [34]. The viscoelastic binder can flow into those cavities, which may result in mechanical interlocking and an increasing binding strength [17]. The used fat mimetic had a rather smooth texture. However, surface roughness studies would be necessary to assess the contribution of mechanical interlocking [19].
- Laccase addition increased binding strength of pea protein–sugar beet pectin mixtures. As mentioned above, the two structural elements, namely TVP and fat mimetic, consist of proteins that can potentially be crosslinked via tyrosine residues to the pea protein and the sugar beet pectin in the binder through laccase action. The higher binding strength (Figure 2B) and the better adhesion of the binder to the fat mimetic upon addition of laccase (Figure 3B), indicates that covalent crosslinks were built that contributed to stronger interactions between the pea protein–sugar beet pectin mixture and textured protein/fat mimetic. In general, covalent bonds are stronger than non-covalent bonds [35], which occurred between the structural elements and pea protein–sugar beet pectin mixture without laccase addition. Herz, Herz, Dreher, Gibis, Ray, Pibarot, Schmitt and Weiss [15] reported that binding between a protein extrudate and the same fat mimetic was improved when the soy-based binder was treated with transglutaminase for covalent bonds, instead of weak physical bonds only, which is in line with our results. Furthermore, an increased hardness of soy-based burger patties was achieved when the textured vegetable proteins were bound together with laccase-treated sugar beet pectin [8]. When laccase was not used, the burger patty was crumbly, and no coherent product was obtained [8]. It should be noted that the pea protein–sugar beet pectin binder should be applied in the unsolidified state, i.e., before the action of laccase action. Otherwise, the binder would be solid already and not be able to deform and penetrate the adherend. Furthermore, possible binding sites between the binder and the adherend would already be used up.
- Binding strength at 25 °C was higher compared to 70 °C. It is known that the share of viscous properties in biopolymer mixtures increases with temperature [36]. Although this can lead to an increase in adhesive strength, due to higher deformability, it is also unfavorable for cohesion. Cohesion is the strength of the binder opposing stresses that would lead to disruption [18], such as the one that is applied during the tensile strength test. In bacon-type analogues, an appropriate balance between adhesion and cohesion of the binder is critical [15], and we suggest that the increased temperature led to a lower cohesive strength of the binder.
- Methylcellulose showed higher binding strength to the fat mimetic. Methylcellulose as a hydrophobic biopolymer [13] can interact hydrophobically with the fat mimetic layer and can thus exhibit stronger interactions than with TVP. This is supported by the fact that the binding strength at 70 °C was equal to that at 25 °C when two fat mimetic layers were bound together, which was unlike the other tested binder system (Figure 2). Hydrophobic forces are known to increase with temperature [37].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boukid, F. Plant-based meat analogues: From niche to mainstream. Eur. Food Res. Technol. 2020, 247, 297–308. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Hoek, A.C.; Luning, P.A.; Weijzen, P.; Engels, W.; Kok, F.J.; de Graaf, C. Replacement of meat by meat substitutes. A survey on person- and product-related factors in consumer acceptance. Appetite 2011, 56, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–10. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Keppler, J.K.; van der Goot, A.J. Functionality of Ingredients and Additives in Plant-Based Meat Analogues. Foods 2021, 10, 600. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Improved functional properties of meat analogs by laccase catalyzed protein and pectin crosslinks. Sci. Rep. 2021, 11, 16631. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, S.J.; Lee, E.Y.; Sabikun, N.; Hwang, Y.H.; Joo, S.T. A Novel Approach for Tuning the Physicochemical, Textural, and Sensory Characteristics of Plant-Based Meat Analogs with Different Levels of Methylcellulose Concentration. Foods 2021, 10, 560. [Google Scholar] [CrossRef]
- Lu, G.H.; Chen, T.C. Application of egg white and plasma powders as muscle food binding agents. J. Food Eng. 1999, 42, 147–151. [Google Scholar] [CrossRef]
- Acton, J.C.; Ziegler, G.R.; Burge, D.L., Jr. Functionality of muscle constituents in the processing of comminuted meat products. Crit. Rev. Food Sci. Nutr. 1983, 18, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Desbrières, J.; Hirrien, M.; Ross-Murphy, S.B. Thermogelation of methylcellulose: Rheological considerations. Polymer 2000, 41, 2451–2461. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Badar, I.H.; Xia, X.; Kong, B.; Chen, Q. Prospects of artificial meat: Opportunities and challenges around consumer acceptance. Trends Food Sci. Technol. 2021, 116, 434–444. [Google Scholar] [CrossRef]
- Herz, E.; Herz, L.; Dreher, J.; Gibis, M.; Ray, J.; Pibarot, P.; Schmitt, C.; Weiss, J. Influencing factors on the ability to assemble a complex meat analogue using a soy-protein-binder. Innov. Food Sci. Emerg. Technol. 2021, 73, 102806. [Google Scholar] [CrossRef]
- Devine, C.; Dikeman, M. Encyclopedia of Meat Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Noren, N.E.; Scanlon, M.G.; Arntfield, S.D. Differentiating between tackiness and stickiness and their induction in foods. Trends Food Sci. Technol. 2019, 88, 290–301. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Understanding stickiness in sugar-rich food systems: A review of mechanisms, analyses, and solutions of adhesion. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5901–5937. [Google Scholar] [CrossRef]
- Laukemper, R.; Becker, T.; Jekle, M. Surface Energy of Food Contact Materials and Its Relation to Wheat Dough Adhesion. Food Bioprocess Technol. 2021, 14, 1142–1154. [Google Scholar] [CrossRef]
- Michalski, M.C.; Desobry, S.; Hardy, J. Food materials adhesion: A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 591–619. [Google Scholar] [CrossRef]
- Varvara, R.A.; Szabo, K.; Vodnar, D.C. 3D Food Printing: Principles of Obtaining Digitally-Designed Nourishment. Nutrients 2021, 13, 3617. [Google Scholar] [CrossRef]
- Moll, P.; Salminen, H.; Roeth, C.; Schmitt, C.; Weiss, J. Concentrated pea protein – apple pectin mixtures as food glue: Influence of biopolymer concentration and pH on stickiness. Food Hydrocoll 2022, 130, 107671. [Google Scholar] [CrossRef]
- Weiss, J.; Salminen, H.; Moll, P.; Schmitt, C. Use of molecular interactions and mesoscopic scale transitions to modulate protein-polysaccharide structures. Adv. Colloid Interface Sci. 2019, 271, 101987. [Google Scholar] [CrossRef] [PubMed]
- Dreher, J.; Blach, C.; Terjung, N.; Gibis, M.; Weiss, J. Formation and characterization of plant-based emulsified and crosslinked fat crystal networks to mimic animal fat tissue. J. Food Sci. 2020, 85, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Moll, P.; Salminen, H.; Seitz, O.; Schmitt, C.; Weiss, J. Characterization of Soluble and Insoluble Fractions Obtained from a Commercial Pea Protein Isolate. J. Dispers. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Kurniawati, S.; Nicell, J.A. Characterization of Trametes versicolor laccase for the transformation of aqueous phenol. Bioresour. Technol. 2008, 99, 7825–7834. [Google Scholar] [CrossRef]
- Chen, H.; Gan, J.; Ji, A.; Song, S.; Yin, L. Development of double network gels based on soy protein isolate and sugar beet pectin induced by thermal treatment and laccase catalysis. Food Chem. 2019, 292, 188–196. [Google Scholar] [CrossRef]
- Phang, H.S.; Bruhn, C.M. Burger preparation: What consumers say and do in the home. J. Food Prot. 2011, 74, 1708–1716. [Google Scholar] [CrossRef]
- Yigit, S.; Mendes, M. Which effect size measure is appropriate for one-way and two-way anovamodels? A Monte Carlo simulation study. Revstat. Stat. J. 2018, 16, 295–313. [Google Scholar]
- Burke, J.; Hartel, R.W. Stickiness of sugar syrups with and without particles. J. Food Eng. 2021, 290. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; He, S.; Li, X.; Jin, R.; Liu, Q.; Chen, S.; Sun, H. High-moisture Extrusion Technology Application in the Processing of Textured Plant Protein Meat Analogues: A Review. Food Rev. International. Int. 2022, 1–36. [Google Scholar] [CrossRef]
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Synergistic effects of laccase and pectin on the color changes and functional properties of meat analogs containing beet red pigment. Sci. Rep. 2022, 12, 1168. [Google Scholar] [CrossRef] [PubMed]
- Dreher, J.; Blach, C.; Terjung, N.; Gibis, M.; Weiss, J. Influence of protein content on plant-based emulsified and crosslinked fat crystal networks to mimic animal fat tissue. Food Hydrocoll 2020, 106, 105864. [Google Scholar] [CrossRef]
- Osen, R. Texturization of Pea Protein Isolates Using High Moisture Extrusion Cooking; Technische Universität München: Singapore, 2017. [Google Scholar]
- Cerny, J.; Hobza, P. Non-covalent interactions in biomacromolecules. Phys. Chem. Chem. Phys. 2007, 9, 5291–5303. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Sethi, R. Viscoelastic gels of guar and xanthan gum mixtures provide long-term stabilization of iron micro- and nanoparticles. J. Nanoparticle Res. 2012, 14, 1239. [Google Scholar] [CrossRef] [Green Version]
- Jones, O.G.; McClements, D.J. Functional Biopolymer Particles: Design, Fabrication, and Applications. Compr. Rev. Food Sci. Food Saf. 2010, 9, 374–397. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moll, P.; Salminen, H.; Stadtmueller, L.; Schmitt, C.; Weiss, J. Comparison of Binding Properties of a Laccase-Treated Pea Protein–Sugar Beet Pectin Mixture with Methylcellulose in a Bacon-Type Meat Analogue. Foods 2023, 12, 85. https://doi.org/10.3390/foods12010085
Moll P, Salminen H, Stadtmueller L, Schmitt C, Weiss J. Comparison of Binding Properties of a Laccase-Treated Pea Protein–Sugar Beet Pectin Mixture with Methylcellulose in a Bacon-Type Meat Analogue. Foods. 2023; 12(1):85. https://doi.org/10.3390/foods12010085
Chicago/Turabian StyleMoll, Pascal, Hanna Salminen, Lucie Stadtmueller, Christophe Schmitt, and Jochen Weiss. 2023. "Comparison of Binding Properties of a Laccase-Treated Pea Protein–Sugar Beet Pectin Mixture with Methylcellulose in a Bacon-Type Meat Analogue" Foods 12, no. 1: 85. https://doi.org/10.3390/foods12010085




