Proteomic Analysis of Honey: Peptide Profiling as a Novel Approach for New Zealand Mānuka (Leptospermum scoparium) Honey Authentication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Chemicals and Reagents
2.3. Honey Protein Extraction
2.4. Total Protein Assay
2.5. Trypsin Digest
2.6. Solid-Phase Extraction
2.7. PRM Analysis
2.8. Spectral Library Data Acquisition
2.9. Statistical Analysis
3. Results & Discussion
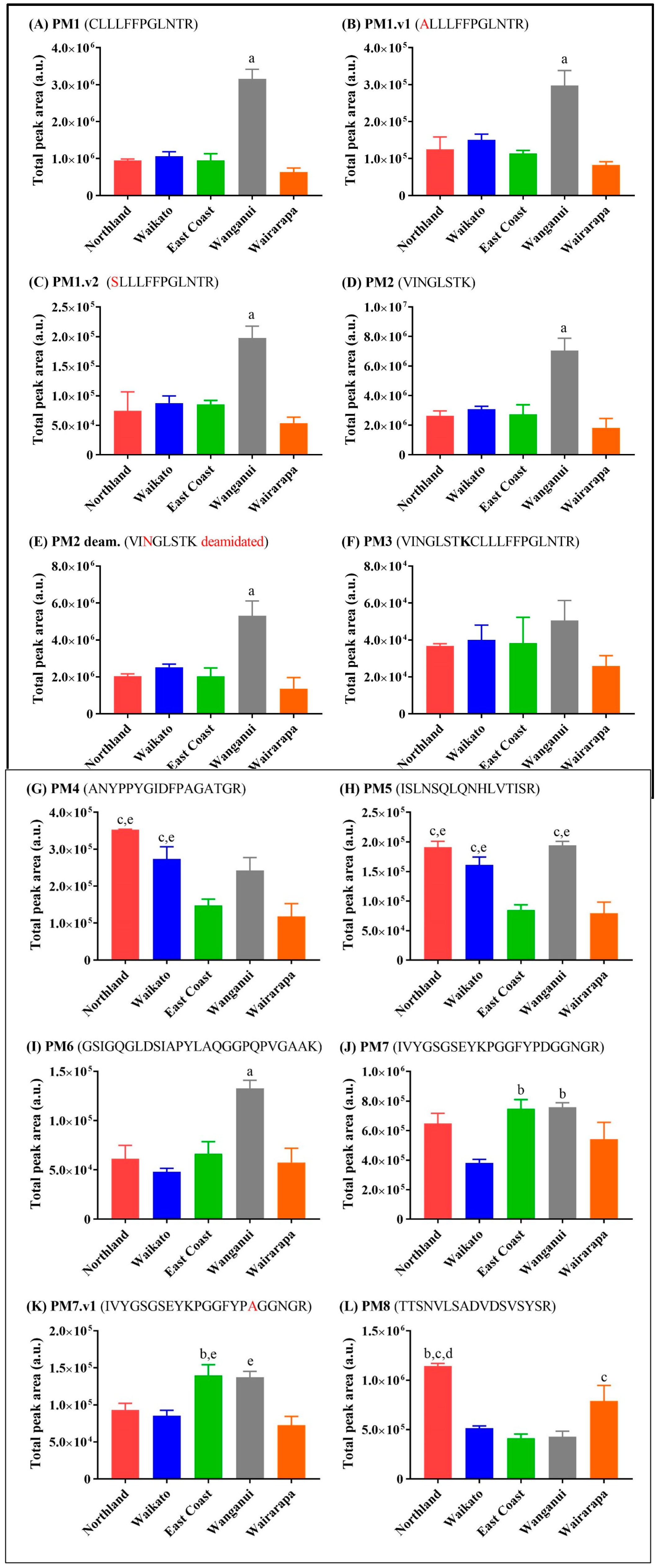
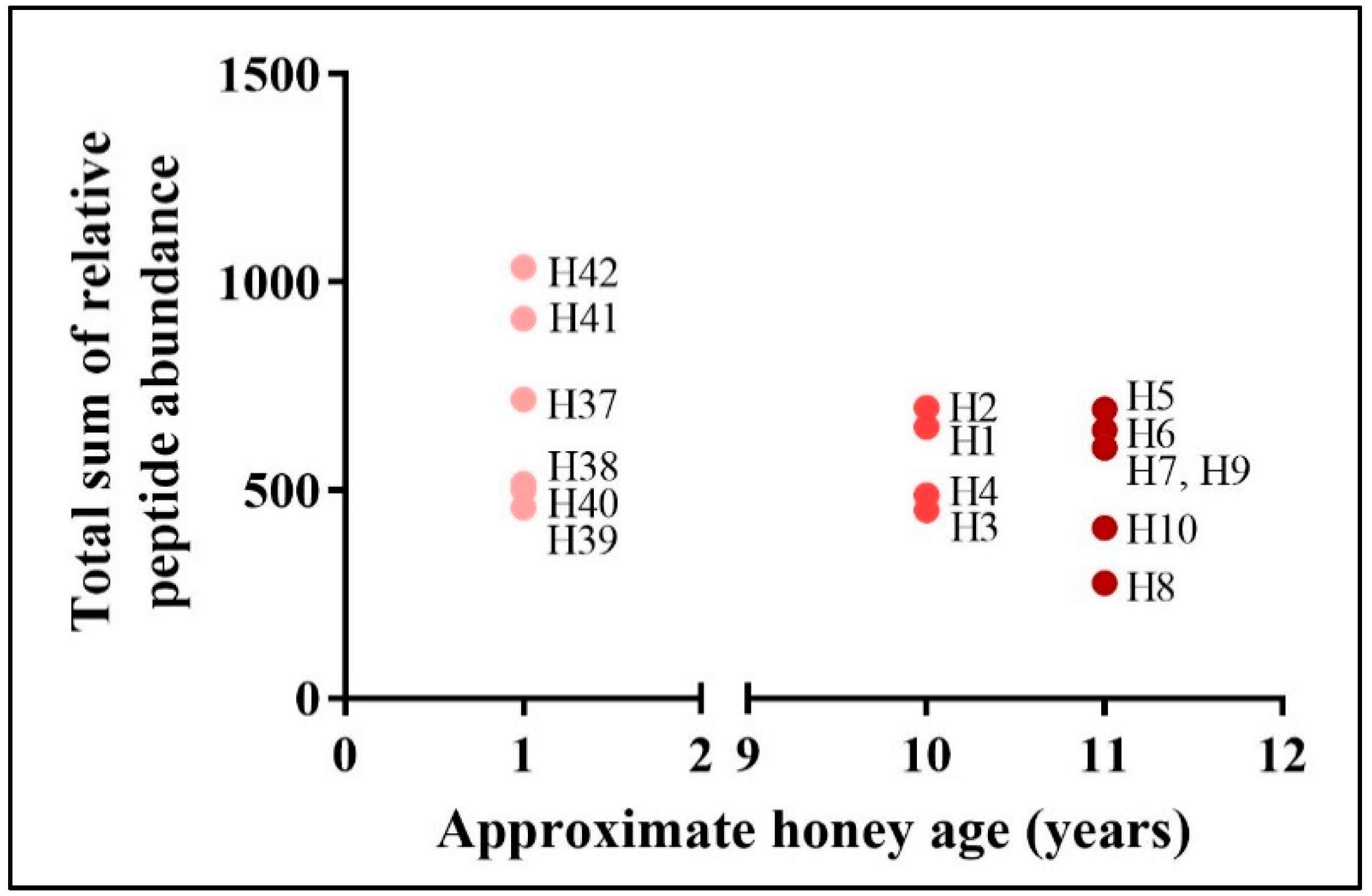
3.1. Relative Abundance of Peptide Markers in Mānuka Honey
3.2. Comparison to Other New Zealand Honeys
3.3. MRJP Peptide Relative Abundance across Honey Types
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Honey Samples | Floral Type | Concentration (mg/kg) | |||
|---|---|---|---|---|---|
| 2′-MAP | 2-MB | 3-PLA | 4-HPA | ||
| H1 | Mānuka | 17 | 8 | 1073 | 8 |
| H2 | Mānuka | 15 | 11 | 1488 | 13 |
| H3 | Mānuka | 4 | 13 | 585 | 7 |
| H4 | Mānuka | 4 | 13 | 607 | 7 |
| H5 | Mānuka | 12 | 17 | 1149 | 11 |
| H6 | Mānuka | 10 | 15 | 1082 | 12 |
| H7 | Mānuka | 7 | 15 | 949 | 11 |
| H8 | Mānuka | 13 | 6 | 1089 | 8 |
| H9 | Mānuka | 14 | 5 | 1082 | 8 |
| H10 | Mānuka | 13 | 5 | 1093 | 8 |
| H11 | Kānuka | ND | 6 | 3118 | 9 |
| H12 | Kānuka | ND | 6 | 3262 | 8 |
| H13 | Kānuka | ND | 7 | 2440 | 10 |
| H14 | Kānuka | ND | ND | 868 | 6 |
| H15 | Rewarewa | ND | ND | 44 | ND |
| H16 | Rewarewa | ND | ND | 9 | ND |
| H17 | Rewarewa | ND | ND | ND | ND |
| H18 | Kāmahi | ND | ND | 12 | ND |
| H19 | Kāmahi | ND | ND | ND | ND |
| H20 | Kāmahi | ND | ND | 25 | ND |
| H21 | Kāmahi | ND | ND | ND | ND |
| H22 | Clover | ND | ND | ND | ND |
| H23 | Clover | ND | ND | ND | ND |
| H24 | Clover | ND | ND | ND | ND |
| H25 | Clover | ND | 1 | ND | ND |
| H26 | Pōhutukawa | ND | 3 | 190 | ND |
| H27 | Pōhutukawa | ND | ND | ND | ND |
| H28 | Pōhutukawa | ND | 7 | 348 | ND |
| H29 | Honeydew | ND | 4 | ND | ND |
| H30 | Honeydew | ND | 2 | 56 | ND |
| H31 | Honeydew | ND | 1 | 40 | ND |
| H32 | Ling | ND | ND | 1029 | 6 |
| H33 | Ling | ND | ND | 969 | 7 |
| H34 | Ling | ND | ND | 823 | 6 |
| H35 | Vipers bugloss | ND | ND | ND | ND |
| H36 | Vipers bugloss | ND | ND | 38 | ND |
| H37 | Mānuka | 10 | ND | 221 | 2 |
| H38 | Mānuka | 4 | ND | 232 | 2 |
| H39 | Mānuka | 1 | ND | 765 | 2 |
| H40 | Mānuka | 1 | ND | 719 | 2 |
| H41 | Mānuka | 3 | ND | 709 | 3 |
| H42 | Mānuka | 2 | ND | 710 | 3 |
| Sample | Honey Type | Floral Species | Region | Harvest Year | Protein Content of Precipitate (μg/μL) |
|---|---|---|---|---|---|
| H1 | Mānuka | L. scoparium | Northland | 2008/2009 | 0.776 |
| H2 | Mānuka | L. scoparium | Northland | 2008/2009 | 0.646 |
| H3 | Mānuka | L. scoparium | Waikato | 2008/2009 | 0.749 |
| H4 | Mānuka | L. scoparium | Waikato | 2008/2009 | 0.820 |
| H5 | Mānuka | L. scoparium | Waikato | 2007 | 0.627 |
| H6 | Mānuka | L. scoparium | Waikato | 2007 | 0.633 |
| H7 | Mānuka | L. scoparium | Waikato | 2007 | 0.670 |
| H8 | Mānuka | L. scoparium | Wairarapa | 2007 | 0.676 |
| H9 | Mānuka | L. scoparium | Wairarapa | 2007 | 0.629 |
| H10 | Mānuka | L. scoparium | Wairarapa | 2007 | 0.624 |
| H11 | Kānuka | K. ericoides | Northland | - | 0.639 |
| H12 | Kānuka | K. ericoides | Northland | - | 0.731 |
| H13 | Kānuka | K. ericoides | Northland | - | 0.630 |
| H14 | Kānuka | K. ericoides | Northland | - | 0.875 |
| H15 | Rewarewa | K. excelsa | - | - | 0.869 |
| H16 | Rewarewa | K. excelsa | - | - | 0.631 |
| H17 | Rewarewa | K. excelsa | - | - | 1.056 |
| H18 | Kāmahi | W. racemosa | - | - | 2.060 |
| H19 | Kāmahi | W. racemosa | - | - | 2.332 |
| H20 | Kāmahi | W. racemosa | - | - | 1.955 |
| H21 | Kāmahi | W. racemosa | - | - | 2.042 |
| H22 | Clover | Trifolium spp. | - | - | 2.099 |
| H23 | Clover | Trifolium spp. | - | - | 2.308 |
| H24 | Clover | Trifolium spp. | - | - | 1.566 |
| H25 | Clover | Trifolium spp. | - | - | 2.304 |
| H26 | Pōhutukawa | M. excelsa | - | - | 1.259 |
| H27 | Pōhutukawa | M. excelsa | - | - | 0.983 |
| H28 | Pōhutukawa | M. excelsa | - | - | 3.385 |
| H29 | Honeydew | F. solandri | - | - | 2.184 |
| H30 | Honeydew | F. solandri | - | - | 2.269 |
| H31 | Honeydew | F. solandri | - | - | 1.468 |
| H32 | Ling | C. vulgaris | - | - | 6.955 |
| H33 | Ling | C. vulgaris | - | - | 5.872 |
| H34 | Ling | C. vulgaris | - | - | 5.698 |
| H35 | Vipers bugloss | E. vulgare | - | - | 1.108 |
| H36 | Vipers bugloss | E. vulgare | - | - | 1.117 |
| H37 | Mānuka | L. scoparium | East Coast | 2017/2018 | 1.194 |
| H38 | Mānuka | L. scoparium | East Coast | 2017/2018 | 1.736 |
| H39 | Mānuka | L. scoparium | East Coast | 2017/2018 | 1.138 |
| H40 | Mānuka | L. scoparium | East Coast | 2017/2018 | 1.634 |
| H41 | Mānuka | L. scoparium | Whanganui | 2017/2018 | 0.429 |
| H42 | Mānuka | L. scoparium | Whanganui | 2017/2018 | 0.318 |

References
- Molan, P.; Rhodes, T. Honey: A Biologic Wound Dressing. Wounds 2015, 27, 141–151. [Google Scholar]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef]
- Kato, Y.; Umeda, N.; Maeda, A.; Matsumoto, D.; Kitamoto, N.; Kikuzaki, H. Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J. Agric. Food Chem. 2012, 60, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kawai, M.; Kawai, S.; Okano, Y.; Rokkaku, N.; Ishisaka, A.; Murota, K.; Nakamura, T.; Nakamura, Y.; Ikushiro, S. Dynamics of the Cellular Metabolism of Leptosperin Found in Manuka Honey. J. Agric. Food Chem. 2019, 67, 10853–10862. [Google Scholar] [CrossRef] [PubMed]
- Daniels, B.J.; Prijic, G.; Meidinger, S.; Loomes, K.M.; Stephens, J.M.; Schlothauer, R.C.; Furkert, D.P.; Brimble, M.A. Isolation, Structural Elucidation, and Synthesis of Lepteridine From Mānuka (Leptospermum scoparium) Honey. J. Agric. Food Chem. 2016, 64, 5079–5084. [Google Scholar] [CrossRef] [PubMed]
- Beitlich, N.; Lübken, T.; Kaiser, M.; Ispiryan, L.; Speer, K. Fluorescent Pteridine Derivatives as New Markers for the Characterization of Genuine Monofloral New Zealand Manuka (Leptospermum scoparium) Honey. J. Agric. Food Chem. 2016, 64, 8886–8891. [Google Scholar] [CrossRef]
- Stephens, J.M.; Schlothauer, R.C.; Morris, B.D.; Yang, D.; Fearnley, L.; Greenwood, D.R.; Loomes, K.M. Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chem. 2010, 120, 78–164. [Google Scholar] [CrossRef]
- Gallien, S.; Duriez, E.; Domon, B. Selected reaction monitoring applied to proteomics. J. Mass Spectrom. 2011, 46, 298–312. [Google Scholar] [CrossRef]
- Guo, S.; Xu, X.; Zhou, X.; Huang, Y. A rapid and simple UPLC-MS/MS method using collagen marker peptides for identification of porcine gelatin. RSC Adv. 2018, 8, 3768–3773. [Google Scholar] [CrossRef]
- Lange, V.; Picotti, P.; Domon, B.; Aebersold, R. Selected reaction monitoring for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2008, 4, 222. [Google Scholar] [CrossRef]
- Peterson, A.C.; Russell, J.D.; Bailey, D.J.; Westphall, M.S.; Coon, J.J. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol. Cell. Proteom. 2012, 11, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Rauniyar, N. Parallel Reaction Monitoring: A Targeted Experiment Performed Using High Resolution and High Mass Accuracy Mass Spectrometry. Int. J. Mol. Sci. 2015, 16, 28566–28581. [Google Scholar] [CrossRef]
- Schilling, B.; MacLean, B.; Held, J.M.; Sahu, A.K.; Rardin, M.J.; Sorensen, D.J.; Peters, T.; Wolfe, A.J.; Hunter, C.L.; MacCoss, M.J.; et al. Multiplexed, Scheduled, High-Resolution Parallel Reaction Monitoring on a Full Scan QqTOF Instrument with Integrated Data-Dependent and Targeted Mass Spectrometric Workflows. Anal. Chem. 2015, 87, 10222–10229. [Google Scholar] [CrossRef]
- Ronsein, G.E.; Pamir, N.; von Haller, P.D.; Kim, D.S.; Oda, M.N.; Jarvik, G.P.; Vaisar, T.; Heinecke, J.W. Parallel reaction monitoring (PRM) and selected reaction monitoring (SRM) exhibit comparable linearity, dynamic range and precision for targeted quantitative HDL proteomics. J. Proteom. 2015, 113, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.D.; Chen, J.; Chen, Q.; Huang, B.F.; Han, J.L. Authentication of pork in meat mixtures using PRM mass spectrometry of myosin peptides. RSC Adv. 2018, 8, 11157–11162. [Google Scholar] [CrossRef]
- Lin, B.; Daniels, B.J.; Middleditch, M.J.; Furkert, D.P.; Brimble, M.A.; Bong, J.; Stephens, J.M.; Loomes, K.M. Utility of the Leptospermum scoparium Compound Lepteridine as a Chemical Marker for Manuka Honey Authenticity. ACS Omega 2020, 5, 8858–8866. [Google Scholar] [CrossRef]
- Bong, J.; Loomes, K.M.; Lin, B.; Stephens, J.M. New approach: Chemical and fluorescence profiling of NZ honeys. Food Chem. 2018, 267, 355–367. [Google Scholar] [CrossRef]
- Stephens, J.M.; Loomes, K.M.; Braggins, T.J.; Bong, J.; Lin, B.; Prijic, G. Flourescence: A novel method for determining manuka honey floral purity. In Honey Analysis; Toledo, V.A.A., Ed.; Intech: Rijeka, Croatia, 2017; pp. 95–113. [Google Scholar]
- Lin, B.; Loomes, K.M.; Prijic, G.; Schlothauer, R.; Stephens, J.M. Lepteridine as a unique fluorescent marker for the authentication of manuka honey. Food Chem. 2017, 225, 175–180. [Google Scholar] [CrossRef]
- Bong, J.; Prijic, G.; Braggins, T.J.; Schlothauer, R.C.; Stephens, J.M.; Loomes, K.M. Leptosperin is a distinct and detectable fluorophore in Leptospermum honeys. Food Chem. 2017, 214, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Bong, J.; Loomes, K.M.; Schlothauer, R.C.; Stephens, J.M. Fluorescence markers in some New Zealand honeys. Food Chem. 2016, 192, 1006–1014. [Google Scholar] [CrossRef]
- Bong, J.; Middleditch, M.; Loomes, K.M.; Stephens, J.M. Proteomic analysis of honey. Identification of unique peptide markers for authentication of NZ manuka (Leptospermum scoparium) honey. Food Chem. 2021, 350, 128442. [Google Scholar] [CrossRef] [PubMed]
- Thrimawithana, A.H.; Jones, D.; Hilario, E.; Grierson, E.; Ngo, H.M.; Liachko, I.; Sullivan, S.; Bilton, T.P.; Jacobs, J.M.E.; Bicknell, R.; et al. A whole genome assembly of Leptospermum scoparium (Myrtaceae) for mānuka research. N. Z. J. Crop. Hortic. Sci. 2019, 47, 233–260. [Google Scholar] [CrossRef]
- Agnew, B.J.; Murray, D.; Patton, W.F. A rapid solid-phase fluorescence-based protein assay for quantitation of protein electrophoresis samples containing detergents, chaotropes, dyes, and reducing agents. Electrophoresis 2004, 25, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.E.A.; Azim, M.K. Characterisation of natural honey proteins: Implications for the floral and geographical origin of honey. Int. J. Food Sci. Technol. 2012, 47, 362–368. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C.; Maldonado-Alvarez, L. A new look on protein-polyphenol complexation during honey storage: Is this a random or organized event with the help of dirigent-like proteins? PLoS ONE 2013, 8, e72897. [Google Scholar] [CrossRef]
- Hellwig, M.; Ruckriemen, J.; Sandner, D.; Henle, T. Unique Pattern of Protein-Bound Maillard Reaction Products in Manuka (Leptospermum scoparium) Honey. J. Agric. Food Chem. 2017, 65, 3532–3540. [Google Scholar] [CrossRef]
- Davies, A.M.C. Proline in Honey: An Osmoregulatory Hypothesis. J. Apic. Res. 1978, 17, 227–233. [Google Scholar] [CrossRef]
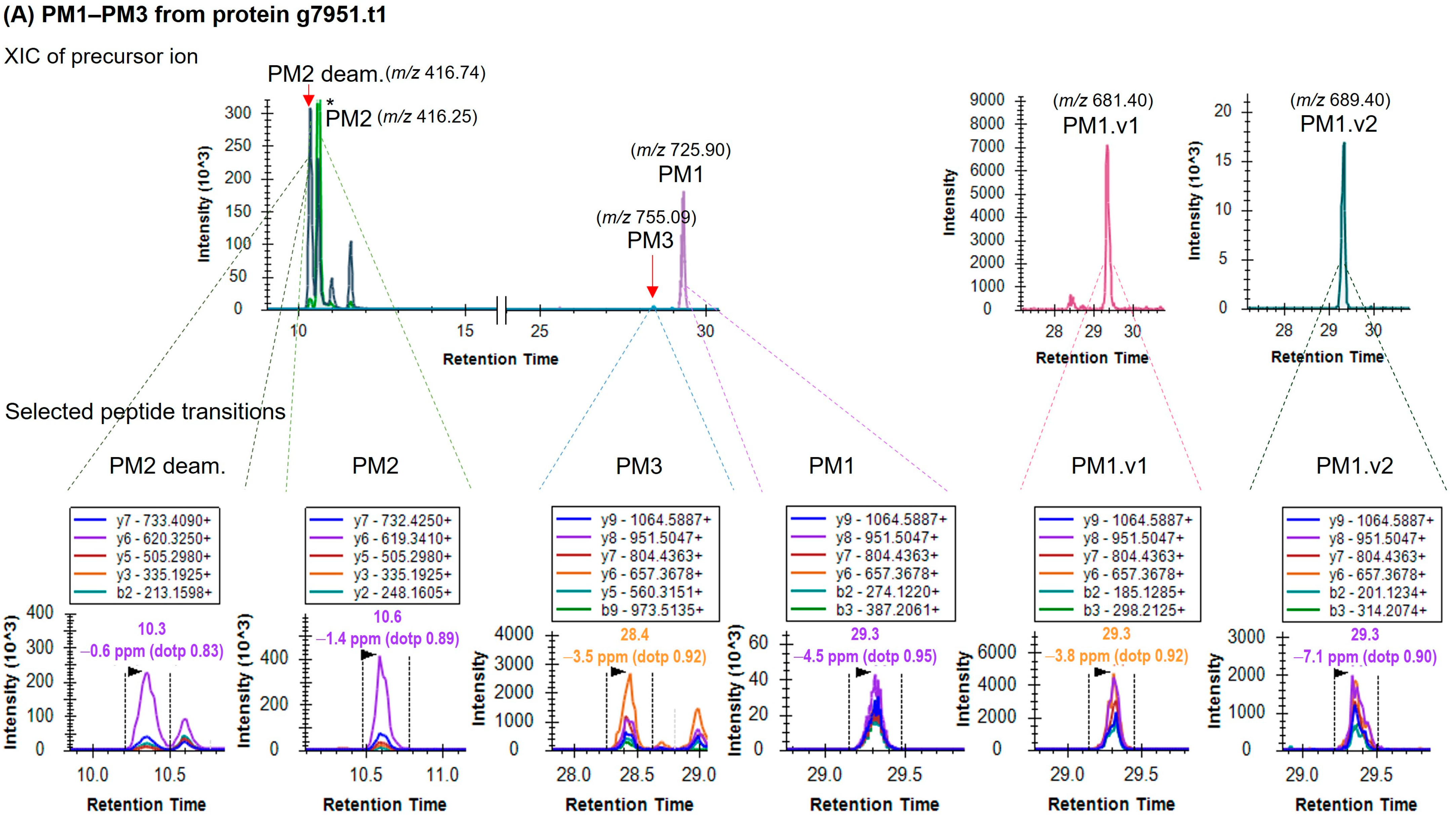
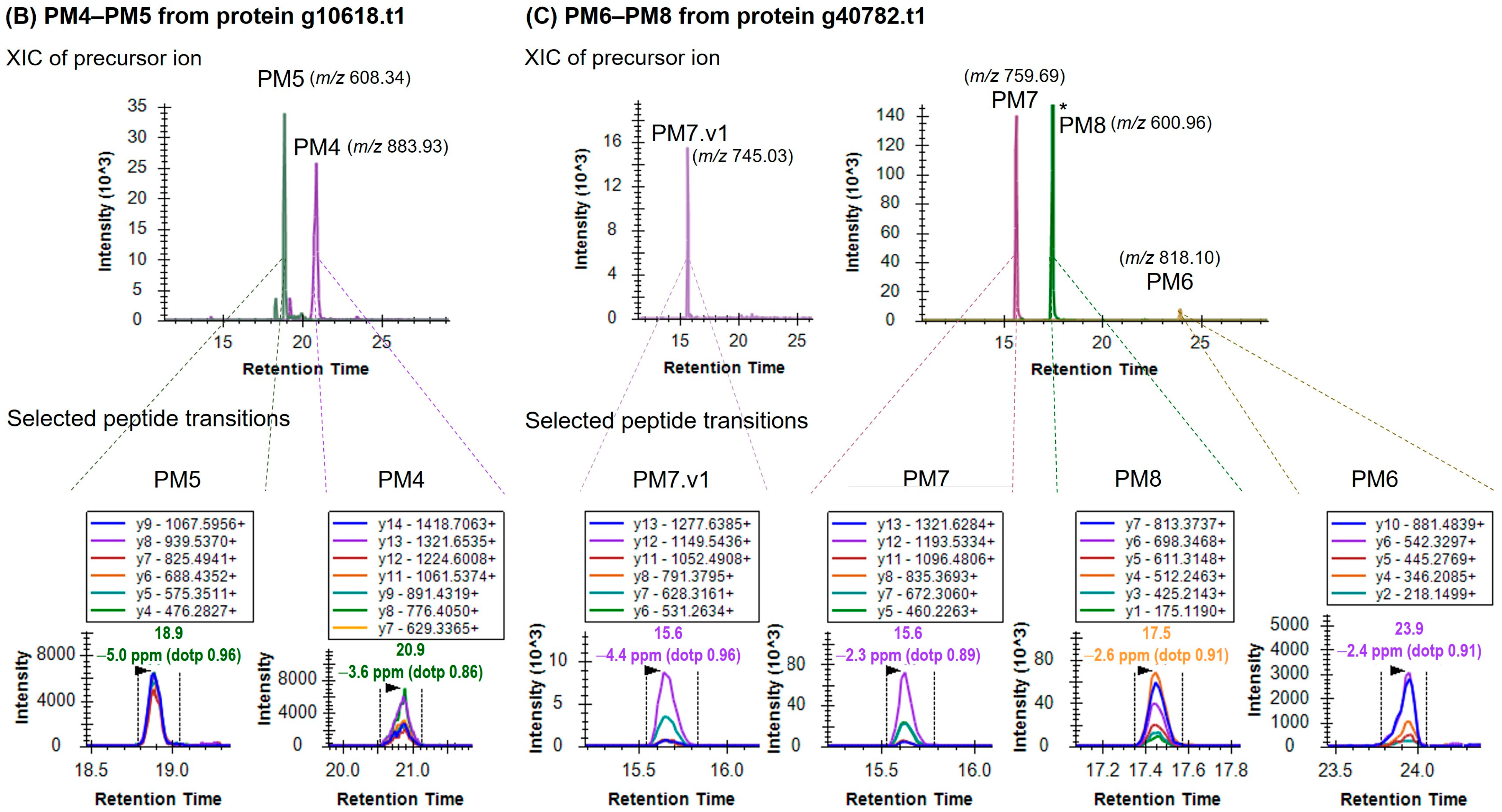
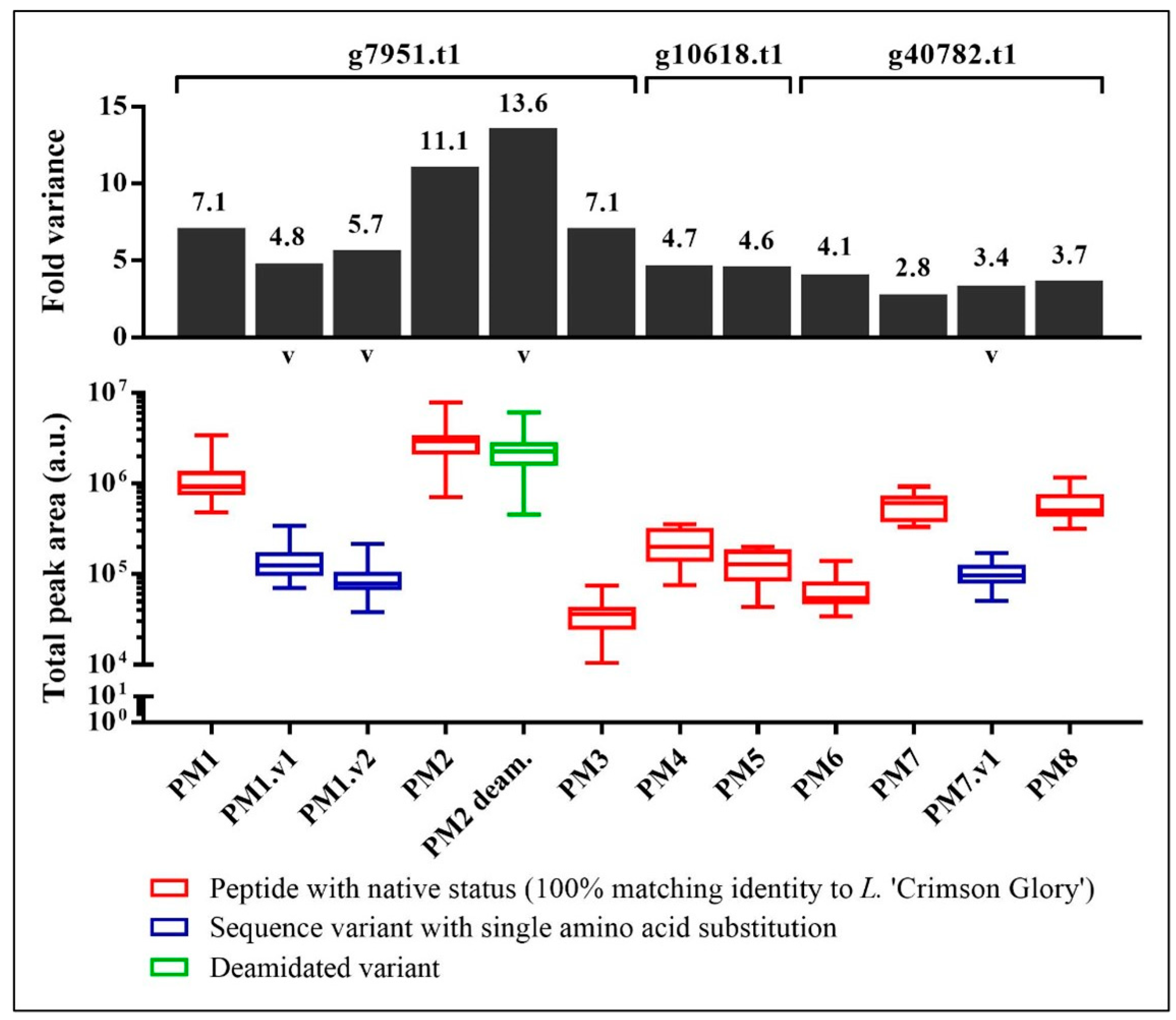
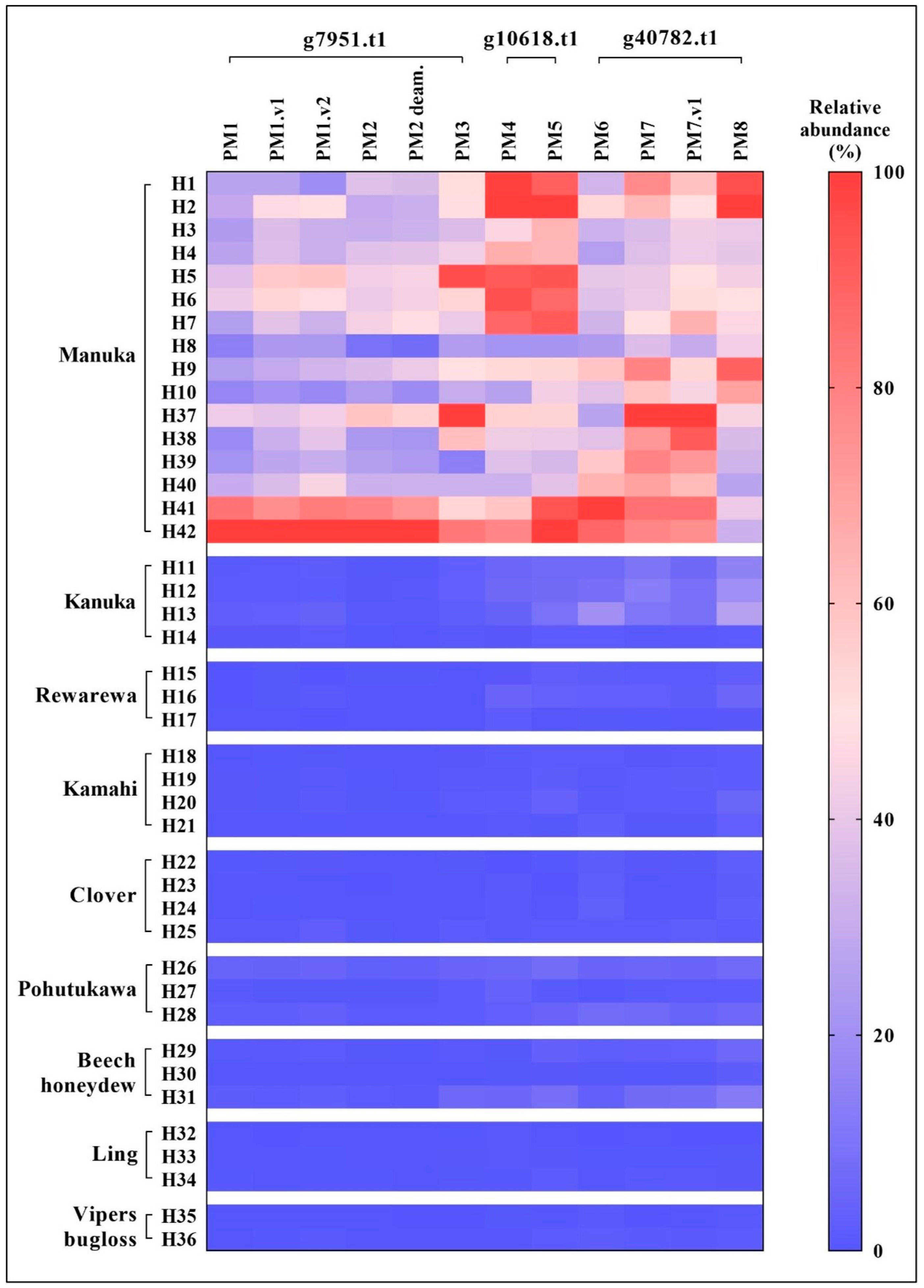
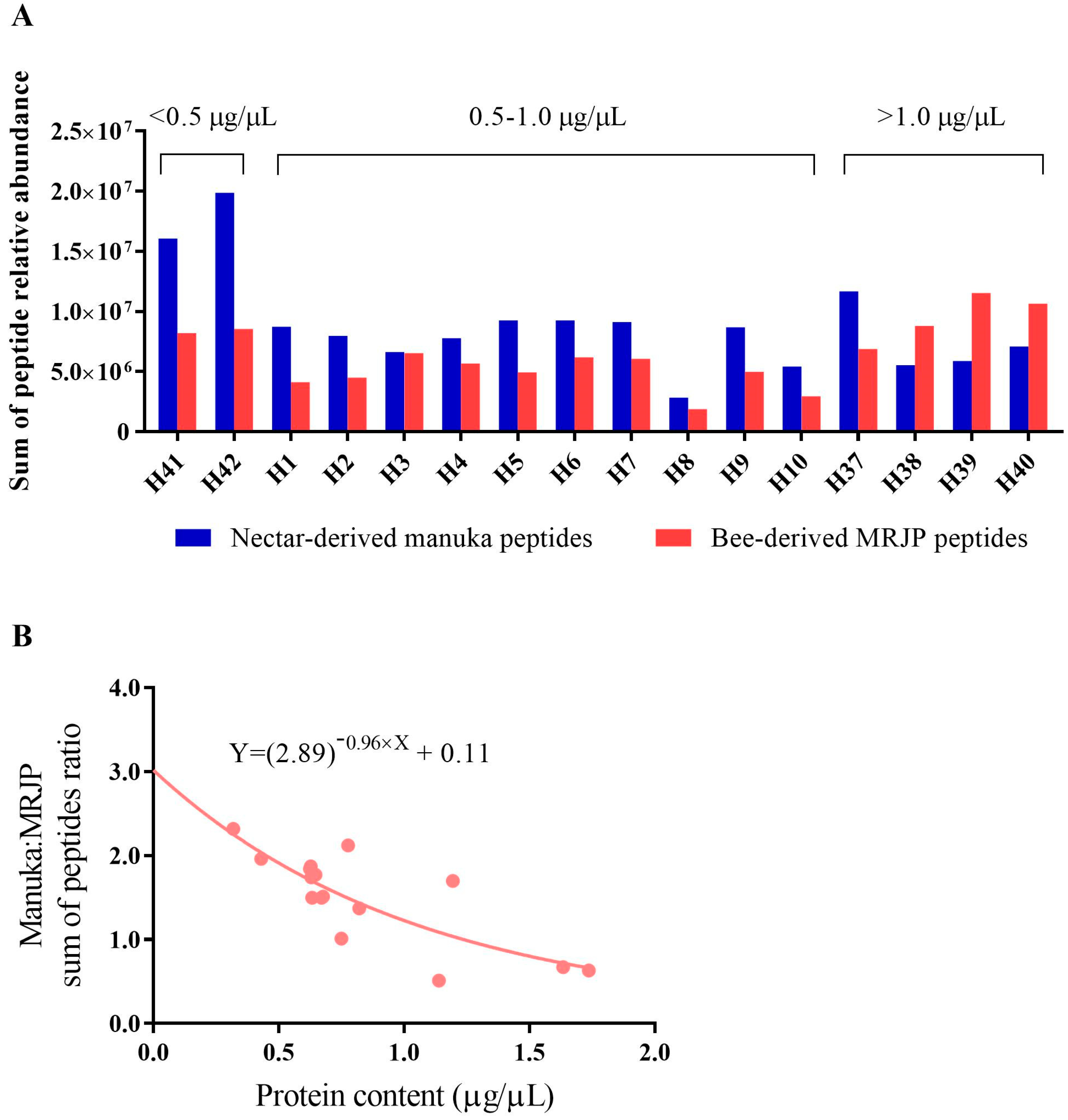
| No. | Targeted Peptide | Sequence a | Protein | Precursor m/z | z | Collision Energy (kV) | Accumulation Time (ms) |
|---|---|---|---|---|---|---|---|
| 1 | PM1 | CLLLFFPGLNTR | g7951.t1 | 725.90 | 2 | 42 | 20.0 |
| 2 | PM1.v1 | ALLLFFPGLNTR | g7951.t1 | 681.40 | 2 | 40 | 15.0 |
| 3 | PM1.v2 | SLLLFFPGLNTR | g7951.t1 | 689.40 | 2 | 43 | 20.0 |
| 4 | PM2 | VINGLSTK | g7951.t1 | 416.25 | 2 | 23 | 10.0 |
| 5 | PM2 deam. | VINGLSTK deamidated | g7951.t1 | 416.74 | 2 | 23 | 10.0 |
| 9 | PM3 | VINGLSTKCLLLFFPGLNTR | g7951.t1 | 755.09 | 3 | 42 | 100.0 |
| 7 | PM4 | ANYPPYGIDFPAGATGR | g10618.t1 | 883.93 | 2 | 52 | 30.0 |
| 8 | PM5 | ISLNSQLQNHLVTISR | g10618.t1 | 608.34 | 3 | 34 | 25.0 |
| 9 | PM6 | GSIGQGLDSIAPYLAQGGPQPVGAAK | g40782.t1 | 818.10 | 3 | 46 | 30.0 |
| 10 | PM7 | IVYGSGSEYKPGGFYPDGGNGR | g40782.t1 | 759.69 | 3 | 42 | 20.0 |
| 11 | PM7.v1 | IVYGSGSEYKPGGFYPAGGNGR | g40782.t1 | 745.03 | 3 | 42 | 30.0 |
| 12 | PM8 | TTSNVLSADVDSVSYSR | g40782.t1 | 600.96 | 3 | 33 | 20.0 |
| 13 | BP1 | SLPILHEWK | MRJP1 | 561.82 | 2 | 32 | 10.0 |
| 14 | BP2 | LLTFDLTTSQLLK | MRJP1 | 746.93 | 2 | 44 | 10.0 |
| 16 | BP3 | HIDFDFGSDER | MRJP3 | 669.29 | 2 | 39 | 20.0 |
| 17 | BP4 | GGPLLRPYPDWSFAK | MRJP3 | 568.64 | 3 | 31 | 20.0 |
| 18 | BP5 | IINNDFNFNDVNFR | MRJP3 | 871.42 | 2 | 51 | 20.0 |
| 19 | BP6 | YLDYDFGSDER | MRJP5 | 690.29 | 2 | 40 | 20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bong, J.; Middleditch, M.; Stephens, J.M.; Loomes, K.M. Proteomic Analysis of Honey: Peptide Profiling as a Novel Approach for New Zealand Mānuka (Leptospermum scoparium) Honey Authentication. Foods 2023, 12, 1968. https://doi.org/10.3390/foods12101968
Bong J, Middleditch M, Stephens JM, Loomes KM. Proteomic Analysis of Honey: Peptide Profiling as a Novel Approach for New Zealand Mānuka (Leptospermum scoparium) Honey Authentication. Foods. 2023; 12(10):1968. https://doi.org/10.3390/foods12101968
Chicago/Turabian StyleBong, Jessie, Martin Middleditch, Jonathan M. Stephens, and Kerry M. Loomes. 2023. "Proteomic Analysis of Honey: Peptide Profiling as a Novel Approach for New Zealand Mānuka (Leptospermum scoparium) Honey Authentication" Foods 12, no. 10: 1968. https://doi.org/10.3390/foods12101968





