Neutral Electrolyzed Water in Chicken Breast—A Preservative Option in Poultry Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluated Solutions
2.2. Chemical Analysis of Solutions
2.3. In Vitro Microbicidal Assay
2.4. Chicken Breast
2.5. Experimental Design
2.6. Chicken Contamination and Treatments
2.7. Bacterial Survival Counts and Chicken Breast Storage
2.8. Color
2.9. Chicken Breast pH
2.10. Lactic Acid
2.11. Total Volatile Basic Nitrogen
2.12. Thiobarbituric Acid Reactive Substances (TBARS)
2.13. Instrumental Texture Analysis
2.13.1. Texture Profile Analysis (TPA)
2.13.2. Warner–Bratzler (WB) Test
2.14. Sensory Analysis
2.15. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis of Evaluated Solutions
3.2. In Vitro Bactericidal Assay
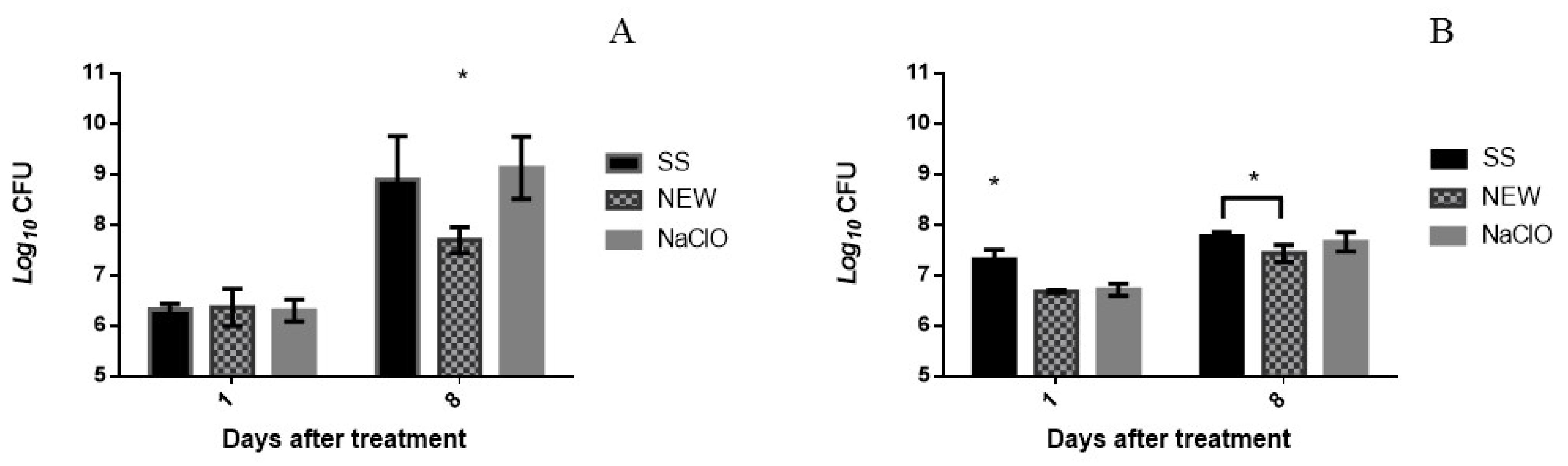
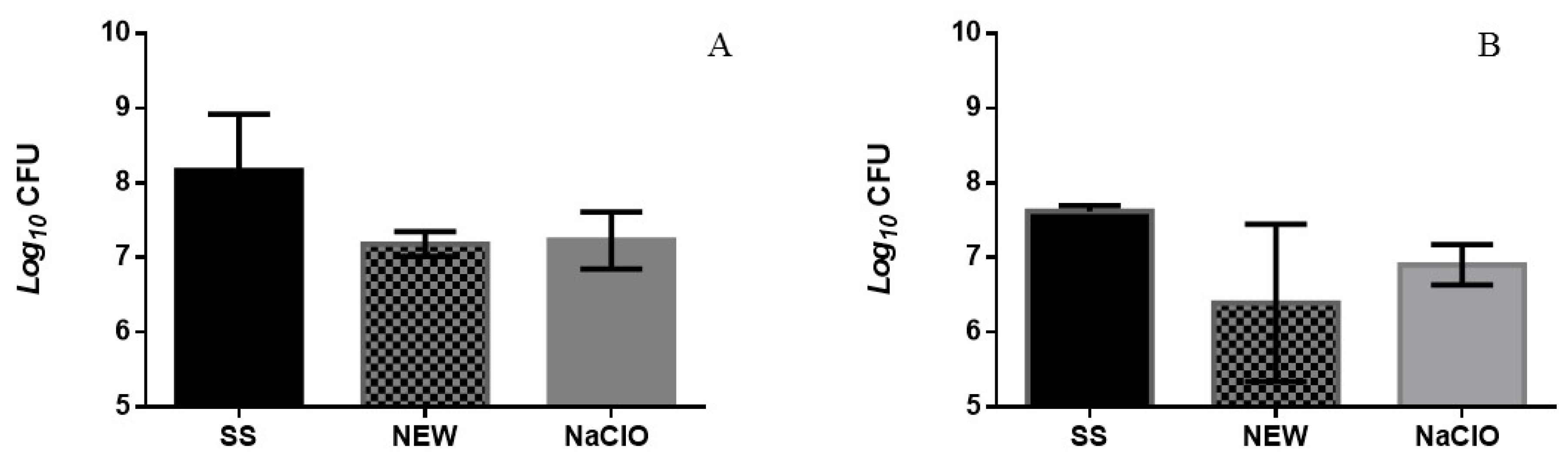
3.3. Bactericidal Assay on Chicken Breast
3.4. Physicochemical Properties of Chicken Breast
3.4.1. pH
3.4.2. Lactic Acid
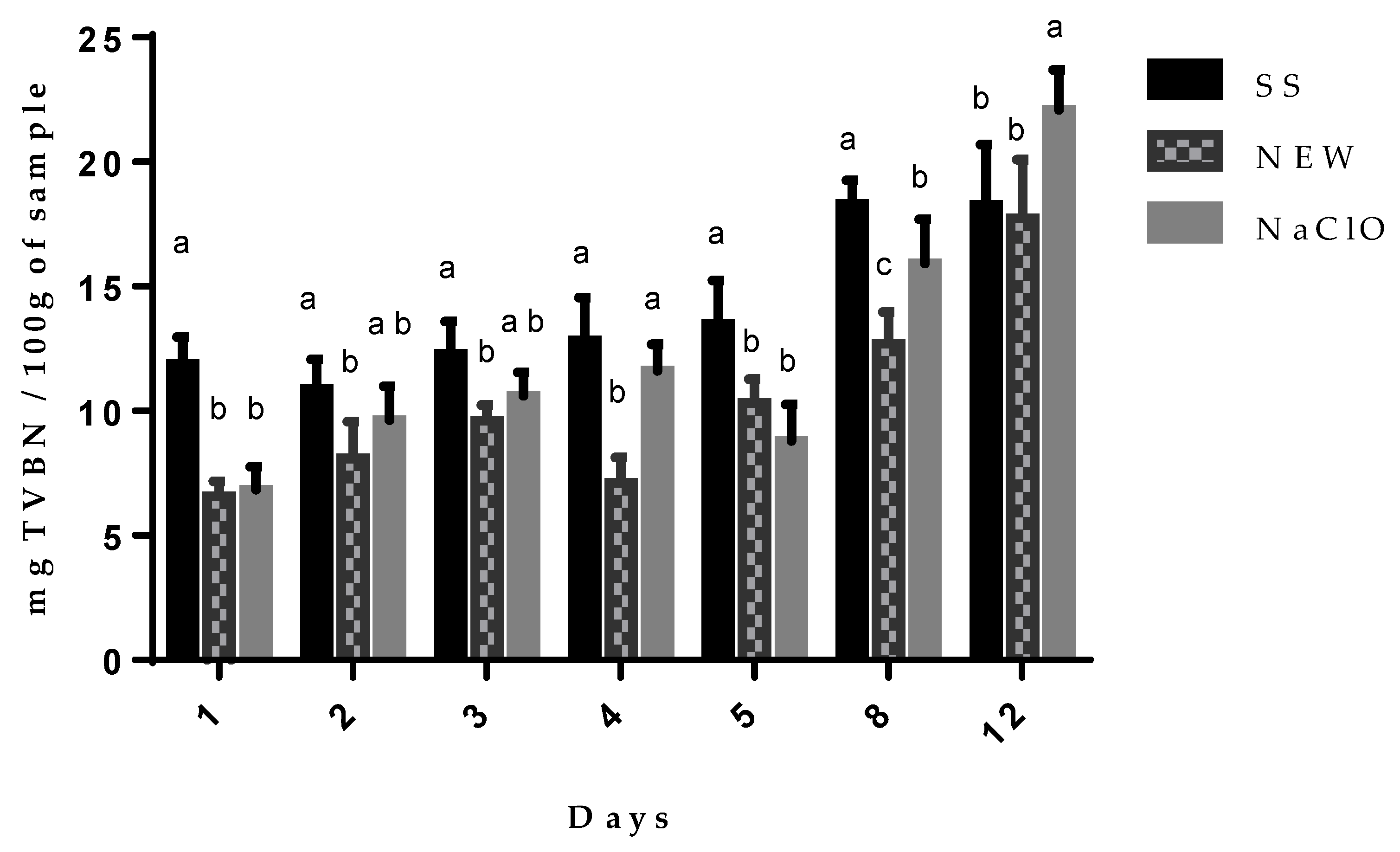
3.4.3. Total Volatile Basic Nitrogen Content (TVBN)
3.4.4. TBARS
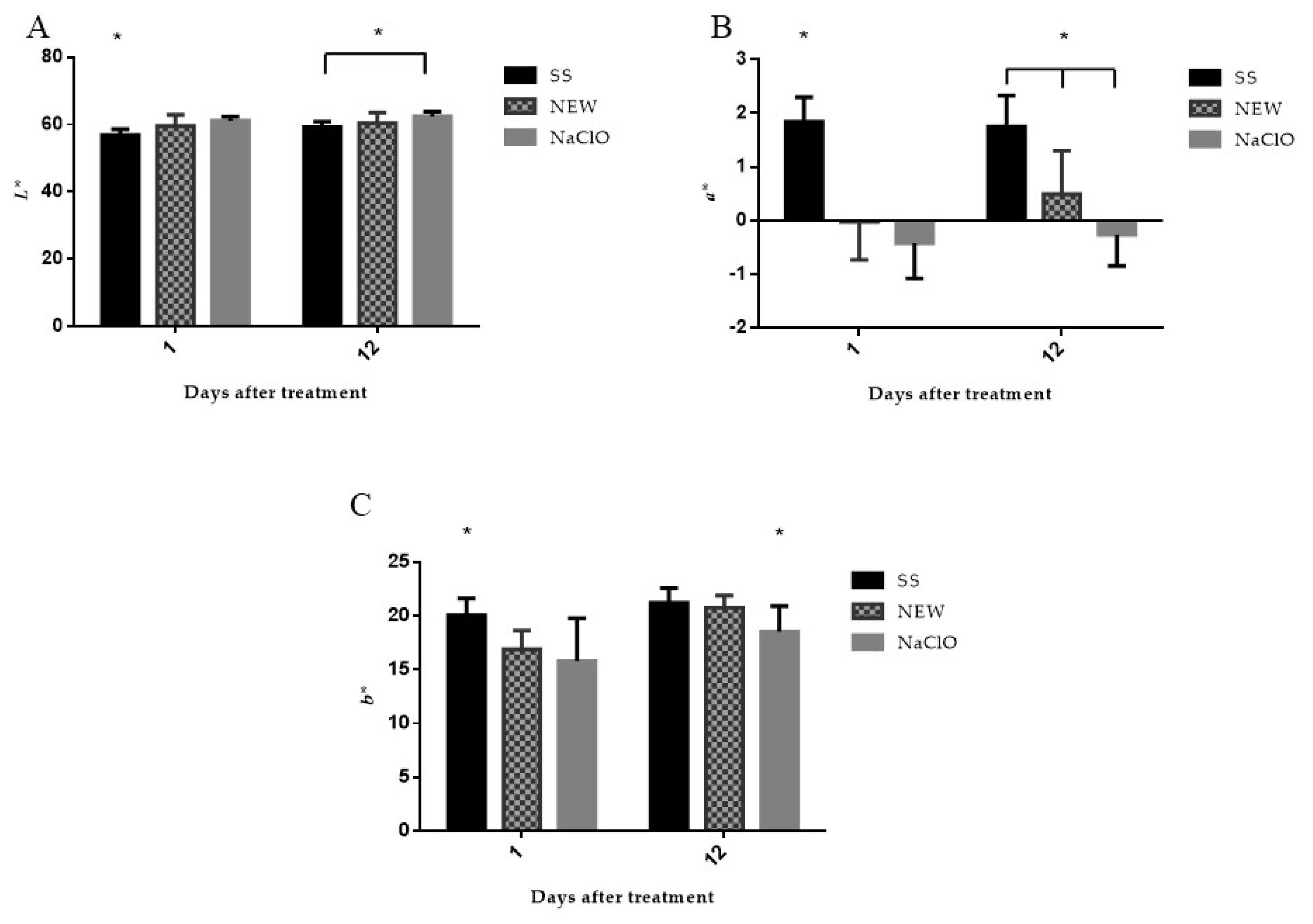
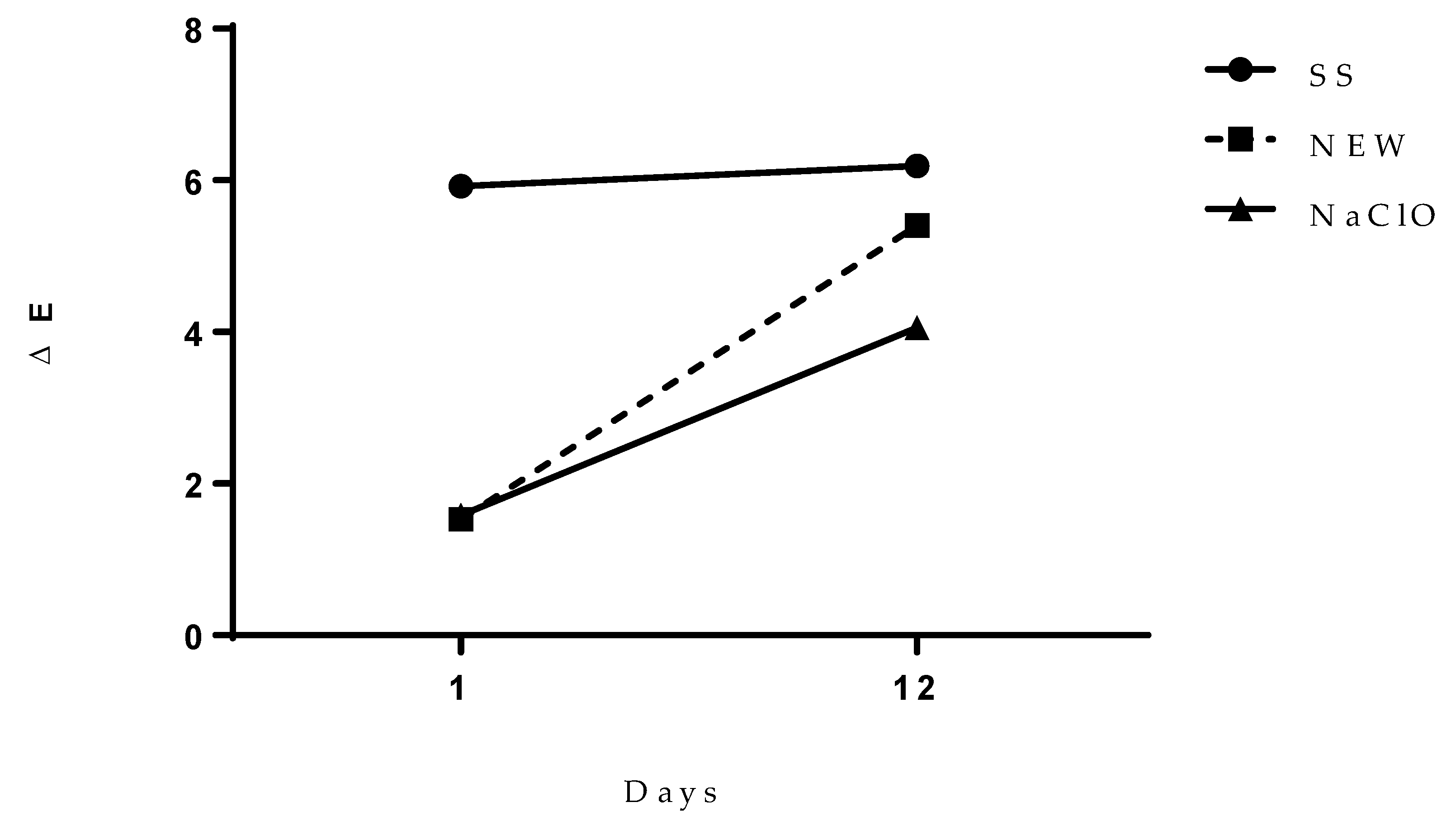
3.4.5. Color
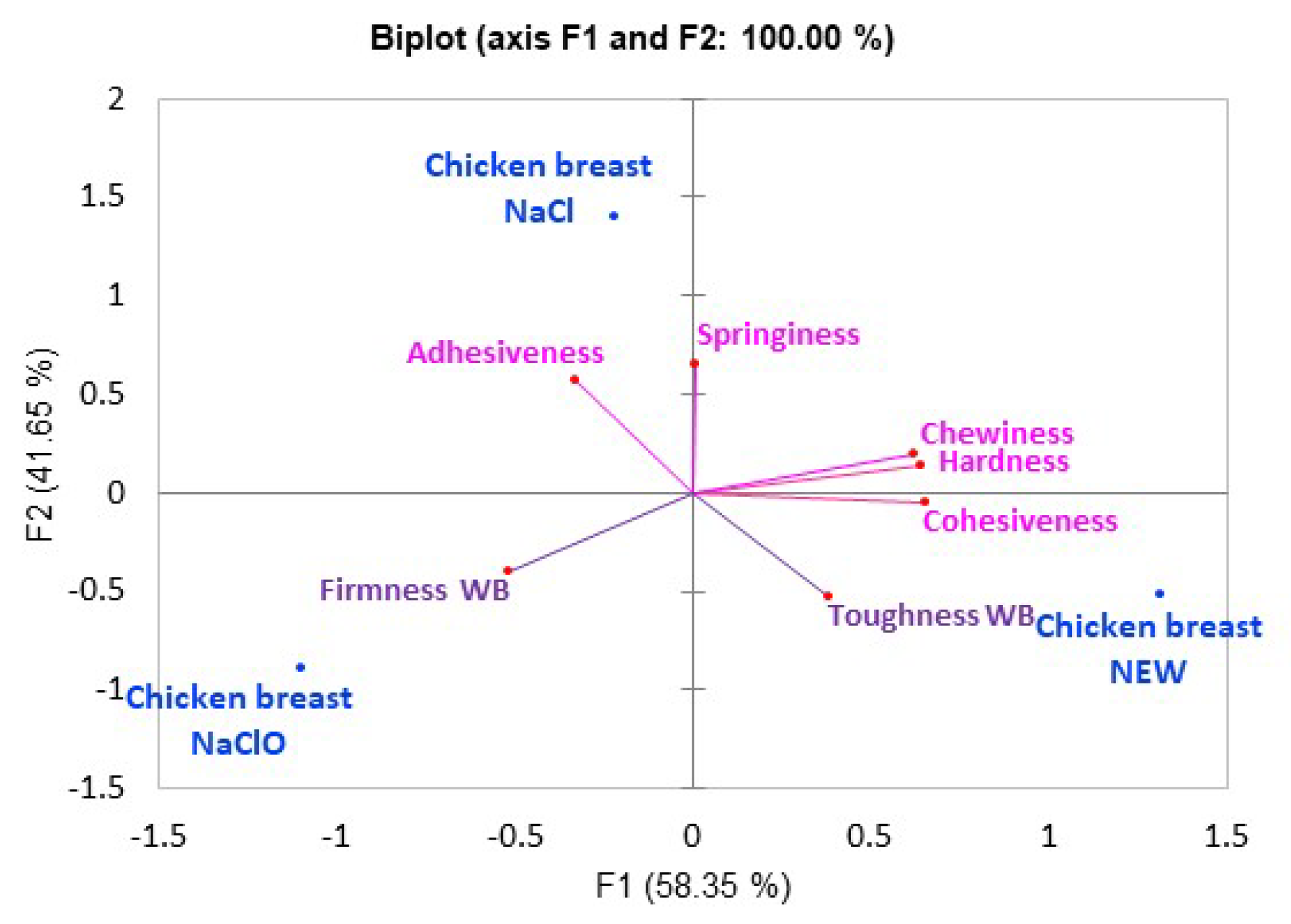
3.5. Texture
3.6. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Macelline, S.P.; Chrystal, P.V.; Liu, S.Y.; Selle, P.H. The Dynamic Conversion of Dietary Protein and Amino Acids into Chicken-Meat Protein. Animals 2021, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Bhuiyan, M.M.; Iji, P.A. Nutritive value of vegetable protein diets for broiler chickens and selection of diets containing different vegetable or animal proteins. World’s Poult. Sci. J. 2015, 71, 15–26. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Rounsevell, M.D. Human appropriation of land for food: The role of diet. Glob. Environ. Chang. 2016, 41, 88–98. [Google Scholar] [CrossRef]
- USDA Livestock and Poultry. World Markets and Trade Mixed Global 2023 Trade Outlook for Beef, Pork, and Chicken Meat; 2023. Available online: https://apps.fas.usda.gov/psdonline/circulars/livestock_poultry.pdf (accessed on 5 May 2023).
- Cao, C.; Xiao, Z.; Tong, H.; Tao, X.; Gu, D.; Wu, Y.; Xu, Z.; Ge, C. Effect of ultrasound-assisted enzyme treatment on the quality of chicken breast meat. Food Bioprod. Process. 2020, 125, 193–203. [Google Scholar] [CrossRef]
- Luckose, F. The Rising Popularity of Chicken Meat. Acta Sci. Nutr. Health 2019, 3, 1–2. [Google Scholar] [CrossRef]
- Kralik, G.; Kralik, Z.; Grčević, M.; Hanžek, D. Quality of Chicken Meat. In Animal Husbandry and Nutrition; InTech: London, United Kingdom, 2018. [Google Scholar]
- Bogosavljević-Bošković, S.; Pavlovski, Z.; Petrović, M.D.; Dosković, V.; Rakonjac, S. Broiler Meat Quality: Proteins and Lipids of Muscle Tissue. Afr. J. Biotechnol. 2010, 9, 9177–9182. [Google Scholar]
- Ponce Alquicira, E.; Braña Varela, D.; López Hernández, L.H.; Delgado Suárez, E. Evaluación de La Frescura de La Carne, 1st ed.; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Ciudad de México, Mexico, 2013; ISBN 978-607-37-0093-1. [Google Scholar]
- Wazir, H.; Chay, S.Y.; Zarei, M.; Hussin, F.S.; Mustapha, N.A.; Ibadullah, W.Z.W.; Saari, N. Effects of Storage Time and Temperature on Lipid Oxidation and Protein Co-Oxidation of Low-Moisture Shredded Meat Products. Antioxidants 2019, 8, 486. [Google Scholar] [CrossRef]
- Klaharn, K.; Pichpol, D.; Meeyam, T.; Harintharanon, T.; Lohaanukul, P.; Punyapornwithaya, V. Bacterial contamination of chicken meat in slaughterhouses and the associated risk factors: A nationwide study in Thailand. PLoS ONE 2022, 17, e0269416. [Google Scholar] [CrossRef]
- CDC. List of Multistate Foodborne Outbreak Notices. 2023. Available online: https://www.cdc.gov/foodsafety/outbreaks/lists/outbreaks-list.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Ffoodsafety%2Foutbreaks%2Fmultistate-outbreaks%2Foutbreaks-list.html#print (accessed on 5 May 2023).
- Ölmez, H.; Kretzschmar, U. Potential Alternative Disinfection Methods for Organic Fresh-Cut Industry for Minimizing Water Consumption and Environmental Impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Bilek, S.E.; Turantaş, F. Decontamination Efficiency of High Power Ultrasound in the Fruit and Vegetable Industry, a Review. Int. J. Food Microbiol. 2013, 166, 155–162. [Google Scholar] [CrossRef]
- Fallik, E. Microbial Quality and Safety of Fresh Produce. In Postharvest Handling. A system Approach; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Elsevier Inc.: New York, NY, USA, 2014; pp. 313–339. [Google Scholar]
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Hung, Y.C.; Bailly, D.; Kim, C.; Zhao, Y.; Wang, X. Effect of electrolyzed oxidizing water and chlorinated water treatments on strawberry and broccoli quality. J. Food Qual. 2010, 33, 578–598. [Google Scholar] [CrossRef]
- Afari, G.K.; Hung, Y.-C.; King, C.H. Efficacy of Neutral pH Electrolyzed Water in Reducing Escherichia coli O157: H7 and Salmonella Typhimurium DT 104 on Fresh Produce Items using an Automated Washer at Simulated Food Service Conditions. J. Food Sci. 2015, 80, M1815–M1822. [Google Scholar] [CrossRef]
- de Jong, A.E.I.; Verhoeff-Bakkenes, L.; Nauta, M.J.; de Jonge, R. Cross-contamination in the kitchen: Effect of hygiene measures. J. Appl. Microbiol. 2008, 105, 615–624. [Google Scholar] [CrossRef]
- Xuan, X.-T.; Fan, Y.-F.; Ling, J.-G.; Hu, Y.Q.; Liu, D.-H.; Chen, S.G.; Ye, X.-Q.; Ding, T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control. 2017, 73, 1483–1489. [Google Scholar] [CrossRef]
- Athayde, D.R.; Flores, D.R.M.; da Silva, J.S.; Genro, A.L.G.; Silva, M.S.; Klein, B.; Mello, R.; Campagnol, P.C.B.; Wagner, R.; de Menezes, C.R.; et al. Application of electrolyzed water for improving pork meat quality. Food Res. Int. 2017, 100, 757–763. [Google Scholar] [CrossRef]
- Kim, C.; Hung, Y.-C.; Brackett, R.E. Efficacy of electrolyzed oxidizing (EO) and chemically modified water on different types of foodborne pathogens. Int. J. Food Microbiol. 2000, 61, 199–207. [Google Scholar] [CrossRef]
- Tabernero de Paz, M.J.; Bodas, R.; Bartolomé, D.; Posado, R.; García, J.J.; Olmedo, S. Electrolyzed Water as a Cleaning Agent in Animal Production: Effects on Health and Performance. Arch. Zootec. 2013, 62, 13–23. [Google Scholar] [CrossRef]
- Orejel, J.C.R.; Cano-Buendía, J.A. Applications of Electrolyzed Water as a Sanitizer in the Food and Animal-By Products Industry. Processes 2020, 8, 534. [Google Scholar] [CrossRef]
- Rivera-Garcia, A.; Santos-Ferro, L.; Ramirez-Orejel, J.C.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Paez-Esquiliano, D.; Andrade-Esquivel, E.; Cano-Buendia, J.A. The effect of neutral electrolyzed water as a disinfectant of eggshells artificially contaminated with Listeria monocytogenes. Food Sci. Nutr. 2019, 7, 2252–2260. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Park, J.; Bin Song, K.; Al-Harbi, N.A.; Oh, D.-H. Effects of Slightly Acidic Low Concentration Electrolyzed Water on Microbiological, Physicochemical, and Sensory Quality of Fresh Chicken Breast Meat. J. Food Sci. 2011, 77, M35–M41. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Shinke, M.; Hiraishi, M.; Tsuchiya, Y.; Masuda, S. The application of alkaline and acidic electrolyzed water in the sterilization of chicken breasts and beef liver. Food Sci. Nutr. 2015, 4, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Cichoski, A.J.; Flores, D.R.M.; De Menezes, C.R.; Jacob-Lopes, E.; Zepka, L.Q.; Wagner, R.; Barin, J.S.; de Moraes Flores, É.M.; da Cruz Fernandes, M.; Campagnol, P.C.B. Ultrasound and slightly acid electrolyzed water application: An efficient combination to reduce the bacterial counts of chicken breast during pre-chilling. Int. J. Food Microbiol. 2019, 301, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pimentel, V.M.; Regalado-González, C.; Nava-Morales, G.M.; Meas-Vong, Y.; Castañeda-Serrano, M.P.; Gar-cía-Almendárez, B.E. Effect of neutral electrolyzed water as antimicrobial intervention treatment of chicken meat and on trihalomethanes formation. J. Appl. Poult. Res. 2020, 29, 622–635. [Google Scholar] [CrossRef]
- Sarjit, A.; Dykes, G.A. Trisodium phosphate and sodium hypochlorite are more effective as antimicrobials against Campylobacter and Salmonella on duck as compared to chicken meat. Int. J. Food Microbiol. 2015, 203, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Corkran, S.; McKee, S.R.; Bilgili, S.F.; Singh, M. Evaluation of post-chill applications of antimicrobials against Campylobacter jejuni on poultry carcasses. J. Appl. Poult. Res. 2015, 24, 451–456. [Google Scholar] [CrossRef]
- Torres-Rosales, E.; Rivera-Garcia, A.; Rosario-Perez, P.J.; Ramirez-Orejel, J.C.; Paez-Esquiliano, D.; Martinez-Vidal, S.; Guzman-Olea, E.; Cano-Buendia, J.A. Application of Neutral Electrolyzed Water on pork chops and its impact on meat quality. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Secretaría de Economía. NMX-AA-008-SCFI-2016; 2016. Available online: https://www.gob.mx/cms/uploads/attachment/file/166767/NMX-AA-008-SCFI-2016.pdf (accessed on 5 May 2023).
- Secretaría de Economía. NMX-AA-100-1987, Calidad Del Agua-Determinación de Cloro Total-Método Iodométrico; 1992. Available online: https://www.gob.mx/cms/uploads/attachment/file/166803/NMX-AA-100-1987.pdf (accessed on 5 May 2023).
- American Public Health Association, American Water Works Association and Water Environment Federation. Iodometric Method I. In Standard Methods for the Examination of Water and Wastewater; Greenberg, A.E., Ed.; American Public Health Association: Baltimore, MD, USA, 2012; pp. 36–37. ISBN 978-0875532356. [Google Scholar]
- Medina-Gudiño, J.; Rivera-Garcia, A.; Santos-Ferro, L.; Ramirez-Orejel, J.C.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Paez-Esquiliano, D.; Martinez-Vidal, S.; Andrade-Esquivel, E.; Cano-Buendia, J.A. International Journal of Food Microbiology Analysis of Neutral Electrolyzed Water anti-bacterial activity on contaminated eggshells with Salmonella enterica or Escherichia coli. Int. J. Food Microbiol. 2020, 320, 108538. [Google Scholar] [CrossRef]
- NMX-BB-040-SCFI-1999 Métodos Generales de Análisis-Determinación de La Actividad Antimicrobiana En Productos Ger-Micidas; Mexico. 1999. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=4955916&fecha=03/11/1999#gsc.tab=0 (accessed on 5 May 2023).
- FAO; WHO. Benefits and Risks of the Use of Chlorine-Containing Disinfectants in Food Production and Food Processing; FAO: Washington, DC, USA, 2008. [Google Scholar]
- Bialka, K.L.; Demirci, A.; Knabel, S.J.; Patterson, P.H.; Puri, V.M. Efficacy of electrolyzed oxidizing water for the microbial safety and quality of eggs. Poult. Sci. 2004, 83, 2071–2078. [Google Scholar] [CrossRef]
- Bügener, E.; Kump, A.W.-S.; Casteel, M.; Klein, G. Benefits of neutral electrolyzed oxidizing water as a drinking water additive for broiler chickens. Poult. Sci. 2014, 93, 2320–2326. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Conte-Júnior, C.A.; Monteiro, M.L.G.; Canto, A.C.V.S.; Costa-Lima, B.R.C.; Mano, S.B.; Franco, R.M. Effects of ultraviolet light on biogenic amines and other quality indicators of chicken meat during refrigerated storage. Poult. Sci. 2014, 93, 2304–2313. [Google Scholar] [CrossRef]
- NMX-F-362-S-1980. In Productos de La Pesca; Determinación de Bases Volátiles Totales: Ciudad de México, México, 1998; Volume 3, pp. 1–18.
- Hernández-Hernández, E.; Castillo-Hernández, G.; González-Gutiérrez, C.J.; Silva-Dávila, A.J.; Gracida-Rodríguez, J.N.; García-Almendárez, B.E.; Di Pierro, P.; Vázquez-Landaverde, P.; Regalado-González, C. Microbiological and Physicochemical Properties of Meat Coated with Microencapsulated Mexican Oregano (Lippia graveolens Kunth) and Basil (Ocimum basilicum L.) Essential Oils Mixture. Coatings 2019, 9, 414. [Google Scholar] [CrossRef]
- Secretaría de Economía. NMX-F-589 Determinación Del Valor de TBA—Método de Prueba. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5097039&fecha=01/07/2009#gsc.tab=0 (accessed on 5 May 2023).
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA Test by an Extractive Method Applied to ‘paté’. Meat Sci. 1995, 42, 103–110. [Google Scholar] [CrossRef]
- Mancinelli, A.C.; Silletti, E.; Mattioli, S.; Bosco, A.D.; Sebastiani, B.; Menchetti, L.; Koot, A.; van Ruth, S.; Castellini, C. Fatty acid profile, oxidative status, and content of volatile organic compounds in raw and cooked meat of different chicken strains. Poult. Sci. 2021, 100, 1273–1282. [Google Scholar] [CrossRef]
- U-Chupaj, J.; Malila, Y.; Gamonpilas, C.; Kijroongrojana, K.; Petracci, M.; Benjakul, S.; Visessanguan, W. Differences in textural properties of cooked caponized and broiler chicken breast meat. Poult. Sci. 2017, 96, 2491–2500. [Google Scholar] [CrossRef]
- Takei, R.; Hayashi, M.; Umene, S.; Kobayashi, Y.; Masunaga, H. Texture and Microstructure of Enzyme-Treated Chicken Breast Meat for People with Difficulties in Mastication. J. Texture Stud. 2016, 47, 231–238. [Google Scholar] [CrossRef]
- Dawson, P.L.; Sheldon, B.W.; Miles, J.J. Effect of Aseptic Processing on the Texture of Chicken Meat. Poult. Sci. 1991, 70, 2359–2367. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture Profile Analysis. Food Technol. 1978, 32, 62–65. [Google Scholar]
- Herrero, A.M.; Ordóñez, J.A.; de Avila, R.; Herranz, B.; de la Hoz, L.; Cambero, M.I. Breaking strength of dry fermented sausages and their correlation with texture profile analysis (TPA) and physico-chemical characteristics. Meat Sci. 2007, 77, 331–338. [Google Scholar] [CrossRef]
- Kim, C.; Hung, Y.-C.; Brackett, R.E. Roles of Oxidation-Reduction Potential in Electrolyzed Oxidizing and Chemically Modified Water for the Inactivation of Food-Related Pathogens. J. Food Prot. 2000, 63, 19–24. [Google Scholar] [CrossRef]
- Guerra Sierra, B.E.; Villalba, R.D.; Contreras, S.; Debasis, M.; Sandoval, A. Neutral Electrolyzed Water in Chillers: A Viable Option in the Microbiological Disinfection of Giblets Chicken. Energy Nexus 2022, 7, 100096. [Google Scholar] [CrossRef]
- Cori, M.E.; Michelangeli, C.; De Basilio, V.; Figueroa, R.; Rivas, N. Solubilidad Proteica, Contenido de Mioglobina, Color y PH de La Carne de Pollo, Gallina y Codorniz. Arch. Zootec. 2014, 63, 25–34. [Google Scholar] [CrossRef]
- Uribe, C.; Cecilia Suárez, M.C. Salmonelosis No Tifoidea y Su Transmisión a Través de Alimentos de Origen Aviar. Colomb. Médica 2006, 37, 151–158. [Google Scholar]
- Duan, D.; Wang, H.; Xue, S.; Li, M.; Xu, X. Application of disinfectant sprays after chilling to reduce the initial microbial load and extend the shelf-life of chilled chicken carcasses. Food Control. 2017, 75, 70–77. [Google Scholar] [CrossRef]
- Wang, H.; Qi, J.; Duan, D.; Dong, Y.; Xu, X.; Zhou, G. Combination of a novel designed spray cabinet and electrolyzed water to reduce microorganisms on chicken carcasses. Food Control. 2017, 86, 200–206. [Google Scholar] [CrossRef]
- Rasschaert, G.; Piessens, V.; Scheldeman, P.; Leleu, S.; Stals, A.; Herman, L.; Heyndrickx, M.; Messens, W. Efficacy of electrolyzed oxidizing water and lactic acid on the reduction of Campylobacter on naturally contaminated broiler carcasses during processing. Poult. Sci. 2013, 92, 1077–1084. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, H.; Zhao, D.; Zheng, W.; Tian, W.; Ma, H.; Liu, K.; Hu, H.; Wang, T.; Soupir, M. Free chlorine loss during spraying of membraneless acidic electrolyzed water and its antimicrobial effect on airborne bacteria from poultry house. Ann. Agric. Environ. Med. 2014, 21, 249–255. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Kim, H.; Beuchat, L.R. Attachment and Biofilm Formation by Escherichia Coli O157:H7 on Stainless Steel as Influenced by Exopolysaccharide Production, Nutrient Availability, and Temperature. J. Food Prot. 2004, 67, 2123–2131. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Beuchat, L.R. Biofilm Formation by Escherichia coli O157:H7 on Stainless Steel: Effect of Exopolysaccharide and Curli Production on Its Resistance to Chlorine. Appl. Environ. Microbiol. 2005, 71, 247–254. [Google Scholar] [CrossRef]
- Wilks, S.A.; Michels, H.; Keevil, C.W. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005, 105, 445–454. [Google Scholar] [CrossRef]
- Kong, D.; Quan, C.; Xi, Q.; Han, R.; Koseki, S.; Li, P.; Du, Q.; Yang, Y.; Forghani, F.; Wang, J. Study on the quality and myofibrillar protein structure of chicken breasts during thawing of ultrasound-assisted slightly acidic electrolyzed water (SAEW). Ultrason. Sonochem. 2022, 88, 106105. [Google Scholar] [CrossRef]
- Sharma, H.; Fidan, H.; Özogul, F.; Rocha, J.M. Recent development in the preservation effect of lactic acid bacteria and essential oils on chicken and seafood products. Front. Microbiol. 2022, 13, 1092248. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Xia, W.; Regenstein, J.M.; Zhou, P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at −3 and 0 °C. Food Chem. 2013, 140, 105–114. [Google Scholar] [CrossRef]
- Indergård, E.; Tolstorebrov, I.; Larsen, H.; Eikevik, T.M. The influence of long-term storage, temperature and type of packaging materials on the quality characteristics of frozen farmed Atlantic Salmon (Salmo Salar). Int. J. Refrig. 2014, 41, 27–36. [Google Scholar] [CrossRef]
- Fletcher, D. Color de La Carne de Aves de Granja. In Ciencia de La Carne de Ave; Acribia: Zaragoza, España, 2001. [Google Scholar]
- Sun, X.B.; Huang, J.C.; Li, T.T.; Ang, Y.; Xu, X.L.; Huang, M. Effects of preslaughter shackling on postmortem glycolysis, meat quality, changes of water distribution, and protein structures of broiler breast meat. Poult. Sci. 2019, 98, 4212–4220. [Google Scholar] [CrossRef]
- Tokur, B.; Polat, A.; Beklevik, G.; Özkütük, S. Changes in the quality of fishburger produced from Tilapia (Oreochromis niloticus) during frozen storage (−18 °C). Eur. Food Res. Technol. 2004, 218, 420–423. [Google Scholar] [CrossRef]
- Zhang, Y.; Holman, B.W.B.; Ponnampalam, E.N.; Kerr, M.G.; Bailes, K.L.; Kilgannon, A.K.; Collins, D.; Hopkins, D.L. Understanding beef flavour and overall liking traits using two different methods for determination of thiobarbituric acid reactive substance (TBARS). Meat Sci. 2019, 149, 114–119. [Google Scholar] [CrossRef]
- Kruk, Z.A.; Yun, H.; Rutley, D.L.; Lee, E.J.; Kim, Y.J.; Jo, C. The effect of high pressure on microbial population, meat quality and sensory characteristics of chicken breast fillet. Food Control. 2011, 22, 6–12. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.-W.; Pu, H.; Xie, A.; Han, Z.; Luo, M. Non-destructive prediction of thiobarbituricacid reactive substances (TBARS) value for freshness evaluation of chicken meat using hyperspectral imaging. Food Chem. 2015, 179, 175–181. [Google Scholar] [CrossRef]
- Mohan, C.C.; Krishnan, K.R.; Babuskin, S.; Sudharsan, K.; Aafrin, V.; Priya, U.L.; Mariyajenita, P.; Harini, K.; Madhushalini, D.; Sukumar, M. Active compound diffusivity of particle size reduced S. aromaticum and C. cassia fused starch edible films and the shelf life of mutton (Capra aegagrus hircus) meat. Meat Sci. 2017, 128, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Portilla, M.; Gomez, N.; Martínez-Benavides, J.; De Nariño, U. Evaluación de las características organolépticas, físicas y químicas de pechuga de pollo, en San Juan de Pasto (Nariño). Rev. Veter. Zootec. 2016, 10, 62–71. [Google Scholar] [CrossRef]
- Ko, J.; Ma, Y.; Song, K. Bin Effect of Chlorine Dioxide Treatment on Microbial Growth and Qualities of Chicken Breast. J. Food Sci. Nutr. 2005, 10, 122–129. [Google Scholar]
- Rahman, U.U.; Sahar, A.; Pasha, I.; Rahman, S.U.; Sohaib, M.; Ishaq, A.; Chughtai, M.F.J.; Zafar, H. Augmenting Quality and Microbial Safety of Broiler Meat at Refrigeration Storage by Applying Chemical Interventions. J. Food Process. Preserv. 2016, 41, e13030. [Google Scholar] [CrossRef]
- Oh, S.R.; Kang, I.; Oh, M.H.; Ha, S.D. Inhibitory effect of chlorine and ultraviolet radiation on growth of Listeria monocytogenes in chicken breast and development of predictive growth models. Poult. Sci. 2014, 93, 200–207. [Google Scholar] [CrossRef]
- Yi, G.; Grabež, V.; Bjelanovic, M.; Slinde, E.; Olsen, K.; Langsrud, O.; Phung, V.T.; Haug, A.; Oostindjer, M.; Egelandsdal, B. Lipid oxidation in minced beef meat with added Krebs cycle substrates to stabilise colour. Food Chem. 2015, 187, 563–571. [Google Scholar] [CrossRef]
- Shao, L.; Qin, Y.; Xu, X.; Wang, H. Assessment of the di- and tri-chlorinated haloacetic acids during broiler prechilling. J. Food Sci. 2021, 86, 5495–5502. [Google Scholar] [CrossRef]
- Al-Haq, M.I.; Sugiyama, J.; Isobe, S. Applications of Electrolyzed Water in Agriculture & Food Industries. Food Sci. Technol. Res. 2005, 11, 135–150. [Google Scholar] [CrossRef]

| SS | NEW | NaClO | |
|---|---|---|---|
| pH | 5.86 ± 0.09 c | 6.70 ± 0.19 b | 7.58 ± 0.16 a |
| ORP (mV) | 365.67 ± 7.57 c | 895.67 ± 13.05 a | 692.33 ± 7.51 b |
| Cl2 (ppm) | ND | 55.73 ± 0.56 a | 35.02 ± 1.06 b |
| Treatment | |||
|---|---|---|---|
| SS | NEW | NaClO | |
| Escherichia coli | 9.27 ± 0.18 * | ˂3.00 | ˂3.00 |
| Salmonella Typhimurium | 8.14 ± 0.03 * | ˂3.00 | ˂3.00 |
| Treatment | |||
|---|---|---|---|
| Day | SS | NEW | NaClO |
| 1 | 6.10 ± 0.41 aC | 6.02 ± 0.16 aB | 6.10 ± 0.11 aB |
| 2 | 6.14 ± 0.33 aC | 6.11 ± 0.12 aB | 6.30 ± 0.33 aB |
| 3 | 6.29 ± 0.04 abC | 6.18 ± 0.11 bB | 6.57 ± 0.14 aA |
| 4 | 6.31 ± 0.07 abC | 6.22 ± 0.09 bB | 6.67 ± 0.07 aA |
| 5 | 6.27 ± 0.17 abC | 6.19 ± 0.03 bB | 6.63 ± 0.08 aA |
| 8 | 6.66 ± 0.63 abB | 6.43 ± 0.17 bB | 6.90 ± 0.09 aA |
| 12 | 7.30 ± 0.22 aA | 6.53 ± 0.05 bA | 7.10 ± 0.21 aA |
| Treatment | |||
|---|---|---|---|
| Day | SS | NEW | NaClO |
| 1 | 0.161 ± 0.05 aA | 0.154 ± 0.03 aA | 0.127 ± 0.01 aA |
| 2 | 0.110 ± 0.01 aB | 0.136 ± 0.00 aA | 0.109 ± 0.02 aA |
| 3 | 0.129 ± 0.03 abA | 0.144 ± 0.01 aA | 0.100 ± 0.01 bA |
| 4 | 0.112 ± 0.01 aB | 0.135 ± 0.01 aA | 0.118 ± 0.01 aA |
| 5 | 0.136 ± 0.02 aA | 0.128 ± 0.00 aA | 0.114 ± 0.00 aA |
| 8 | 0.161 ± 0.04 aA | 0.120 ± 0.00 bA | 0.100 ± 0.02 bA |
| 12 | 0.107 ± 0.01 aB | 0.129 ± 0.00 aA | 0.108 ± 0.03 aA |
| Treatment | |||
|---|---|---|---|
| Day | SS | NEW | NaClO |
| 1 | 0.757 ± 0.35 aA | 0.591 ± 0.25 aA | 0.821 ± 0.33 aA |
| 2 | 0.377 ± 0.16 aB | 0.431 ± 0.22 aA | 0.365 ± 0.12 aB |
| 3 | 0.249 ± 0.03 aB | 0.360 ± 0.19 aA | 0.686 ± 0.25 bA |
| 4 | 0.297 ± 0.12 aB | 0.533 ± 0.14 aA | 0.417 ± 0.08 aB |
| 5 | 0.339 ± 0.16 aB | 0.344 ± 0.14 aA | 0.345 ± 0.12 aB |
| 8 | 0.129 ± 0.02 aB | 0.101 ± 0.04 aB | 0.347 ± 0.10 aB |
| 12 | 0.180 ± 0.03 aB | 0.304 ± 0.18 aA | 0.308 ± 0.12 aB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosario-Pérez, P.J.; Rodríguez-Sollano, H.E.; Ramírez-Orejel, J.C.; Severiano-Pérez, P.; Cano-Buendía, J.A. Neutral Electrolyzed Water in Chicken Breast—A Preservative Option in Poultry Industry. Foods 2023, 12, 1970. https://doi.org/10.3390/foods12101970
Rosario-Pérez PJ, Rodríguez-Sollano HE, Ramírez-Orejel JC, Severiano-Pérez P, Cano-Buendía JA. Neutral Electrolyzed Water in Chicken Breast—A Preservative Option in Poultry Industry. Foods. 2023; 12(10):1970. https://doi.org/10.3390/foods12101970
Chicago/Turabian StyleRosario-Pérez, Patricia J., Héctor E. Rodríguez-Sollano, Juan C. Ramírez-Orejel, Patricia Severiano-Pérez, and José A. Cano-Buendía. 2023. "Neutral Electrolyzed Water in Chicken Breast—A Preservative Option in Poultry Industry" Foods 12, no. 10: 1970. https://doi.org/10.3390/foods12101970





