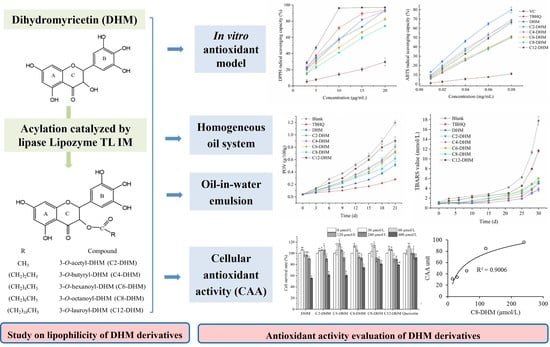
Antioxidant Activities of Dihydromyricetin Derivatives with Different Acyl Donor Chain Lengths Synthetized by Lipozyme TL IM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Acylated Derivatives of DHM
2.3. Determination of the Partition Coefficient of Lipids and Water (log P)
2.4. Antioxidant Activity
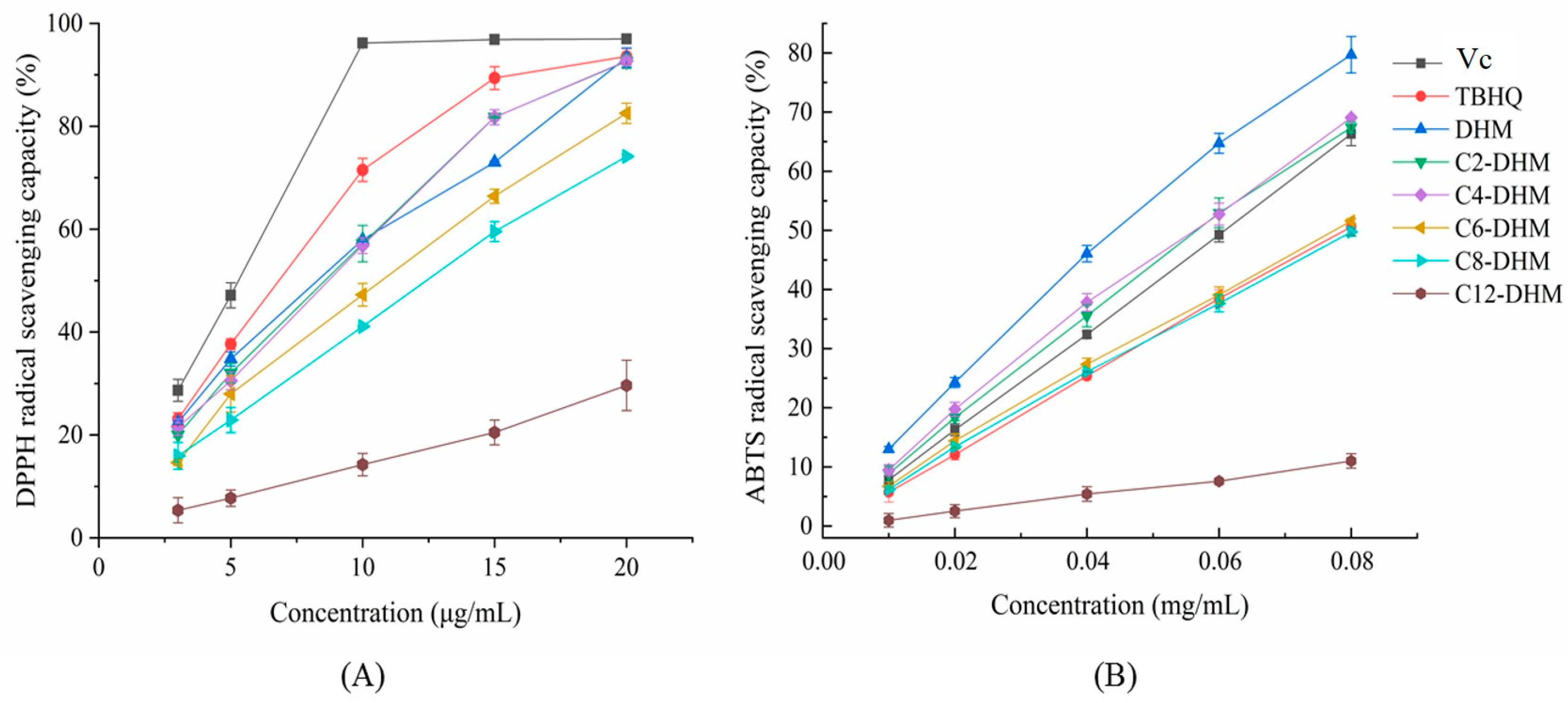
2.4.1. DPPH Radical Scavenging Ability
2.4.2. ABTS Radical Scavenging Ability
2.5. Antioxidant Activity in Sunflower Oil
2.6. Antioxidant Activity in the O/W Emulsion
2.6.1. Emulsion Preparation
2.6.2. Determination of Peroxide Value
2.6.3. Determination of TBARS Value
2.6.4. Determination of Antioxidant Partitioning in the O/W Emulsion
2.7. Cellular Antioxidant Activity (CAA)
2.7.1. Cell Culture
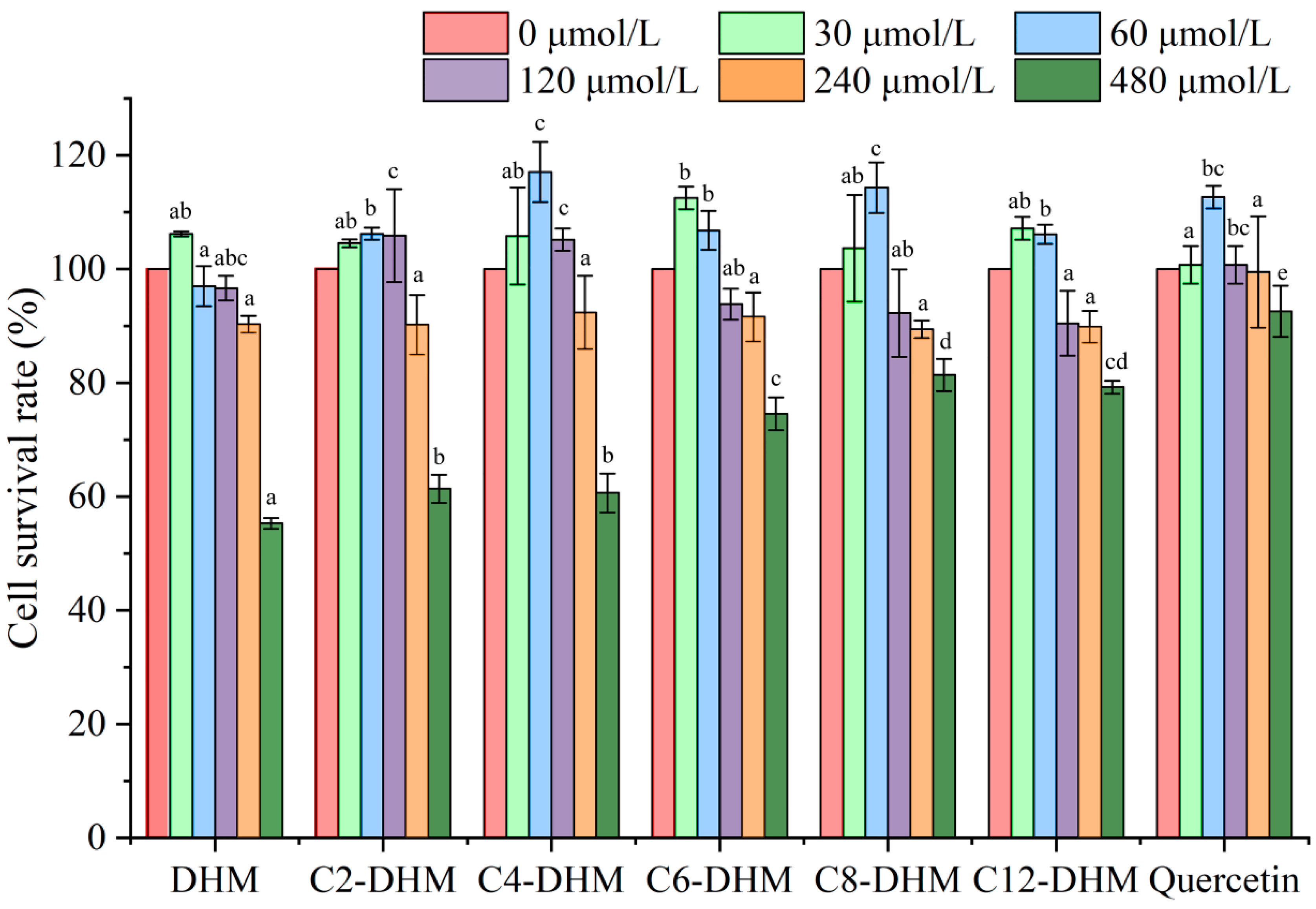
2.7.2. Cytotoxicity
2.7.3. Cellular Antioxidant Activity (CAA) Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Antioxidant Activity
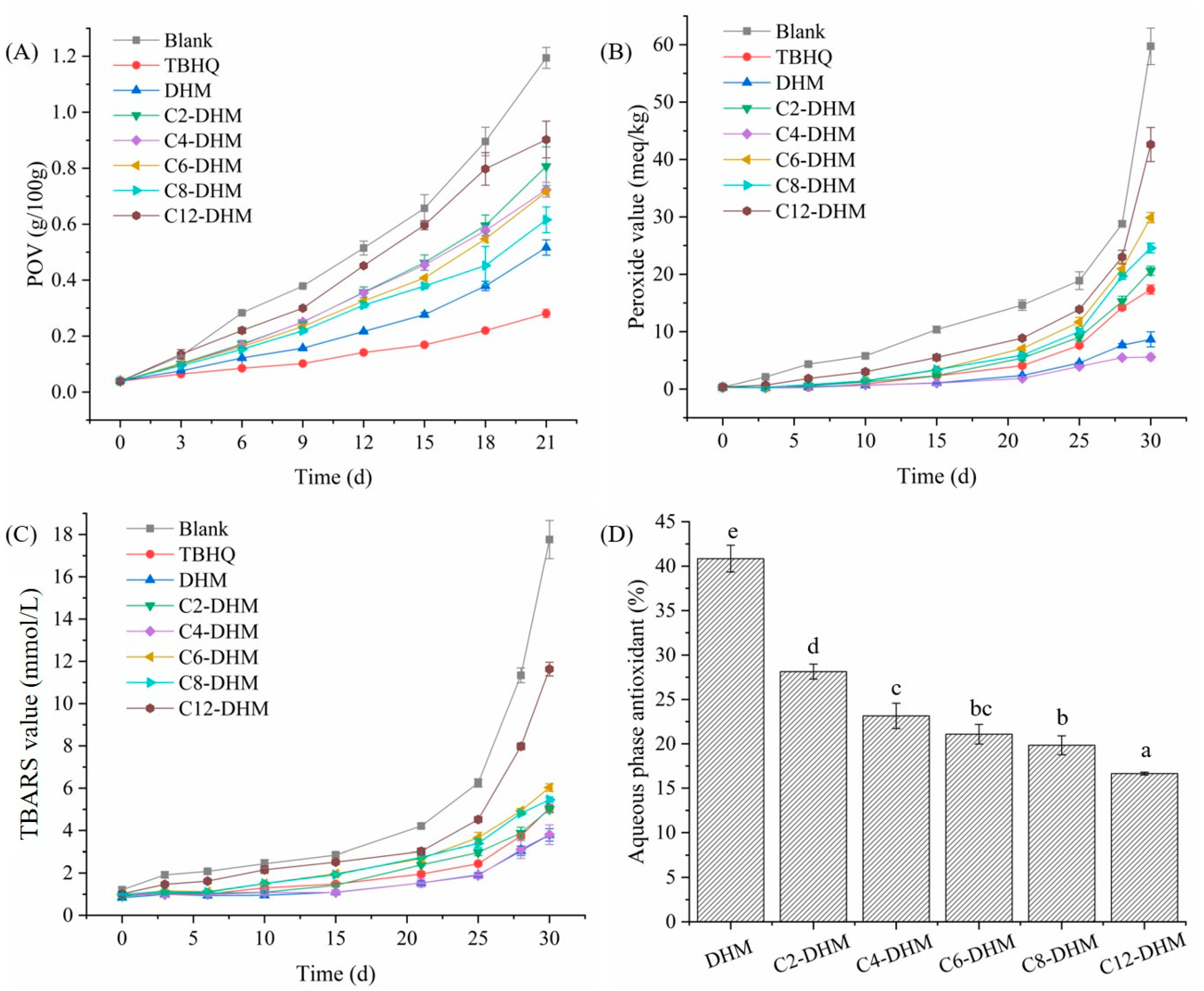
3.2. Antioxidant Activity in the Sunflower Oil System
3.3. Antioxidant Activity in the O/W Emulsion
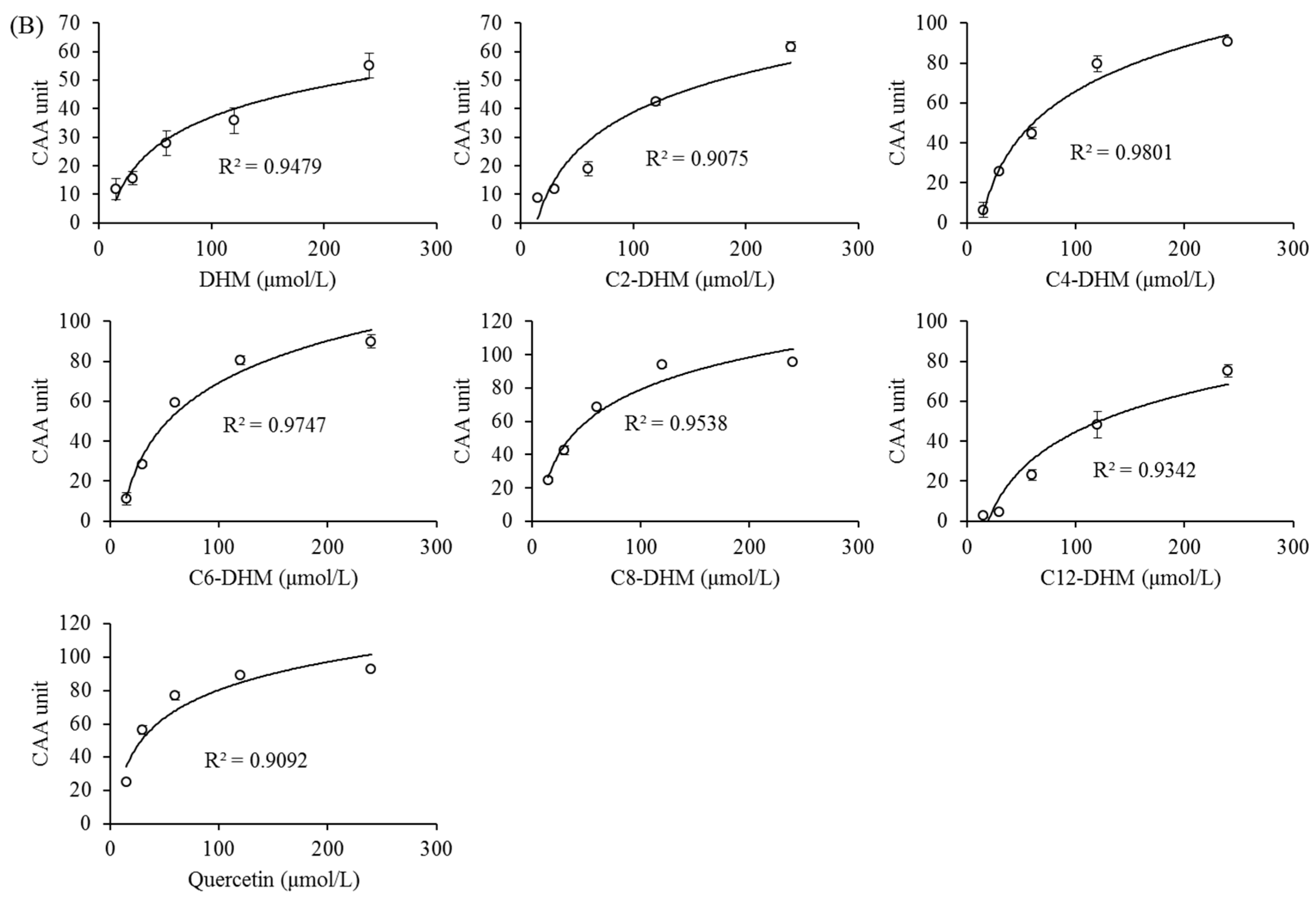
3.4. Cellular Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, H.; Xu, H.; Li, M.; Zhang, D. Molecular mechanism and therapeutic significance of dihydromyricetin in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2022, 935, 175325. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.G.; Du, J.Q.; Zeng, J.; Chen, C.G.; Niu, S.Y. Characterization and antioxidant activity of dihydromyricetin–lecithin complex. Eur. Food Res. Technol. 2009, 230, 325–331. [Google Scholar] [CrossRef]
- Liu, D.; Mao, Y.Q.; Ding, L.J.; Zeng, X.A. Dihydromyricetin: A review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2019, 91, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, S.; Chen, S.W.; Xia, Y.M.; Li, Y. Lipase-catalyzed highly regioselective synthesis of acylated chlorogenic acid. Food Biosci. 2020, 37, 100706. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, N.; Li, Y.; Chen, S.W.; Xia, Y.M. Antioxidant activities of lipophilic (−)-epigallocatechin gallate derivatives in vitro and in lipid-based food systems. Food Biosci. 2021, 42, 101055. [Google Scholar] [CrossRef]
- Kumar, V.; Jahan, F.; Mahajan, R.V.; Saxena, R.K. Efficient regioselective acylation of quercetin using Rhizopus oryzae lipase and its potential as antioxidant. Bioresource Technol. 2016, 218, 1246–1248. [Google Scholar] [CrossRef]
- Saik, A.Y.H.; Lim, Y.Y.; Stanslas, J.; Choo, W.S. Lipase-catalyzed acylation of quercetin with cinnamic acid. Biocatal. Biotransfor. 2016, 34, 33–43. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.X.; Zheng, L.; Cui, X.Y.; Huang, H.; Geng, X.; Xie, X.N. Regioselective acylation of resveratrol catalyzed by lipase under microwave. Green Chem. Lett. Rev. 2018, 11, 312–317. [Google Scholar] [CrossRef]
- Hyatt, J.R.; Zhang, S.Y.; Akoh, C.C. Comparison of antioxidant activities of selected phenolic compounds in O/W emulsions and bulk oil. Food Chem. 2021, 349, 129037. [Google Scholar] [CrossRef]
- Poter, W.L.; Black, E.D.; Drolet, A.M. Use of polyamide oxidative fluoresence test on lipid emulsions: Contrast in relative effectiveness of antioxidants in bulk versus dispersed systems. J. Agric. Food Chem. 1989, 37, 615–624. [Google Scholar] [CrossRef]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Villeneuve, P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. 2014, 55, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Villeneuve, P. Chain length affects antioxidant properties of chlorogenate esters in emulsion: The cutoff theory behind the polar paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef]
- Panya, A.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; McClements, D.J.; Decker, E.A. An investigation of the versatile antioxidant mechanisms of action of rosmarinate alkyl esters in oil-in-water emulsions. J. Agric. Food Chem. 2012, 60, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Meireles, M.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Diaz, C.; Monteiro, L.S. Control of antioxidant efficiency of chlorogenates in emulsions: Modulation of antioxidant interfacial concentrations. J. Sci. Food Agric. 2019, 99, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.S.; Coupland, J.N.; Elias, R.J. Effect of alkyl chain length on the antioxidant activity of alkylresorcinol homologues in bulk oils and oil-in-water emulsions. Food Chem. 2021, 346, 128885. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.X.; Brock, A.A.; Jackson, B.T.; Greenspan, P.; Pegg, R.B. The cellular antioxidant and anti-glycation capacities of phenolics from Georgia peaches. Food Chem. 2020, 316, 126234. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Meng, X.; Chen, S.; Huang, D.; Xia, Y.; Zhu, S. Antioxidant activities of chlorogenic acid derivatives with different acyl donor chain lengths and their stabilities during in vitro simulated gastrointestinal digestion. Food Chem. 2021, 357, 129904. [Google Scholar] [CrossRef]
- Zhu, S.; Du, B.; Huang, D.; Xia, Y.; Chen, S.; Li, Y. Highly efficient regioselective acylation of dihydromyricetin catalyzed by lipase in nonaqueous solvents. Processes 2022, 10, 1368. [Google Scholar] [CrossRef]
- Sun, J.; Jing, H.; Liu, T.M.; Dong, S.J.; Obadi, M.; Xu, B. Evaluation of antioxidant modification on the functional and structural properties of EWP conjugates. RSC Adv. 2020, 10, 10666–10672. [Google Scholar] [CrossRef] [PubMed]
- Noon, J.; Mills, T.B.; Norton, I.T. The use of natural antioxidants to combat lipid oxidation in O/W emulsions. J. Food Eng. 2020, 281, 110006. [Google Scholar] [CrossRef]
- Liu, R.R.; Xu, Y.; Chang, M.; Liu, R.J.; Wang, X.G. Interactions between α-tocopherol and γ-oryzanol in oil-in-water emulsions. Food Chem. 2021, 356, 129648. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Liu, R.H. Cellar antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Laguna, O.; Durand, E.; Baréa, B.; Dauguet, S.; Fine, F.; Lecomte, J. Synthesis and evaluation of antioxidant activities of novel hydroxyalkyl esters and bis-aryl esters based on sinapic and caffeic acids. J. Agric. Food Chem. 2020, 68, 9308–9318. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhang, M.M.; Li, X.F.; Lai, F.R.; Zhao, G.L. Biocatalytic synthesis of acylated derivatives of troxerutin: Their bioavailability and antioxidant properties in vitro. Microb. Cell Fact. 2018, 17, 130. [Google Scholar] [CrossRef]
- Wang, M.F.; Zhang, X.C.; Zhong, Y.J.; Perera, N.S.; Shahidi, F. Antiglycation activity of lipophilized epigallocatechin gallate (EGCG) derivatives. Food Chem. 2016, 190, 1022–1026. [Google Scholar] [CrossRef]
- Romsted, L.S.; Bravo-Díaz, C. Modeling chemical reactivity in emulsions. Curr. Opin. Colloid Interface 2013, 18, 3–14. [Google Scholar] [CrossRef]
- Bayrasy, C.; Chabi, B.; Laguerre, M.; Lecomte, J.; Jublanc, E.; Villeneuve, P.; Cabello, G. Boosting antioxidants by lipophilization: A strategy to increase cell uptake and target mitochondria. Pharm. Res. 2013, 30, 1979–1989. [Google Scholar] [CrossRef]
- Lu, M.Y.; Zhang, T.; Jiang, Z.R.; Guo, Y.W.; Qiu, F.C.; Liu, R.R.; Zhang, L.L.; Chang, M.; Liu, R.J.; Jin, Q.Z.; et al. Physical properties and cellular antioxidant activity of vegetable oil emulsions with different chain lengths and saturation of triglycerides. LWT-Food Sci. Technol. 2020, 121, 108948. [Google Scholar] [CrossRef]

| Sample | IC50 | EC50 (μmol/L) | ||
|---|---|---|---|---|
| DPPH (μg/mL) | ABTS (μg/mL) | CAA Unit | ||
| No Wash | Wash | |||
| Vc | 4.23 ± 1.38 a | 61.02 ± 2.15 a | - | - |
| TBHQ | 7.55 ± 0.18 ab | 79.34 ± 3.37 a | - | - |
| DHM | 9.04 ± 0.09 bc | 46.51 ± 2.19 a | 90.17 ± 5.02 e | 226.26 ± 4.67 e |
| C2-DHM | 9.07 ± 0.10 bc | 58.29 ± 2.20 a | 77.79 ± 3.81 d | 171.62 ± 8.84 d |
| C4-DHM | 9.08 ± 0.25 bc | 56.30 ± 1.03 a | 60.81 ± 6.23 c | 60.70 ± 0.64 b |
| C6-DHM | 11.17 ± 0.04 bc | 77.22 ± 1.46 a | 65.24 ± 3.00 c | 52.91 ± 2.69 b |
| C8-DHM | 12.69 ± 0.09 c | 80.15 ± 0.42 a | 45.43 ± 0.74 b | 35.14 ± 0.29 a |
| C12-DHM | 35.93 ± 5.63 d | 360.69 ± 50.13 b | 233.96 ± 9.69 f | 122.44 ± 9.48 c |
| Quercetin | - | - | 33.91 ± 3.67 a | 29.03 ± 0.36 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Wang, S.; Zhu, S.; Li, Y.; Huang, D.; Chen, S. Antioxidant Activities of Dihydromyricetin Derivatives with Different Acyl Donor Chain Lengths Synthetized by Lipozyme TL IM. Foods 2023, 12, 1986. https://doi.org/10.3390/foods12101986
Du B, Wang S, Zhu S, Li Y, Huang D, Chen S. Antioxidant Activities of Dihydromyricetin Derivatives with Different Acyl Donor Chain Lengths Synthetized by Lipozyme TL IM. Foods. 2023; 12(10):1986. https://doi.org/10.3390/foods12101986
Chicago/Turabian StyleDu, Baoshuang, Shan Wang, Song Zhu, Yue Li, Dejian Huang, and Shangwei Chen. 2023. "Antioxidant Activities of Dihydromyricetin Derivatives with Different Acyl Donor Chain Lengths Synthetized by Lipozyme TL IM" Foods 12, no. 10: 1986. https://doi.org/10.3390/foods12101986