Physicochemical and In Vitro Starch Residual Digestion Structures of Extruded Maize and Sorghum Starches Added with Sodium Stearoyl Lactylate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Extrusion
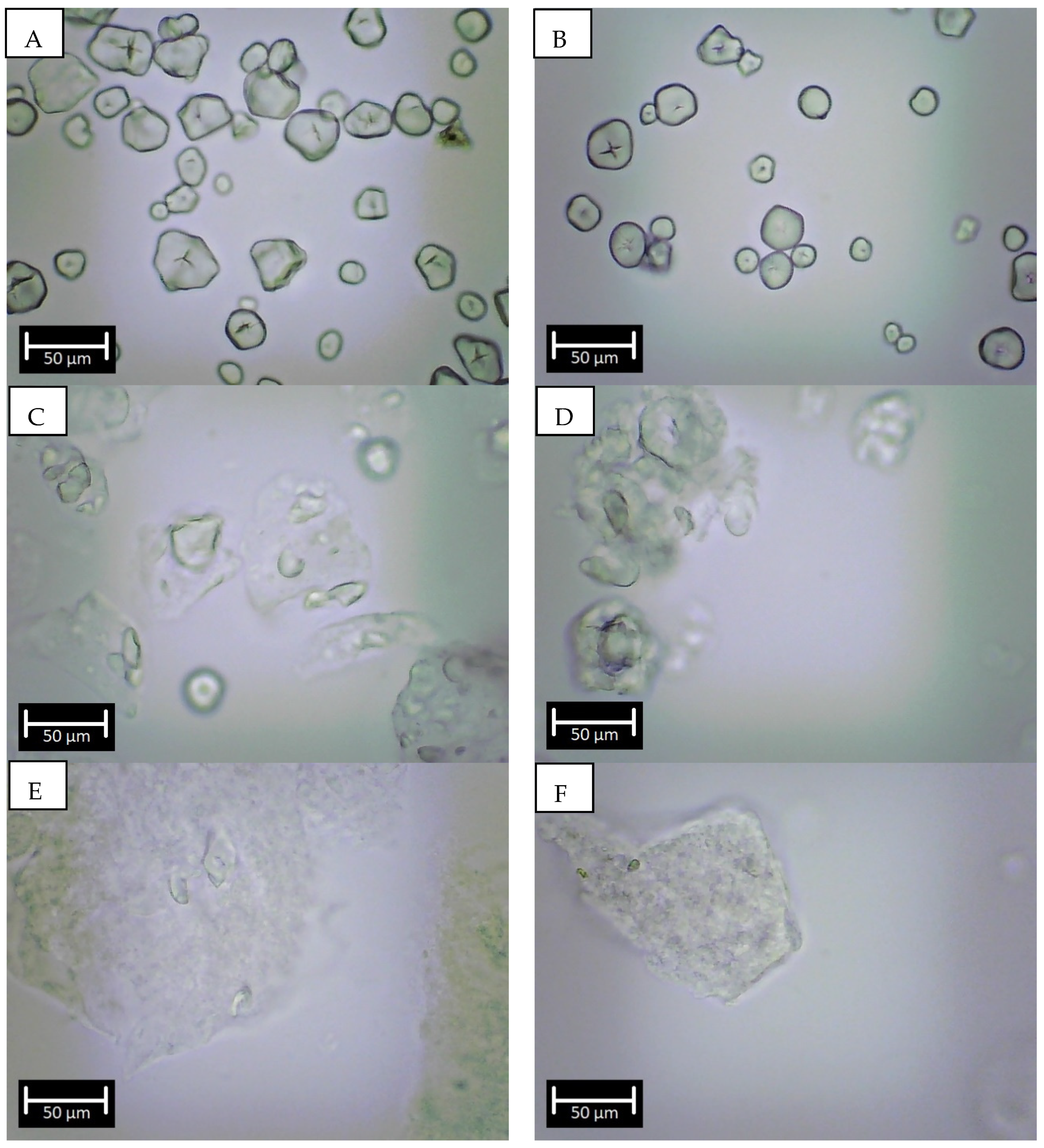
2.3. Starch Granule Morphology
2.4. Starch and Amylose Contents
2.5. Thermal Properties
2.6. X-ray Diffraction
2.7. Starch Digestion Fractions
2.8. In Vitro Starch Digestion Rate and Predicted Glycemic Index
2.9. Transmittance and Wavelength of Maximum Absorption
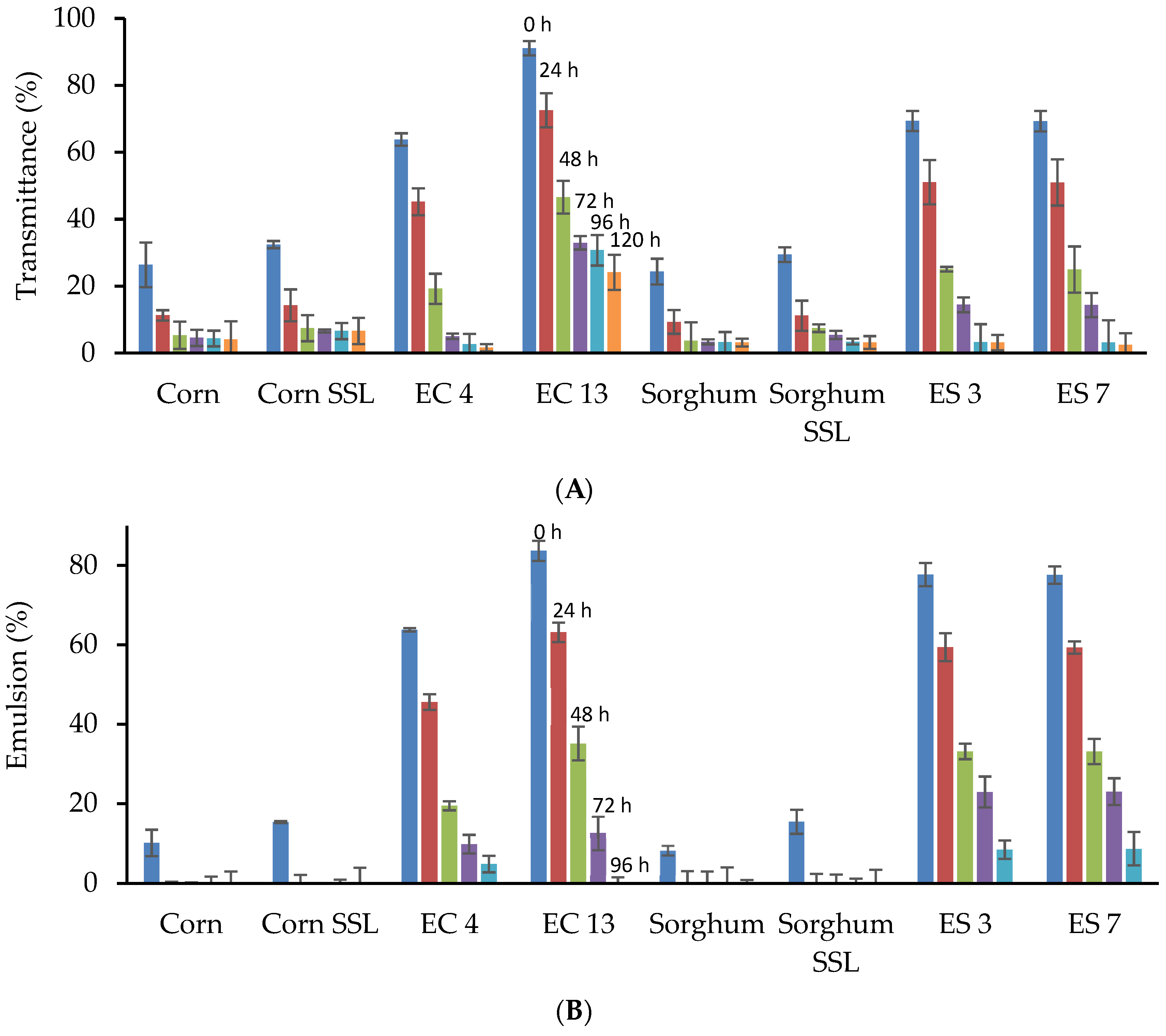
2.10. Emulsifying Characteristics
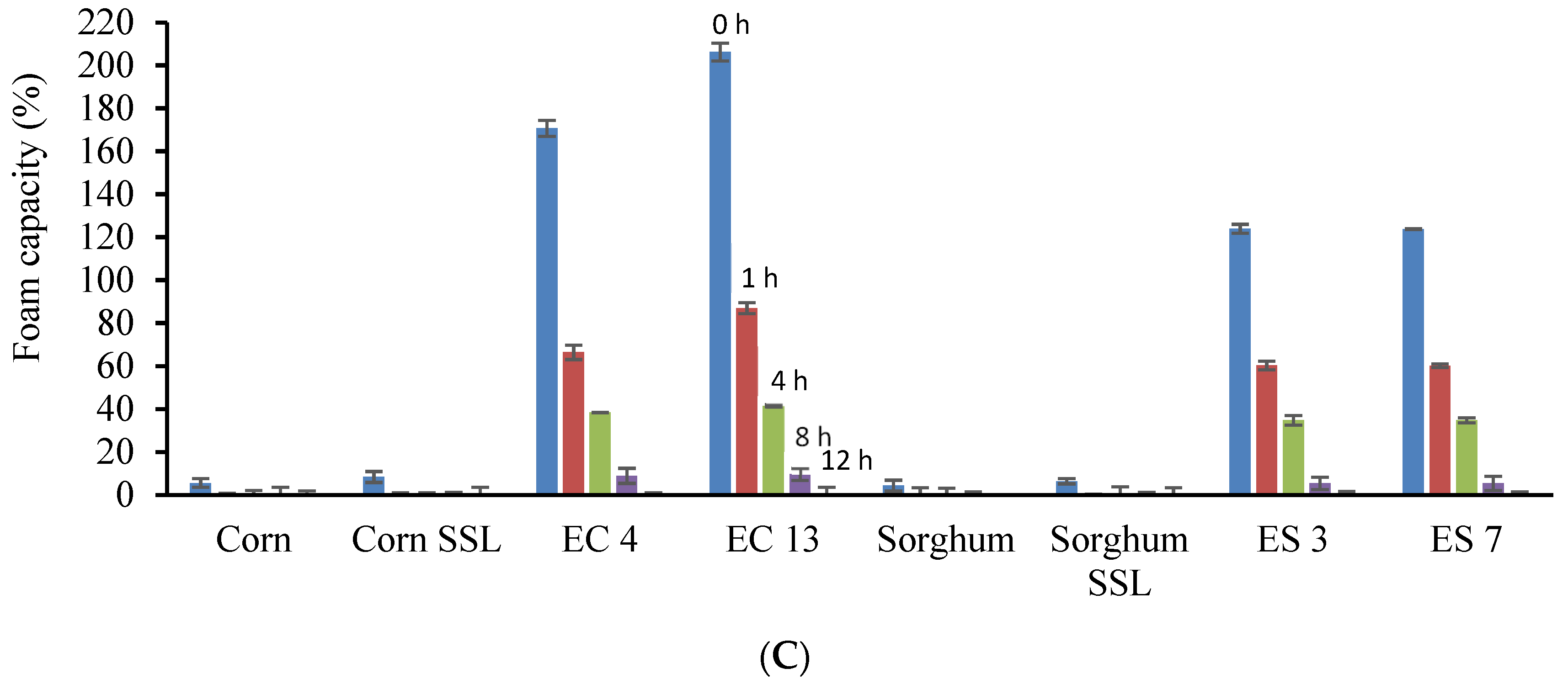
2.11. Foaming Characteristics
2.12. Molecular Characteristics of Starch and Amylopectin Chain Length Distribution
2.13. Statistical Analyses
3. Results and Discussion
3.1. Starch Characteristics
3.2. Thermal Characteristics
3.3. In Vitro Starch Digestion Properties
3.4. Functional Properties
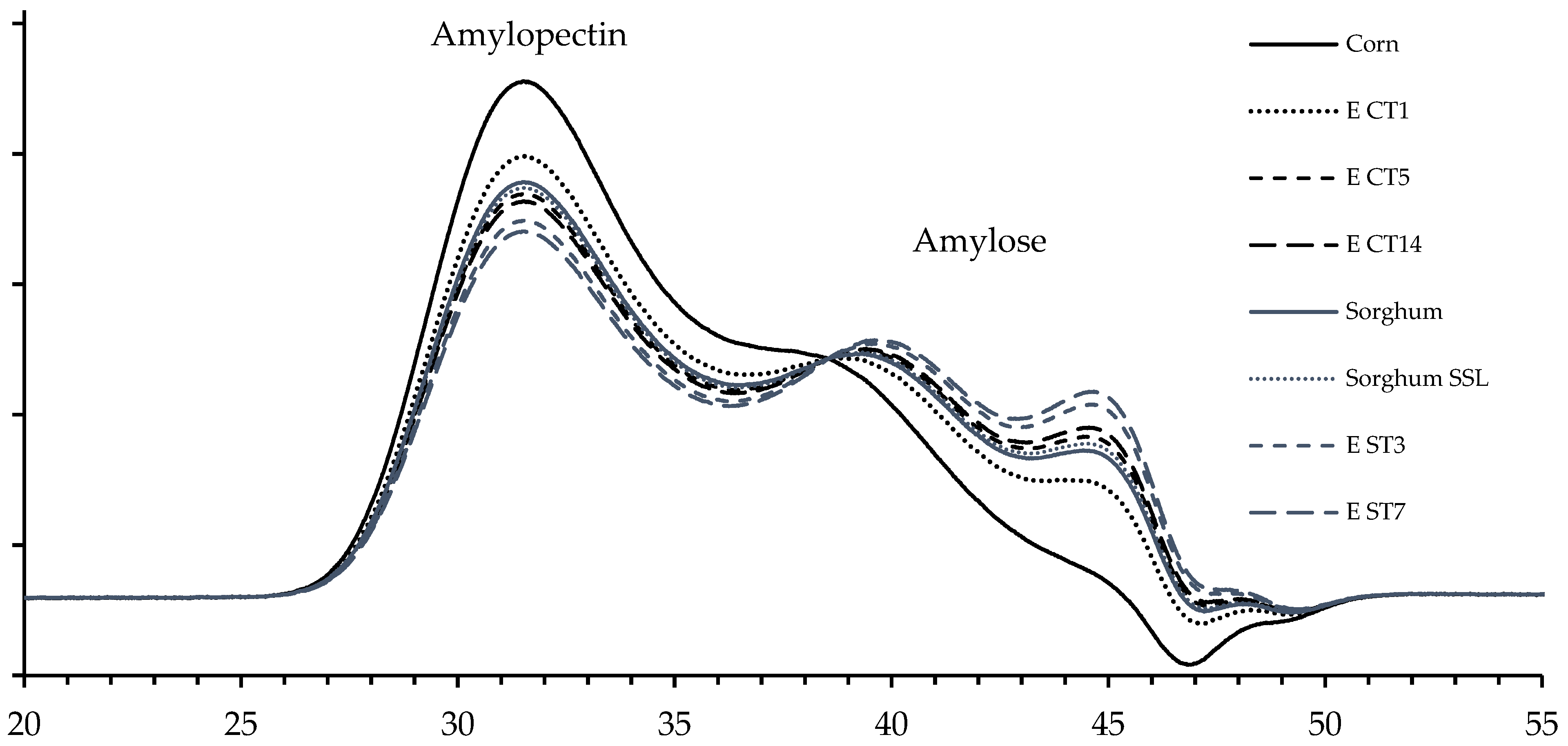
3.5. Molecular Characteristics and Chain Length Distribution
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Mahmood, K.; Kamilah, H.; Sang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A review: Interactions of starch/non-starch hydrocolloid blending and the recent food applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Ji, H.; Yang, P.; Zhan, L.; Wang, X.; Li, X.; Ma, H.; Chen, F. Effects of inulin with short and long-chain on pasting, texture and rheological properties of sweet potato starch. CyTA J. Food 2020, 19, 21–32. [Google Scholar] [CrossRef]
- Zou, J.; Xu, M.; Tang, W.; Wen, L.; Yang, B. Modification of structural, physicochemical, and digestive properties of normal maize starch by thermal treatment. Food Chem. 2020, 309, 125733. [Google Scholar] [CrossRef]
- Panyoo, A.E.; Emmambux, M.N. Effects of screw configuration, screw speed, and stearic acid addition on the functional properties and structural characteristics of maize starch extrudates. Starch 2019, 71, 1800149. [Google Scholar] [CrossRef]
- Ai, J.; Witt, T.; Cowin, G.; Dhital, S.; Turner, M.S.; Stokes, J.R.; Gidley, M.J. Anti-staling of high-moisture starchy foods: Effect of hydrocolloids, emulsifiers and enzyme on mechanics of steamed-rice cakes. Food Hydrocoll. 2018, 83, 454–464. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Luo, S.; Liu, W.; Chen, J.; Zeng, Z.; Liu, C. Properties of starch after extrusion: A Review. Starch 2018, 70, 1700110. [Google Scholar] [CrossRef]
- Wang, J.P.; An, H.Z.; Jin, Z.Y.; Xie, Z.J.; Zhuang, H.N.; Kim, J.M. Emulsifiers and thickeners on extrusion-cooked instant rice products. J. Food Sci. Technol. 2013, 50, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ceballos, E.; Nava-Valdez, Y.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Effect of the use of thermoplastic extruded corn or sorghum starches on the brewing performance of lager beers. J. Am. Soc. Brew. Chem. 2015, 73, 318–322. [Google Scholar] [CrossRef]
- Heredia-Olea, E.; Cortés-Ceballos, E.; Serna-Saldívar, S.O. Malting Sorghum with Aspergillus Oryzae Enhances Gluten-Free Wort Yield and Extract. J. Am. Soc. Brew. Chem. 2017, 75, 116–121. [Google Scholar] [CrossRef]
- de la Rosa-Millán, J. Physicochemical, Molecular, and Digestion Characteristics of Annealed and Heat–Moisture Treated Starches Under Acidic, Neutral, or Alkaline pH. Cereal Chem. 2017, 94, 770–779. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 4, 33–50. [Google Scholar]
- Goñi, I.; García-Alonso, A.; Saura-calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1977, 17, 427–437. [Google Scholar] [CrossRef]
- Tetchi, F.A.; Rolland-Sabaté, A.; Amani, G.N.; Colonna, P. Molecular and physicochemical characterisation of starches from yam, cocoyam, cassava, sweet potato and ginger produced in the Ivory Coast. J. Sci. Food Agric. 2007, 87, 1906–1916. [Google Scholar] [CrossRef]
- Pedroche, J.; Yust, M.M.; Lqari, H.; Girón-Calle, J.; Alaiz, M.; Vioque, J.; Millán, F. Brassica carinata protein isolates: Chemical composition, protein characterization and improvement of functional properties by protein hydrolysis. Food Chem. 2004, 88, 337–346. [Google Scholar] [CrossRef]
- Yoo, S.H.; Jane, J.L. Structural and physical properties of waxy and other wheat starches. Carbohydr. Polym. 2002, 49, 297–305. [Google Scholar] [CrossRef]
- Ao, Z.; Simsek, S.; Zhang, G.; Venkatachalam, M.; Reuhs, B.L.; Hamaker, B.R. Starch with a slow digestion property produced by altering its chain length, branch density, and crystalline structure. J. Agric. Food Chem. 2007, 55, 4540–4547. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Yang, Y.; Bian, S.; Zhou, X.; Zhu, K.; Xu, L.; Jin, Z.; Jiao, A. Effects of extrusion and enzymatic debranching on the structural characteristics and digestibility of corn and potato starches. Food Biosci. 2022, 47, 101679. [Google Scholar] [CrossRef]
- He, H.; Chi, C.; Xie, F.; Li, X.; Liang, Y.; Chen, L. Improving the in vitro digestibility of rice starch by thermomechanically assisted complexation with guar gum. Food Hydrocoll. 2020, 102, 105637. [Google Scholar] [CrossRef]
- Agustiniano-Osornio, J.C.; González-Soto, R.A.; Flores-Huicochea, E. Resistant starch production from mango starch using a single-screw extruder. J. Sci. Food Agric. 2005, 85, 2105–2110. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Kaplan, D.L.; Wang, Q. Protein-amylose/amylopectin molecular interactions during high-moisture extruded texturization toward plant-based meat substitutes applications. Food Hydrocoll. 2022, 127, 107559. [Google Scholar] [CrossRef]
- Dudu, O.E.; Ma, Y.; Olurin, T.O.; Oyedeji, A.B.; Oyeyinka, S.A.; Ogungbemi, J.W. Synergistic effect of hydrothermal and additive treatments on structural and functional characteristics of cassava starch. J. Food Process. Preserv. 2021, 45, e15904. [Google Scholar] [CrossRef]
- Oikonomou, N.A.; Krokida, M.K. Literature data compilation of WAI and WSI of extrudate food products. Int. J. Food Prop. 2011, 14, 199–240. [Google Scholar] [CrossRef]
- Šárka, E.; Dvořáček, V. Waxy starch as a perspective raw material (a review). Food Hydrocoll. 2017, 69, 402–409. [Google Scholar] [CrossRef]
- Tan, X.; Tan, X.; Li, E.; Bai, Y.; Nguyen, T.T.L.; Gilbert, R.G. Starch molecular fine structure is associated with protein composition in chickpea seed. Carbohydr. Polym. 2021, 272, 118489. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gilbert, R.G.; Gidley, M.J. Molecular-structure evolution during in vitro fermentation of granular high-amylose wheat starch is different to in vitro digestion. Food Chem. 2021, 362, 130188. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Atluri, S.; Zhang, Z.; Gidley, M.J.; Li, E.; Gilbert, R.G. Structural reasons for inhibitory effects of pectin on α-amylase enzyme activity and in-vitro digestibility of starch. Food Hydrocoll. 2021, 114, 106581. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Yang, T.; Ma, Y.; McClements, D.J.; Ren, F.; Tian, Y.; Jin, Z. A review of structural transformations and properties changes in starch during thermal processing of foods. Food Hydrocoll. 2021, 113, 106543. [Google Scholar] [CrossRef]

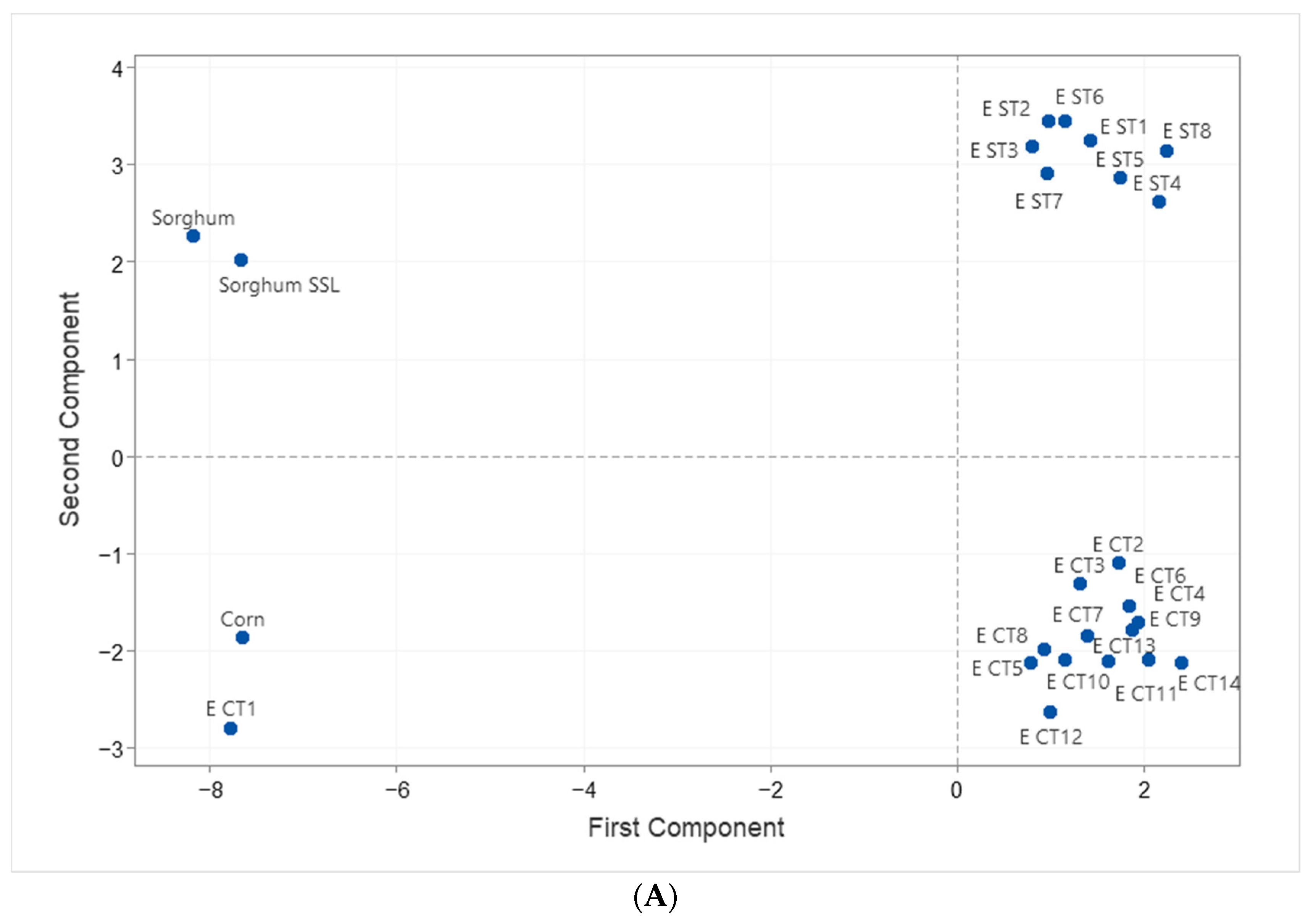
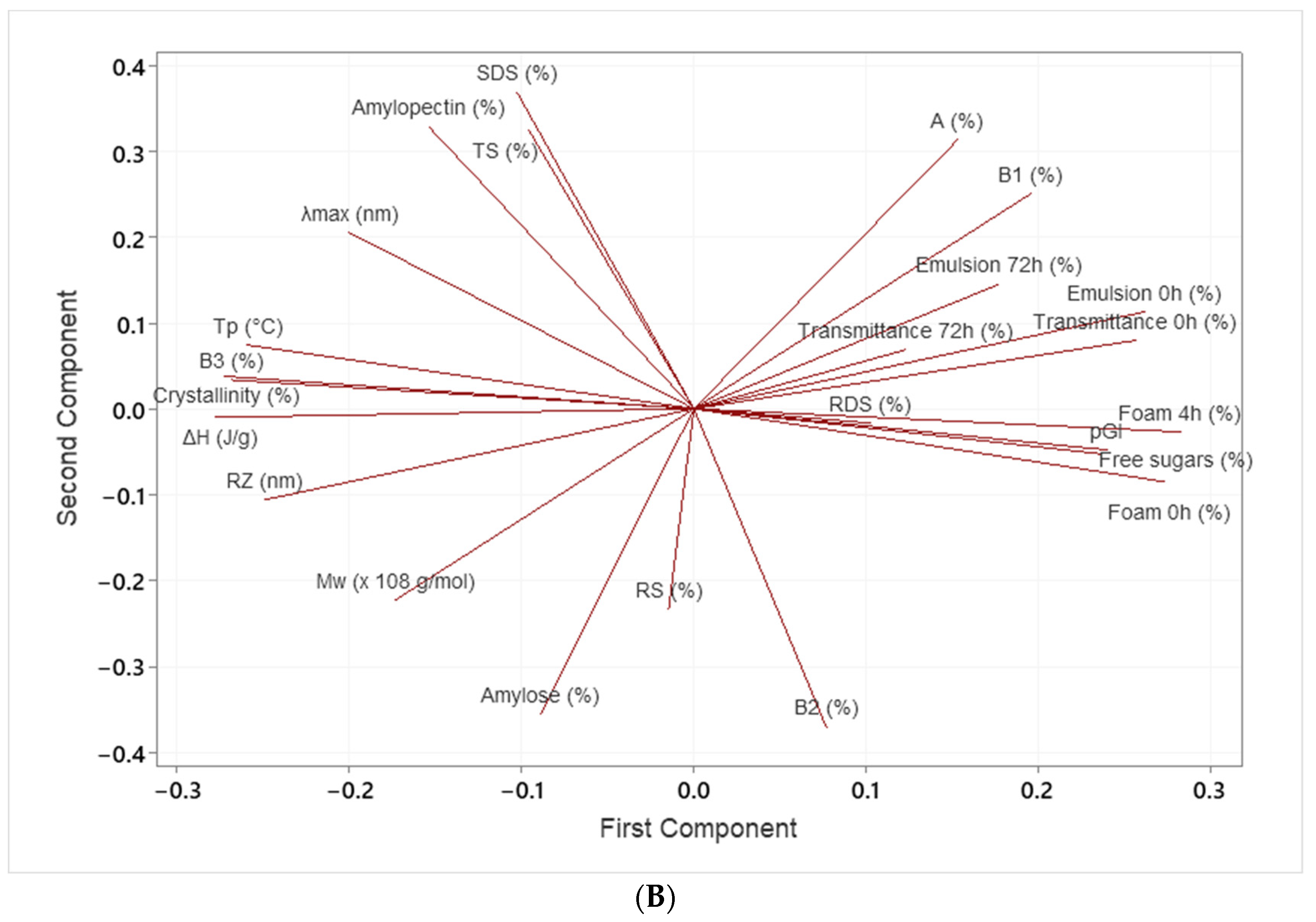
| Treatment | RPM | Moisture (%) | Temp (°C) | SSL (%) |
|---|---|---|---|---|
| E CT1 | 140 | 30 | 60 | 0 |
| E CT 2 | 100 | 30 | 80 | 0 |
| E CT 3 | 180 | 30 | 80 | 0 |
| E CT 4 | 140 | 20 | 80 | 0 |
| E CT 5 | 140 | 40 | 80 | 0 |
| E CT 6 | 140 | 30 | 100 | 0 |
| E CT 7 | 140 | 20 | 100 | 0.5 |
| E CT 8 | 140 | 40 | 100 | 0.5 |
| E CT 9 | 100 | 30 | 100 | 0.5 |
| E CT 10 | 180 | 30 | 100 | 0.5 |
| E CT 11 | 100 | 20 | 80 | 0.5 |
| E CT 12 | 180 | 20 | 80 | 0.5 |
| E CT 13 | 100 | 40 | 80 | 0.5 |
| Treatment | SSL (%) | Temp (°C) | Moisture (%) |
|---|---|---|---|
| E ST1 | 0.5 | 80 | 40 |
| E ST2 | 0.5 | 80 | 20 |
| E ST3 | 0.5 | 100 | 20 |
| E ST4 | 0.5 | 100 | 40 |
| E ST5 | 1 | 80 | 40 |
| E ST6 | 1 | 80 | 20 |
| E ST7 | 1 | 100 | 20 |
| E ST8 | 1 | 100 | 40 |
| Treatment | Total Starch (%) | Amylose (%) | Amylopectin (%) |
|---|---|---|---|
| Maize | 91.23 ± 0.41 b | 27.35 ± 0.15 a | 72.65 ± 0.42 g |
| E CT1 | 88.54 ± 0.30 c | 25.65 ± 0.50 b | 74.35 ± 0.29 f |
| E CT2 | 89.42 ± 0.52 c | 23.24 ± 0.12 c | 76.76 ± 0.17 d |
| E CT3 | 90.07 ± 0.73 b | 24.09 ± 0.77 b | 75.91 ± 0.30 e |
| E CT4 | 88.99 ± 0.16 c | 22.21 ± 0.39 d | 77.79 ± 0.81 c |
| E CT5 | 89.61 ± 0.40 c | 23.33 ± 0.90 c | 76.67 ± 0.53 d |
| E CT6 | 90.22 ± 0.24 b | 23.74 ± 0.03 c | 76.26 ± 0.03 d |
| E CT7 | 89.88 ± 0.03 c | 23.30 ± 0.60 c | 76.70 ± 0.58 d |
| E CT8 | 90.05 ± 0.52 b | 23.57 ± 0.69 c | 76.44 ± 0.18 d |
| E CT9 | 90.13 ± 0.09 b | 23.45 ± 0.12 c | 76.55 ± 0.05 d |
| E CT10 | 90.00 ± 0.06 b | 23.82 ± 0.49 c | 76.19 ± 0.63 d |
| E CT11 | 88.42 ± 0.85 c | 21.43 ± 0.99 d | 78.57 ± 0.53 c |
| E CT12 | 88.59 ± 0.50 c | 22.70 ± 0.09 d | 77.30 ± 0.78 c |
| E CT13 | 89.09 ± 0.17 c | 22.41 ± 0.86 d | 77.59 ± 0.54 c |
| E CT14 | 88.81 ± 0.65 c | 22.73 ± 0.36 d | 77.27 ± 0.84 c |
| Sorghum | 93.23 ± 0.22 a | 21.23 ± 0.22 d | 78.77 ± 0.10 b |
| Sorghum SSL | 91.93 ± 0.62 b | 19.92 ± 0.05 e | 80.08 ± 0.20 b |
| E ST1 | 91.19 ± 0.40 b | 17.98 ± 0.56 f | 82.02 ± 0.67 a |
| E ST2 | 92.00 ± 0.58 b | 19.00 ± 0.51 e | 81.00 ± 0.94 a |
| E ST3 | 91.81 ± 0.75 b | 18.60 ± 0.11 e | 81.40 ± 0.20 a |
| E ST4 | 90.50 ± 0.31 b | 17.09 ± 0.02 f | 82.91 ± 0.79 a |
| E ST5 | 91.82 ± 0.38 b | 18.61 ± 0.99 e | 81.39 ± 0.79 b |
| E ST6 | 92.13 ± 0.16 b | 19.12 ± 0.40 e | 80.88 ± 0.03 b |
| E ST7 | 90.93 ± 0.54 b | 17.72 ± 0.83 f | 82.28 ± 0.48 a |
| E ST8 | 90.54 ± 0.69 b | 17.13 ± 0.43 f | 82.87 ± 0.50 a |
| Treatment | To (°C) | Tp (°C) | Tc (°C) | Tf-To (°C) | ΔH (J/g) | %Gel | Crystallinity (%) |
|---|---|---|---|---|---|---|---|
| Maize | 66.43 ± 1.81 a | 70.31 ± 1.81 b | 73.26 ± 1.81 b | 6.83 ± 1.81 c | 12.43 ± 1.81 b | ND | 27.41 ± 1.81 a |
| E CT1 | 64.93 ± 0.51 b | 68.81 ± 0.51 c | 69.13 ± 0.51 c | 4.20 ± 0.51 d | 10.93 ± 0.51 c | 12.07 ± 0.51 d | 25.36 ± 0.51 b |
| E CT2 | 55.32 ± 0.94 d | 57.41 ± 0.94 e | 59.36 ± 0.94 e | 4.04 ± 0.94 d | 5.36 ± 0.94 d | 56.88 ± 0.94 c | 11.26 ± 0.94 d |
| E CT3 | 54.62 ± 2.46 d | 56.71 ± 2.46 e | 60.24 ± 2.46 e | 5.62 ± 2.46 c | 4.85 ± 2.46 d | 60.97 ± 2.46 b | 10.51 ± 2.46 d |
| E CT4 | 53.65 ± 2.42 d | 55.74 ± 2.42 e | 59.87 ± 2.42 e | 6.22 ± 2.42 c | 4.66 ± 2.42 d | 62.52 ± 2.42 b | 11.42 ± 2.42 d |
| E CT5 | 52.85 ± 2.23 d | 54.94 ± 2.23 e | 58.69 ± 2.23 e | 5.84 ± 2.23 c | 4.78 ± 2.23 d | 61.55 ± 2.23 b | 12.29 ± 2.23 d |
| E CT6 | 51.99 ± 2.28 d | 54.08 ± 2.28 e | 57.98 ± 2.28 e | 5.99 ± 2.28 c | 4.73 ± 2.28 d | 61.94 ± 2.28 b | 12.19 ± 2.28 d |
| E CT7 | 51.09 ± 1.53 d | 53.18 ± 1.53 f | 57.15 ± 1.53 e | 6.07 ± 1.53 c | 4.71 ± 1.53 d | 62.13 ± 1.53 b | 11.84 ± 1.53 d |
| E CT8 | 50.22 ± 0.29 e | 52.31 ± 0.29 f | 56.21 ± 0.29 f | 5.99 ± 0.29 c | 4.73 ± 0.29 d | 61.95 ± 0.29 b | 12.33 ± 0.29 d |
| E CT9 | 49.28 ± 1.76 e | 51.37 ± 1.76 f | 55.43 ± 1.76 f | 6.14 ± 1.76 c | 4.68 ± 1.76 d | 62.34 ± 1.76 b | 8.81 ± 1.76 e |
| E CT10 | 48.52 ± 1.94 e | 50.61 ± 1.94 f | 54.28 ± 1.94 f | 5.77 ± 1.94 c | 4.80 ± 1.94 d | 61.36 ± 1.94 b | 12.89 ± 1.94 d |
| E CT11 | 47.48 ± 0.32 e | 49.57 ± 0.32 g | 53.85 ± 0.32 f | 6.37 ± 0.32 c | 4.61 ± 0.32 d | 62.92 ± 0.32 b | 12.04 ± 0.32 d |
| E CT12 | 46.81 ± 2.19 e | 48.90 ± 2.19 g | 52.36 ± 2.19 f | 5.54 ± 2.19 c | 4.87 ± 2.19 d | 60.78 ± 2.19 b | 13.11 ± 2.19 d |
| E CT13 | 45.88 ± 0.85 e | 47.97 ± 0.85 g | 52.02 ± 0.85 f | 6.14 ± 0.85 c | 4.68 ± 0.85 d | 62.34 ± 0.85 b | 8.81 ± 0.85 e |
| E CT14 | 45.14 ± 0.31 e | 47.23 ± 0.31 g | 50.84 ± 0.31 g | 5.69 ± 0.31 c | 4.83 ± 0.31 d | 61.17 ± 0.31 b | 7.63 ± 0.31 e |
| Sorghum | 69.43 ± 1.87 a | 74.32 ± 1.87 a | 76.26 ± 1.87 a | 6.83 ± 1.87 c | 14.21 ± 1.87 a | ND | 29.34 ± 1.87 a |
| Sorghum SSL | 67.93 ± 1.46 a | 72.82 ± 1.46 a | 73.13 ± 1.46 b | 5.20 ± 1.46 c | 12.71 ± 1.46 b | 10.56 ± 1.46 d | 27.15 ± 1.46 a |
| E ST1 | 57.25 ± 0.20 c | 62.14 ± 0.20 d | 64.15 ± 0.20 d | 6.90 ± 0.20 c | 5.36 ± 0.20 d | 62.28 ± 0.20 b | 13.17 ± 0.20 d |
| E ST2 | 49.72 ± 1.01 e | 54.61 ± 1.01 e | 57.21 ± 1.01 e | 7.49 ± 1.01 b | 4.39 ± 1.01 d | 69.08 ± 1.01 a | 17.41 ± 1.01 c |
| E ST3 | 50.52 ± 1.87 d | 55.41 ± 1.87 e | 60.15 ± 1.87 e | 9.63 ± 1.87 a | 4.20 ± 1.87 d | 70.43 ± 1.87 a | 13.17 ± 1.87 d |
| E ST4 | 48.85 ± 0.98 e | 53.74 ± 1.98 e | 58.43 ± 1.98 e | 9.58 ± 1.98 a | 4.01 ± 1.98 d | 71.79 ± 1.98 a | 7.31 ± 1.98 e |
| E ST5 | 47.24 ± 0.43 e | 52.13 ± 0.43 e | 54.13 ± 0.43 f | 6.89 ± 0.43 c | 4.20 ± 0.43 d | 70.47 ± 0.43 a | 13.03 ± 0.43 d |
| E ST6 | 48.08 ± 1.51 e | 52.97 ± 1.51 e | 55.26 ± 1.51 f | 7.18 ± 1.51 b | 4.39 ± 1.51 d | 69.11 ± 1.51 a | 14.16 ± 1.51 d |
| E ST7 | 48.87 ± 1.46 e | 53.76 ± 1.46 e | 55.12 ± 1.46 f | 6.25 ± 1.46 c | 4.20 ± 1.46 d | 70.47 ± 1.46 a | 13.03 ± 1.46 d |
| E ST8 | 47.20 ± 0.50 e | 52.09 ± 0.50 e | 54.33 ± 0.50 f | 7.13 ± 0.50 b | 4.00 ± 0.50 d | 71.82 ± 0.50 a | 7.16 ± 0.50 e |
| Treatment | RDS (%) | SDS (%) | RS (%) | pGI |
|---|---|---|---|---|
| Maize | 73.43 ± 1.83 b | 22.27 ± 0.18 b | 4.30 ± 0.69 f | 92.18 ± 1.25 a |
| E CT1 | 64.91 ± 2.87 c | 24.43 ± 3.57 b | 10.66 ± 0.46 e | 90.41 ± 3.61 a |
| E CT2 | 72.24 ± 2.65 b | 20.52 ± 3.88 b | 7.24 ± 4.15 e | 93.18 ± 3.10 a |
| E CT3 | 68.90 ± 1.40 c | 20.19 ± 4.22 b | 10.91 ± 4.23 e | 92.80 ± 0.57 a |
| E CT4 | 75.26 ± 2.83 b | 19.78 ± 2.66 b | 4.96 ± 4.26 f | 93.66 ± 2.43 a |
| E CT5 | 66.91 ± 3.44 c | 18.94 ± 2.79 b | 14.15 ± 0.46 d | 92.71 ± 2.43 a |
| E CT6 | 70.25 ± 1.60 b | 19.28 ± 1.69 b | 10.48 ± 3.30 e | 93.09 ± 3.70 a |
| E CT7 | 71.92 ± 3.56 b | 19.44 ± 3.40 b | 8.64 ± 3.00 e | 93.28 ± 2.30 a |
| E CT8 | 70.33 ± 1.90 b | 19.28 ± 3.89 b | 10.38 ± 2.21 e | 93.10 ± 4.19 a |
| E CT9 | 73.67 ± 0.52 b | 19.62 ± 3.68 b | 6.71 ± 0.12 f | 93.48 ± 2.29 a |
| E CT10 | 65.32 ± 1.57 d | 18.78 ± 0.51 c | 15.90 ± 1.11 c | 92.53 ± 3.80 a |
| E CT11 | 78.68 ± 1.07 a | 20.12 ± 3.77 b | 1.20 ± 3.99 g | 94.05 ± 2.59 a |
| E CT12 | 60.31 ± 3.29 e | 18.28 ± 3.15 c | 21.41 ± 3.98 a | 91.95 ± 1.59 a |
| E CT13 | 73.67 ± 0.17 b | 19.62 ± 2.08 b | 6.71 ± 1.88 f | 93.48 ± 0.87 a |
| E CT14 | 63.65 ± 4.05 d | 18.61 ± 4.12 c | 17.73 ± 0.24 b | 92.34 ± 4.17 a |
| Sorghum | 62.71 ± 3.90 d | 27.88 ± 0.15 a | 9.41 ± 3.35 e | 90.37 ± 2.03 a |
| Sorghum SSL | 62.76 ± 0.13 d | 27.88 ± 2.03 a | 9.37 ± 1.74 e | 90.15 ± 3.29 a |
| E ST1 | 71.73 ± 4.00 b | 27.12 ± 0.64 a | 1.15 ± 0.89 g | 93.00 ± 2.10 a |
| E ST2 | 72.11 ± 1.68 b | 27.71 ± 0.60 a | 0.18 ± 2.10 g | 92.94 ± 4.13 a |
| E ST3 | 70.63 ± 3.27 b | 26.85 ± 1.95 a | 2.52 ± 0.80 g | 92.89 ± 1.53 a |
| E ST4 | 65.53 ± 3.49 d | 25.07 ± 2.10 a | 9.40 ± 1.34 e | 92.51 ± 0.85 a |
| E ST5 | 65.73 ± 3.85 d | 25.61 ± 3.82 a | 8.66 ± 1.36 e | 92.43 ± 4.02 a |
| E ST6 | 72.15 ± 1.70 b | 27.71 ± 1.31 a | 0.13 ± 0.01 h | 92.94 ± 2.39 a |
| E ST7 | 69.44 ± 1.20 c | 26.54 ± 1.53 a | 4.03 ± 1.66 f | 92.78 ± 3.26 a |
| E ST8 | 72.27 ± 3.96 b | 26.74 ± 1.71 a | 0.99 ± 0.12 g | 93.15 ± 0.27 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Rosa-Millan, J.; Heredia-Olea, E.; Pérez-Carrillo, E.; Peña-Gómez, R.; Serna-Saldívar, S.O. Physicochemical and In Vitro Starch Residual Digestion Structures of Extruded Maize and Sorghum Starches Added with Sodium Stearoyl Lactylate. Foods 2023, 12, 1988. https://doi.org/10.3390/foods12101988
de la Rosa-Millan J, Heredia-Olea E, Pérez-Carrillo E, Peña-Gómez R, Serna-Saldívar SO. Physicochemical and In Vitro Starch Residual Digestion Structures of Extruded Maize and Sorghum Starches Added with Sodium Stearoyl Lactylate. Foods. 2023; 12(10):1988. https://doi.org/10.3390/foods12101988
Chicago/Turabian Stylede la Rosa-Millan, Julian, Erick Heredia-Olea, Esther Pérez-Carrillo, Raquel Peña-Gómez, and Sergio O. Serna-Saldívar. 2023. "Physicochemical and In Vitro Starch Residual Digestion Structures of Extruded Maize and Sorghum Starches Added with Sodium Stearoyl Lactylate" Foods 12, no. 10: 1988. https://doi.org/10.3390/foods12101988






