Effect of Microbial Transglutaminase Treatment on the Techno-Functional Properties of Mung Bean Protein Isolate
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Preparation of Mung Bean Protein Isolate (MBPI)
2.3. Preparation of MTG-Treated MBPI
2.4. Effects of MTG Treatment on MBPI
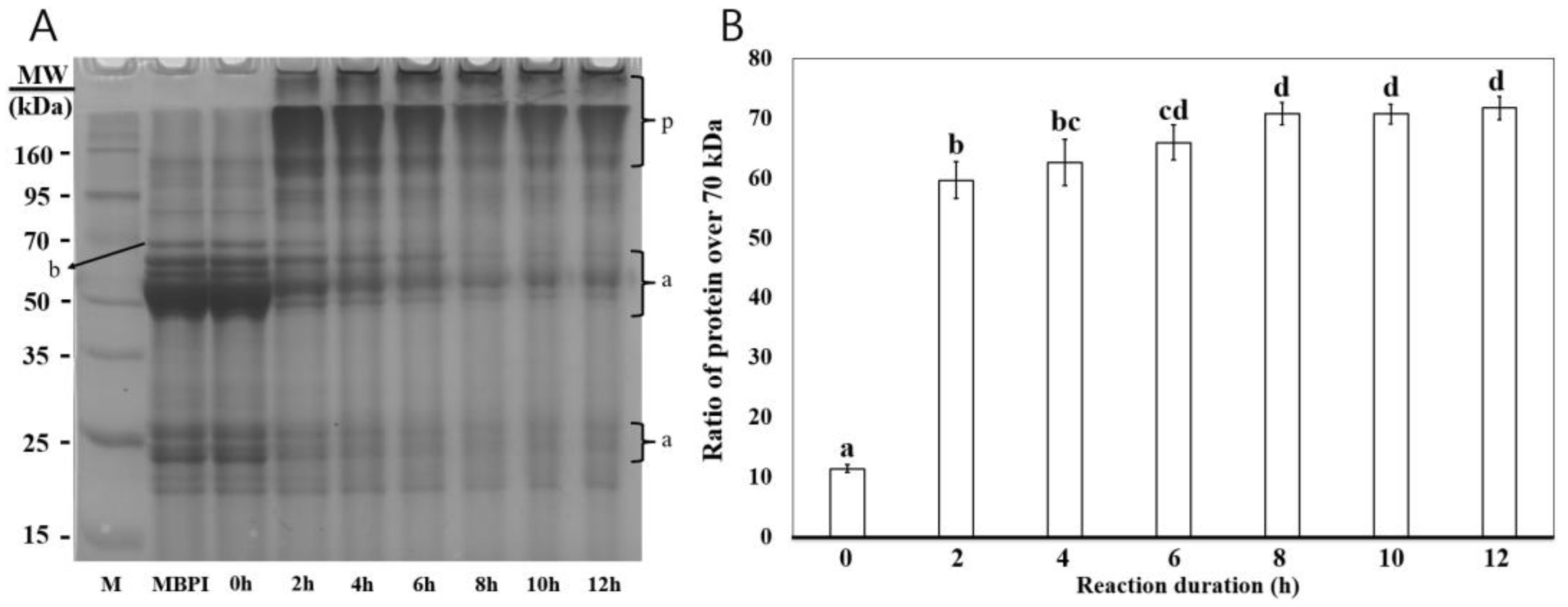
2.4.1. Electrophoresis
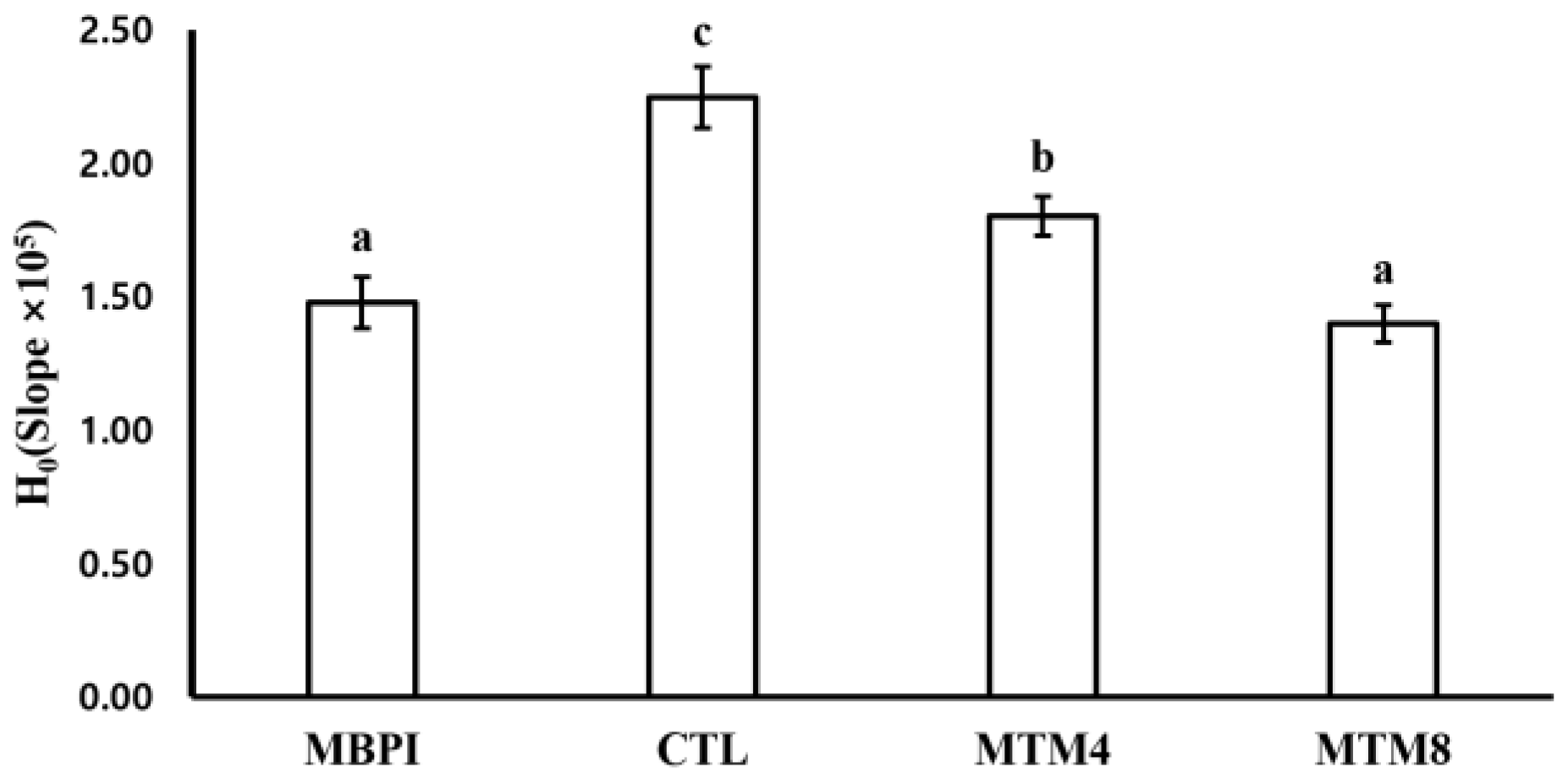
2.4.2. Surface Hydrophobicity (H0)
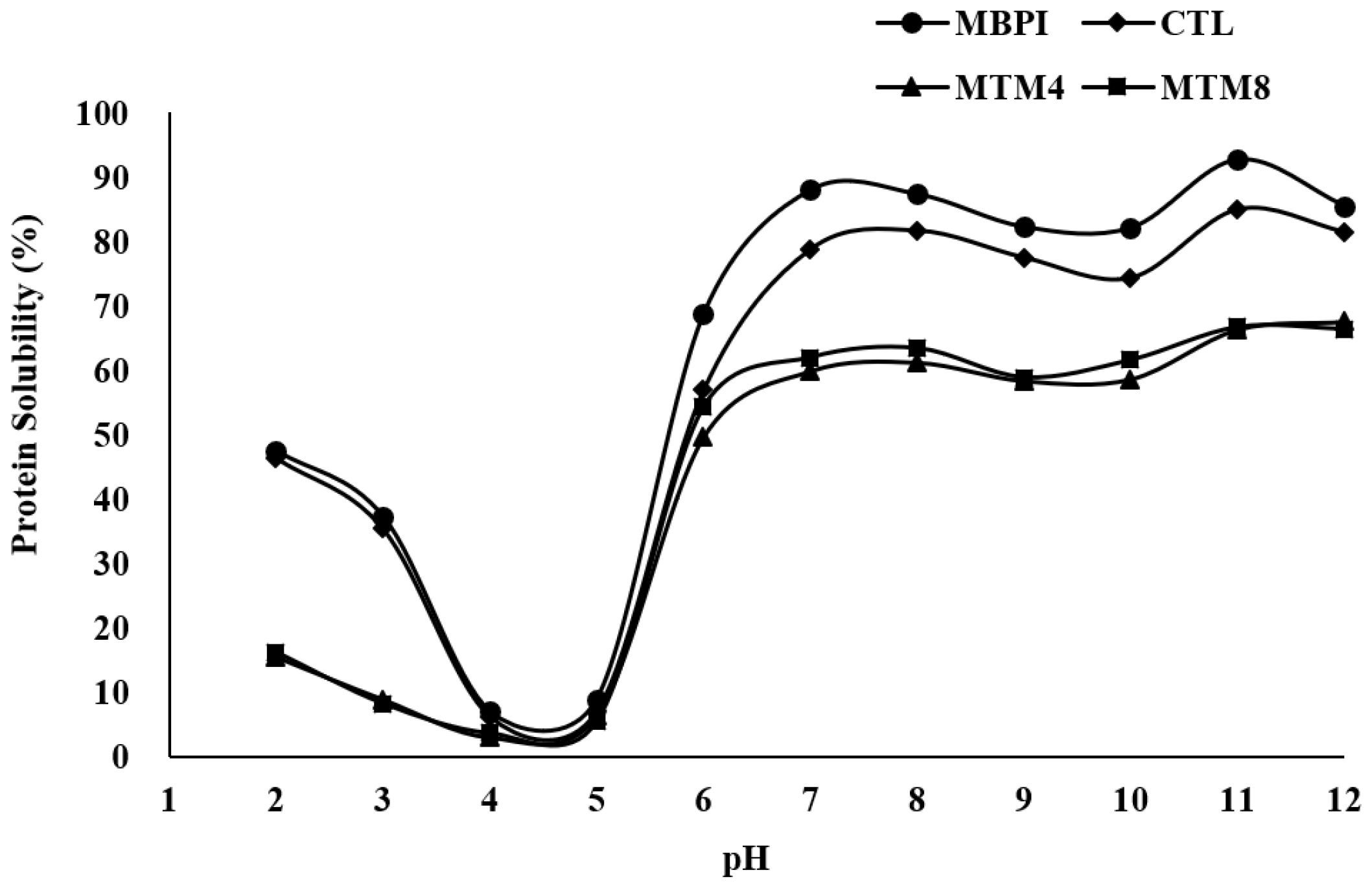
2.4.3. Protein Solubility
2.5. Techno-Functional Properties
2.5.1. Water- and Oil-Holding Capacity (WHC/OHC)
2.5.2. Emulsifying Capacity and Stability (EC/ES)
2.5.3. Foaming Capacity and Stability (FC/FS)
2.5.4. Least Gelling Concentration (LGC)
2.6. Characterization of Heat-Induced Protein Gel
2.6.1. Texture Profile Analysis (TPA)
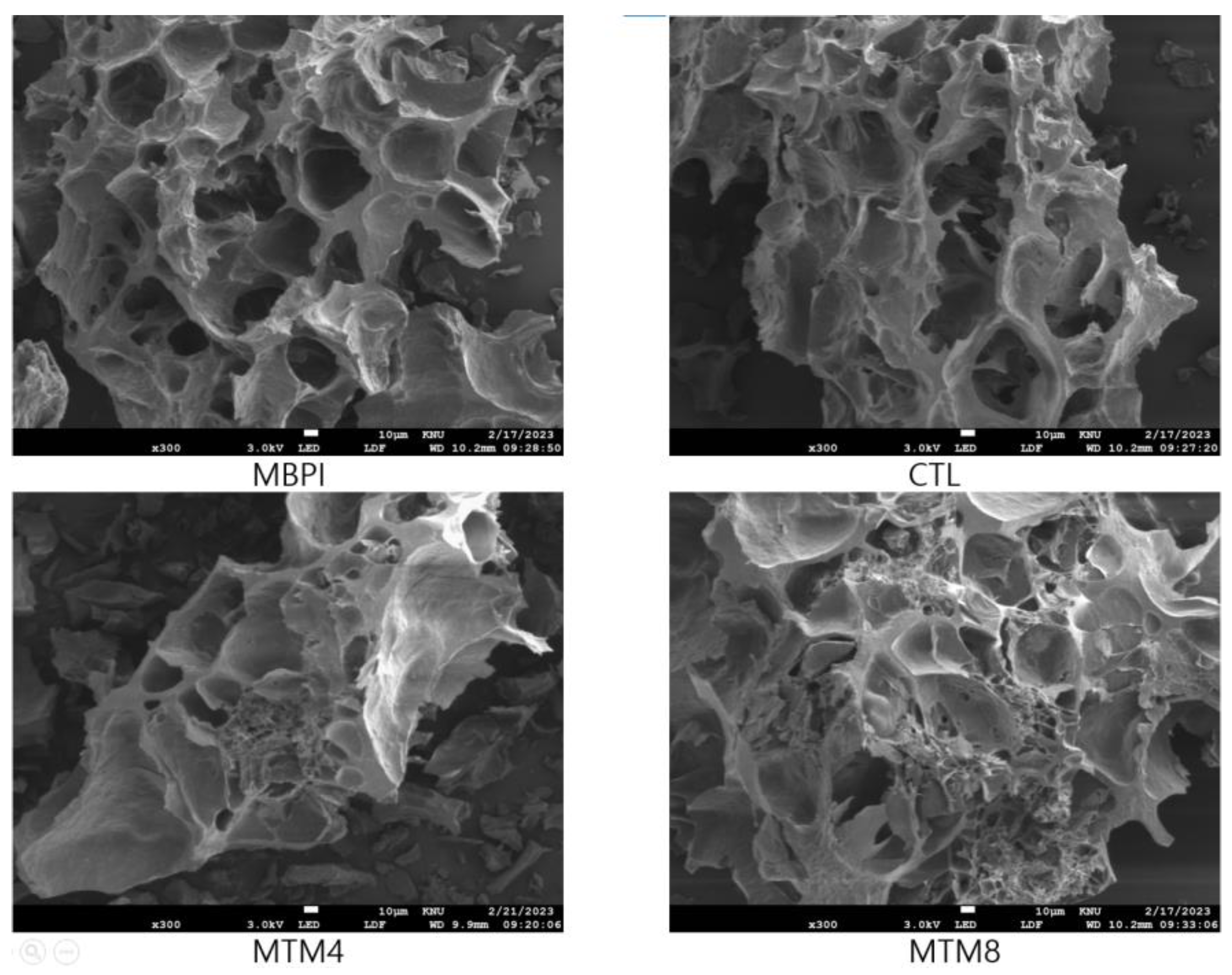
2.6.2. Field Emission Scanning Electron Microscopy (FE-SEM)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Electrophoresis
3.2. Surface Hydrophobicity (H0)
3.3. Protein Solubility
3.4. Techno-Functional Properties
3.4.1. Water- and Oil- Holding Capacity (WHC/OHC)
3.4.2. Emulsifying Capacity and Stability (EC/ES)
3.4.3. Foaming Capacity and Stability (FC/FS)
3.4.4. Least Gelling Concentration (LGC)
3.5. Characterization of Heat-Induced Protein Gels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joshi, V.; Kumar, S. Meat Analogues: Plant Based Alternatives to Meat Products—A Review. Int. J. Food Ferment. Technol. 2015, 5, 107. [Google Scholar] [CrossRef]
- Liu, K. Chemistry and Nutritional Value of Soybean Components. Soybeans 1997, 25–113. [Google Scholar] [CrossRef]
- Cabrera-Orozco, A.; Jimenez-Martinez, C.; Davila-Ortiz, G. Soybean: Non-Nutritional Factors and Their Biological Functionality. Soybean—Bio-Active Compd. 2013, 387–410. [Google Scholar] [CrossRef]
- Pi, X.; Wan, Y.; Yang, Y.; Li, R.; Wu, X.; Xie, M.; Li, X.; Fu, G. Research Progress in Peanut Allergens and Their Allergenicity Reduction. Trends Food Sci. Technol. 2019, 93, 212–220. [Google Scholar] [CrossRef]
- Xia, J.; Zu, Q.; Yang, A.; Wu, Z.; Li, X.; Tong, P.; Yuan, J.; Wu, Y.; Fan, Q.; Chen, H. Allergenicity Reduction and Rheology Property of Lactobacillus-Fermented Soymilk. J. Sci. Food Agric. 2019, 99, 6841–6849. [Google Scholar] [CrossRef]
- Shahrajabian, M.H. A Short Review of Health Benefits and Nutritional Values of Mung Bean in Sustainable Agriculture. Polish J. Agron. 2019, 37, 31–36. [Google Scholar] [CrossRef]
- Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung Bean (Vigna radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits. Nutrients 2019, 11, 1238. [Google Scholar] [CrossRef]
- Kudre, T.G.; Benjakul, S.; Kishimura, H. Comparative Study on Chemical Compositions and Properties of Protein Isolates from Mung Bean, Black Bean and Bambara Groundnut. J. Sci. Food Agric. 2013, 93, 2429–2436. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Mata, A.; Corke, H.; Gan, R.Y.; Fang, Y. Physicochemical and PH-Dependent Functional Properties of Proteins Isolated from Eight Traditional Chinese Beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Zhang, S.; Zhao, X.; Liu, Y.; Jiang, J.; Xiong, Y.L. Structural and Rheological Properties of Mung Bean Protein Emulsion as a Liquid Egg Substitute: The Effect of PH Shifting and Calcium. Food Hydrocoll. 2022, 126, 107485. [Google Scholar] [CrossRef]
- El-Adawy, T.A. Functional Properties and Nutritional Quality of Acetylated and Succinylated Mung Bean Protein Isolate. Food Chem. 2000, 70, 83–91. [Google Scholar] [CrossRef]
- Motoki, M.; Seguro, K. Transglutaminase and Its Use for Food Processing. Trends Food Sci. Technol. 1998, 9, 204–210. [Google Scholar] [CrossRef]
- Gulzar, S.; Tagrida, M.; Patil, U.; Ma, L.; Zhang, B.; Benjakul, S. Mung Bean Protein Isolate Treated with High-Intensity Pulsed Electric Field: Characteristics and Its Use for Encapsulation of Asian Seabass Oil. J. Microencapsul. 2023, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Sani, I.K.; Alizadeh, M. Isolated Mung Bean Protein-Pectin Nanocomposite Film Containing True Cardamom Extract Microencapsulation/CeO2 Nanoparticles/Graphite Carbon Quantum Dots: Investigating Fluorescence, Photocatalytic and Antimicrobial Properties. Food Packag. Shelf Life 2022, 33, 100912. [Google Scholar] [CrossRef]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Saari, N. Effects of Drying Techniques on the Physicochemical, Functional, Thermal, Structural and Rheological Properties of Mung Bean (Vigna radiata) Protein Isolate Powder. Food Res. Int. 2020, 138, 109783. [Google Scholar] [CrossRef]
- Liu, C.; Damodaran, S.; Heinonen, M. Effects of Microbial Transglutaminase Treatment on Physiochemical Properties and Emulsifying Functionality of Faba Bean Protein Isolate. LWT 2019, 99, 396–403. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.; Wang, Y.; Li, K.; Du, J.; Bai, Y. Effect of PH-Shifting Treatment on Structural and Heat Induced Gel Properties of Peanut Protein Isolate. Food Chem. 2020, 325, 126921. [Google Scholar] [CrossRef]
- Liu, F.F.; Li, Y.Q.; Wang, C.Y.; Zhao, X.Z.; Liang, Y.; He, J.X.; Mo, H.Z. Impact of PH on the Physicochemical and Rheological Properties of Mung Bean (Vigna radiata L.) Protein. Process Biochem. 2021, 111, 274–284. [Google Scholar] [CrossRef]
- Cortés-Ríos, J.; Zárate, A.M.; Figueroa, J.D.; Medina, J.; Fuentes-Lemus, E.; Rodríguez-Fernández, M.; Aliaga, M.; López-Alarcón, C. Protein Quantification by Bicinchoninic Acid (BCA) Assay Follows Complex Kinetics and Can Be Performed at Short Incubation Times. Anal. Biochem. 2020, 608, 113904. [Google Scholar] [CrossRef]
- Ahmedna, M.; Prinyawiwatkul, W.; Rao, R.M. Solubilized Wheat Protein Isolate: Functional Properties and Potential Food Applications. J. Agric. Food Chem. 1999, 47, 1340–1345. [Google Scholar] [CrossRef]
- Jeong, M.S.; Lee, S.D.; Cho, S.J. Effect of Three Defatting Solvents on the Techno-Functional Properties of an Edible Insect (Gryllus bimaculatus) Protein Concentrate. Molecules 2021, 26, 5307. [Google Scholar] [CrossRef] [PubMed]
- O’kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; van Boekel, M.A. Characterization of Pea Vicilin. 2. Consequences of Compositional Heterogeneity on Heat-Induced Gelation Behavior. ACS Publ. 2004, 52, 3149–3154. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Chen, J.; Zhang, J.; Sheng, L. Impact of Phosphates on Heat-Induced Egg White Gel Properties: Texture, Water State, Micro-Rheology and Microstructure. Food Hydrocoll. 2021, 110, 106200. [Google Scholar] [CrossRef]
- Shrestha, S.; van ’t Hag, L.; Haritos, V.; Dhital, S. Comparative Study on Molecular and Higher-Order Structures of Legume Seed Protein Isolates: Lentil, Mungbean and Yellow Pea. Food Chem. 2023, 411, 135464. [Google Scholar] [CrossRef] [PubMed]
- Rahma, E.H.; Dudek, S.; Mothes, R.; Görnitz, E.; Schwenke, K.D. Physicochemical Characterization of Mung Bean (Phaseolus aureus) Protein Isolates. J. Sci. Food Agric. 2000, 80, 477–483. [Google Scholar] [CrossRef]
- Basman, A.; Köksel, H.; Ng, P.K.W. Effects of Transglutaminase on SDS-PAGE Patterns of Wheat, Soy, and Barley Proteins and Their Blends. J. Food Sci. 2002, 67, 2654–2658. [Google Scholar] [CrossRef]
- Han, X.; Liang, Z.; Tian, S.; Liu, L.; Wang, S. Modification of Whey−soybean Mixed Protein by Sequential High-Pressure Homogenization and Transglutaminase Treatment. LWT 2022, 172, 114217. [Google Scholar] [CrossRef]
- Nivala, O.; Nordlund, E.; Kruus, K.; Ercili-Cura, D. The Effect of Heat and Transglutaminase Treatment on Emulsifying and Gelling Properties of Faba Bean Protein Isolate. LWT 2021, 139, 110517. [Google Scholar] [CrossRef]
- Agyare, K.K.; Addo, K.; Xiong, Y.L. Emulsifying and Foaming Properties of Transglutaminase-Treated Wheat Gluten Hydrolysate as Influenced by PH, Temperature and Salt. Food Hydrocoll. 2009, 23, 72–81. [Google Scholar] [CrossRef]
- Gaspar, A.L.C.; De Góes-Favoni, S.P. Action of Microbial Transglutaminase (MTGase) in the Modification of Food Proteins: A Review. Food Chem. 2015, 171, 315–322. [Google Scholar] [CrossRef]
- Shi, A.M.; Jiao, B.; Liu, H.Z.; Zhu, S.; Shen, M.J.; Feng, X.L.; Hu, H.; Liu, L.; Faisal, S.; Wang, Q.; et al. Effects of Proteolysis and Transglutaminase Crosslinking on Physicochemical Characteristics of Walnut Protein Isolate. LWT 2018, 97, 662–667. [Google Scholar] [CrossRef]
- Tang, C.H.; Sun, X.; Yin, S.W.; Ma, C.Y. Transglutaminase-Induced Cross-Linking of Vicilin-Rich Kidney Protein Isolate: Influence on the Functional Properties and in Vitro Digestibility. Food Res. Int. 2008, 41, 941–947. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, J.H.; Ng, P.K.W. Functional and Thermal Properties of Wheat, Barley, and Soy Flours and Their Blends Treated with a Microbial Transglutaminase. J. Food Sci. 2005, 70, c380–c386. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Xu, Z.; Shan, M.; Ge, X.; Zhang, Y.; Shao, S.; Huang, L.; Wang, W.; Lu, F. Effects of Bacillus Subtilis Transglutaminase Treatment on the Functional Properties of Whey Protein. LWT 2019, 116, 108559. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Gafsi, I.M.; Blecker, C.; Danthine, S.; Attia, H.; Besbes, S. Effect of Drying Methods on Physico-Chemical and Functional Properties of Chickpea Protein Concentrates. J. Food Eng. 2015, 165, 179–188. [Google Scholar] [CrossRef]
- Mokni Ghribi, A.; Maklouf Gafsi, I.; Sila, A.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Effects of Enzymatic Hydrolysis on Conformational and Functional Properties of Chickpea Protein Isolate. Food Chem. 2015, 187, 322–330. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, S.; Wu, C.; Qi, B.; Teng, F.; Wang, Z.; Li, Y.; Jiang, L. The Investigation of Protein Flexibility of Various Soybean Cultivars in Relation to Physicochemical and Conformational Properties. Food Hydrocoll. 2020, 103, 105709. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Zhang, S.; Li, Y. Effects of Flexibility and Surface Hydrophobicity on Emulsifying Properties: Ultrasound-Treated Soybean Protein Isolate. LWT 2021, 142, 110881. [Google Scholar] [CrossRef]
- Glusac, J.; Isaschar-Ovdat, S.; Fishman, A. Transglutaminase Modifies the Physical Stability and Digestibility of Chickpea Protein-Stabilized Oil-in-Water Emulsions. Food Chem. 2020, 315, 126301. [Google Scholar] [CrossRef]
- Babiker, E.E. Effect of Transglutaminase Treatment on the Functional Properties of Native and Chymotrypsin-Digested Soy Protein. Food Chem. 2000, 70, 139–145. [Google Scholar] [CrossRef]
- Xu, J.; Yang, L.; Nie, Y.; Yang, M.; Wu, W.; Wang, Z.; Wang, X.; Zhong, J. Effect of Transglutaminase Crosslinking on the Structural, Physicochemical, Functional, and Emulsion Stabilization Properties of Three Types of Gelatins. LWT 2022, 163, 113543. [Google Scholar] [CrossRef]
- Pinterits, A.; Arntfield, S.D. Improvement of Canola Protein Gelation Properties through Enzymatic Modification with Transglutaminase. LWT 2008, 41, 128–138. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation Properties of Salt-Extracted Pea Protein Isolate Catalyzed by Microbial Transglutaminase Cross-Linking. Food Hydrocoll. 2011, 25, 25–31. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Acevedo, N.C. Effects of Pre-Heating Soybean Protein Isolate and Transglutaminase Treatments on the Properties of Egg-Soybean Protein Isolate Composite Gels. Food Chem. 2020, 318, 126421. [Google Scholar] [CrossRef] [PubMed]
| Fraction | Sample | |||
|---|---|---|---|---|
| MBPI | CTL | MTM4 | MTM8 | |
| WHC (g/g) | 1.90 ± 0.03 a | 2.00 ± 0.05 a | 3.74 ± 0.12 b | 4.08 ± 0.08 b |
| OHC (g/g) | 2.16 ± 0.07 ab | 2.28 ± 0.14 b | 2.03 ± 0.12 a | 2.10 ± 0.04 ab |
| EC (%, v/v) | 54.2 ± 3.6 a | 56.3 ± 0.0 a | 66.7 ± 3.6 b | 68.8 ± 0.0 b |
| ES (%, v/v) | 94.6 ± 1.1 a | 94.5 ± 5.1 a | 97.0 ± 5.3 a | 97.3 ± 2.4 a |
| FC (%, v/v) | 40.0 ± 0.0 a | 36.7 ± 2.9 a | 36.7 ± 2.9 a | 38.3 ± 2.9 a |
| FS (%, v/v) | 54.2 ± 7.2 c | 27.4 ± 2.1 a | 45.2 ± 4.1 b | 39.3 ± 3.1 b |
| LGC (%, w/w) | 22.3 ± 0.6 b | 22.3 ± 0.6 b | 12.7 ± 0.6 a | 12.3 ± 0.6 a |
| Parameter | Sample Gel | |||
|---|---|---|---|---|
| MBPI | CTL | MTM4 | MTM8 | |
| Hardness (g) | 1310.9 ± 50.3 a | 1339.9 ± 68.5 a | 1754.6 ± 71.8 b | 1907.5 ± 20.2 c |
| Adhesiveness (g·s) | −32.9 ± 3.1 a | −29.8 ± 1.9 a | −16.4 ± 1.4 b | −11.2 ± 1.6 c |
| Springiness | 1.0 ± 0.0 a | 1.0 ± 0.0 a | 1.0 ± 0.0 a | 1.0 ± 0.0 a |
| Cohesiveness | 1.16 ± 0.07 a | 1.31 ± 0.06 a | 1.08 ± 0.03 a | 1.08 ± 0.03 a |
| Gumminess (g) | 1524.54 ± 133.0 a | 1513.6 ± 40.1 a | 1897.4 ± 85.6 b | 2066.1 ± 41.8 c |
| Chewiness (g) | 1518.44 ± 132.5 a | 1507.5 ± 40.0 a | 1897.1 ± 84.0 b | 2059.2 ± 42.6 c |
| Resilience | 0.14 ± 0.00 ab | 0.15 ± 0.01 bc | 0.13 ± 0.01 a | 0.15 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.-H.; Cho, S.-J. Effect of Microbial Transglutaminase Treatment on the Techno-Functional Properties of Mung Bean Protein Isolate. Foods 2023, 12, 1998. https://doi.org/10.3390/foods12101998
Moon S-H, Cho S-J. Effect of Microbial Transglutaminase Treatment on the Techno-Functional Properties of Mung Bean Protein Isolate. Foods. 2023; 12(10):1998. https://doi.org/10.3390/foods12101998
Chicago/Turabian StyleMoon, Su-Hyeon, and Seong-Jun Cho. 2023. "Effect of Microbial Transglutaminase Treatment on the Techno-Functional Properties of Mung Bean Protein Isolate" Foods 12, no. 10: 1998. https://doi.org/10.3390/foods12101998
APA StyleMoon, S.-H., & Cho, S.-J. (2023). Effect of Microbial Transglutaminase Treatment on the Techno-Functional Properties of Mung Bean Protein Isolate. Foods, 12(10), 1998. https://doi.org/10.3390/foods12101998






