Effect of Insoluble Dietary Fiber Extracted from Feijoa (Acca sellowiana (O. Berg) Burret.) Supplementation on Physicochemical and Functional Properties of Wheat Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of FJI
2.3. FJI Structure Analysis
2.4. Hydration Properties of FJI
2.5. Determination of Mineral Content of FJI
2.6. Bread-Making Process
2.7. Determination of Proximate Composition
2.8. Determination of the Specific Volume
2.9. Color Analysis
2.10. Texture Analysis
2.11. Determination of TPC, TFC, and Antioxidant Activity
2.12. Determination of Adsorption Capacities
2.12.1. Bile Acid-Adsorption Capacity
2.12.2. Nitrite-Adsorption Capacity
2.12.3. Cholesterol-Adsorption Capacity
2.13. In Vitro Starch Digestion
2.14. Sensory Evaluation
2.15. Statistical Analysis
3. Results and Discussion
3.1. Mineral Composition and Physicochemical Properties of FJI
3.2. Structure Analysis of FJI
3.3. Proximate Composition of Bread
3.4. Color Attributes of Bread
3.5. Specific Volume of Bread
3.6. Texture Properties of Bread
3.7. TPC, TFC, and Antioxidant Activities of Bread
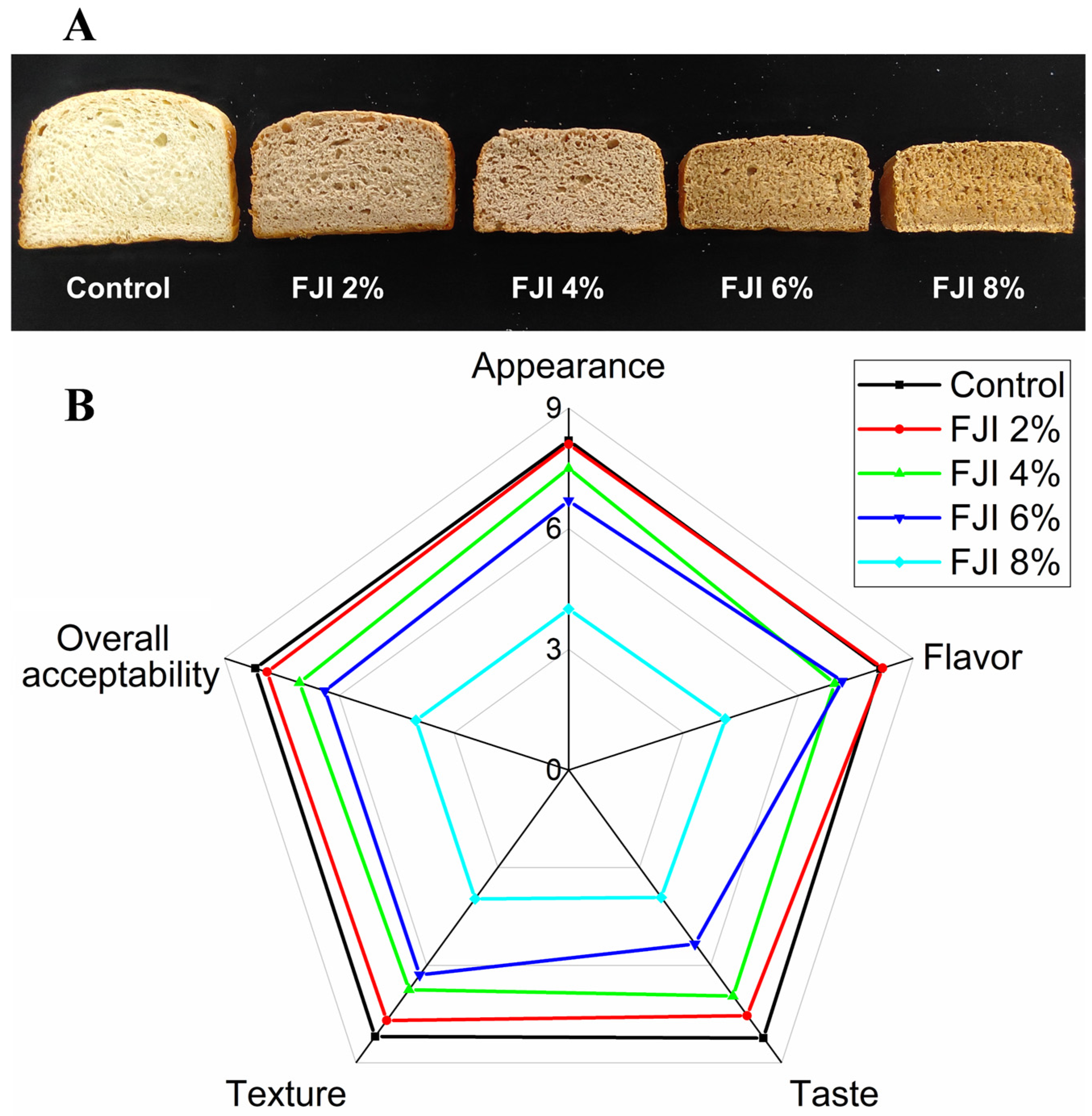
3.8. Sensory Properties of Bread
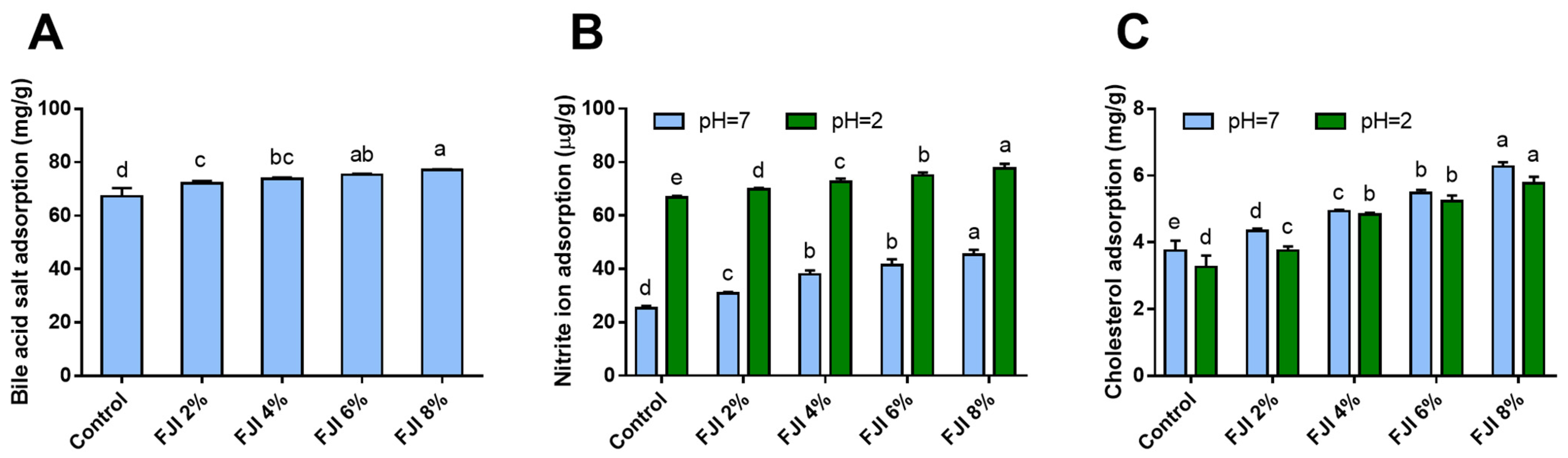
3.9. In Vitro Adsorption Capacities of Bread
3.9.1. Bile Acid Salt Absorption
3.9.2. NO2− Absorption Capacity
3.9.3. Cholesterol Absorption Capacity
3.10. Glucose Release Profile of Bread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Junejo, S.A.; Rashid, A.; Yang, L.; Xu, Y.; Kraithong, S.; Zhou, Y. Effects of spinach powder on the physicochemical and antioxidant properties of durum wheat bread. LWT Food Sci. Technol. 2021, 150, 112058. [Google Scholar] [CrossRef]
- Tebben, L.; Shen, Y.; Li, Y. Improvers and functional ingredients in whole wheat bread: A review of their effects on dough properties and bread quality. Trends Food Sci. Technol. 2018, 81, 10–24. [Google Scholar] [CrossRef]
- Reshmi, S.K.; Sudha, M.L.; Shashirekha, M.N. Starch digestibility and predicted glycemic index in the bread fortified with pomelo (Citrus maxima) fruit segments. Food Chem. 2017, 237, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Feng, X.; Wu, Z.; Li, S.; Bai, X.; Zhao, C.; Ameer, K. Development of wheat bread added with insoluble dietary fiber from ginseng residue and effects on physiochemical properties, in vitro adsorption capacities and starch digestibility. LWT Food Sci. Technol. 2021, 149, 111855. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N. Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Zou, F.; Chen, S.L.; Yuan, D.Y.; Zhang, R.Q.; Zhang, L.; Xiong, H. Microsporogenesis, megasporogensis and male and female gametophyte development in Feijoa sellowiana (Myrtaceae). Int. J. Agric. Biol. 2016, 18, 637–642. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wang, W.; Waterhouse, G.I.N.; Wadhwa, S.S. Utilisation potential of feijoa fruit wastes as ingredients for functional foods. Food Bioprocess Technol. 2012, 6, 3441–3455. [Google Scholar] [CrossRef]
- Zhu, F. Chemical and biological properties of feijoa (Acca sellowiana). Trends Food Sci. Technol. 2018, 81, 121–131. [Google Scholar] [CrossRef]
- Almeida, J.D.S.O.D.; Dias, C.O.; Arriola, N.D.A.; de Freitas, B.S.M.; de Francisco, A.; Petkowicz, C.L.; Araujo, L.; Guerra, M.P.; Nodari, R.O.; Amboni, R.D. Feijoa (Acca sellowiana) peel flours: A source of dietary fibers and bioactive compounds. Food Biosci. 2020, 38, 100789. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D. The development of fruit-based functional foods targeting the health and wellness market: A review. Int. J. Food Sci. Technol. 2011, 46, 899–920. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, M.; Bai, T.; Chen, D.; Zhang, Q.; Lin, D.; Liu, Y.; Liu, A.; Huang, Z.; Qin, W. Comparative study on the structure, physicochemical, and functional properties of dietary fiber extracts from quinoa and wheat. Lwt 2021, 149, 111816. [Google Scholar] [CrossRef]
- Hua, M.; Lu, J.; Qu, D.; Liu, C.; Zhang, L.; Li, S.; Chen, J.; Sun, Y. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Khanpit, V.V.; Tajane, S.P.; Mandavgane, S.A. Dietary fibers from fruit and vegetable waste: Methods of extraction and processes of value addition. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Raczyk, M.; Kruszewski, B.; Zachariasz, E. Effect of tomato, beetroot and carrot juice addition on physicochemical, antioxidant and texture properties of wheat bread. Antioxidants 2022, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Raczyk, M.; Kruszewski, B.; Michalowska, D. Effect of coconut and chestnut flour supplementations on texture, nutritional and sensory properties of baked wheat based bread. Molecules 2021, 26, 4641. [Google Scholar] [CrossRef] [PubMed]
- Bunzel, M.; Ralph, J.; Kim, H.; Lu, F.; Ralph, S.A.; Marita, J.M. Sinapate dehydrodimers and sinapate−ferulate heterodimers in cereal dietary fiber. J. Agric. Food Chem. 2003, 51, 1427–1434. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Tharanathan, R.N. Dietary fiber from coconut residue: Effects of different treatments and particle size on the hydration properties. Eur. Food Res. Technol. 2004, 218, 563–567. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis of AOAC International. Methods 950.46 and 991.43, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- AOAC. AOAC Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- AACC. International. Approved Methods of the AACC; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Chen, Q.; Wang, D.; Tan, C.; Hu, Y.; Sundararajan, B.; Zhou, Z. Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local citrus cultivars. Plants 2020, 9, 196. [Google Scholar] [CrossRef]
- Dong, X.Y.; Hu, Y.; Li, Y.; Zhou, Z.Q. The maturity degree, phenolic compounds and antioxidant activity of Eureka lemon [Citrus limon (L.) Burm. f.]: A negative correlation between total phenolic content, antioxidant capacity and soluble solid content. Sci. Hortic. 2019, 243, 281–289. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, B.; Shi, P.; Tian, H.; Li, Y.; Wang, X.; Wu, S.; Liang, P. The influences of acetylation, hydroxypropylation, enzymatic hydrolysis and crosslinking on improved adsorption capacities and in vitro hypoglycemic properties of millet bran dietary fibre. Food Chem. 2022, 368, 130883. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. Synergistic effect of different dietary fibres in pasta on in vitro starch digestion? Food Chem. 2015, 172, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, Q.; Zheng, B.; Lin, L.; Chen, B.; Zheng, Y.; Xiao, J. Hydration properties and binding capacities of dietary fibers from bamboo shoot shell and its hypolipidemic effects in mice. Food Chem. Toxicol. 2017, 109, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT Food Sci. Technol. 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Tang, X.; Wei, Z.; Zhang, M. Particle size of insoluble dietary fiber from rice bran affects its phenolic profile, bioaccessibility and functional properties. LWT Food Sci. Technol. 2018, 87, 450–456. [Google Scholar] [CrossRef]
- Zhu, Y.; Chu, J.; Lu, Z.; Lv, F.; Bie, X.; Zhang, C.; Zhao, H. Physicochemical and functional properties of dietary fiber from foxtail millet (Setaria italic) bran. J. Cereal Sci. 2018, 79, 456–461. [Google Scholar] [CrossRef]
- Alba, K.; MacNaughtan, W.; Laws, A.P.; Foster, T.J.; Campbell, G.M.; Kontogiorgos, V. Fractionation and characterisation of dietary fibre from blackcurrant pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Pappas, C.S.; Malovikova, A.; Hromadkova, Z.; Tarantilis, P.A.; Ebringerova, A.; Polissiou, M.G. Determination of the degree of esterification of pectinates with decyl and benzyl ester groups by diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and curve-fitting deconvolution method. Carbohydrate Polymers 2004, 56, 465–469. [Google Scholar] [CrossRef]
- Sena Neto, A.R.; Araujo, M.A.M.; Souza, F.V.D.; Mattoso, L.H.C.; Marconcini, J.M. Characterization and comparative evaluation of thermal, structural, chemical, mechanical and morphological properties of six pineapple leaf fiber varieties for use in composites. Ind. Crops Prod. 2013, 43, 529–537. [Google Scholar] [CrossRef]
- Qi, J.; Yokoyama, W.; Masamba, K.G.; Majeed, H.; Zhong, F.; Li, Y. Structural and physico-chemical properties of insoluble rice bran fiber: Effect of acid–base induced modifications. RSC Adv. 2015, 5, 79915–79923. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef]
- Alamprese, C.; Cappa, C.; Ratti, S.; Limbo, S.; Signorelli, M.; Fessas, D.; Lucisano, M. Shelf life extension of whole-wheat breadsticks: Formulation and packaging strategies. Food Chem. 2017, 230, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Rayas-Duarte, P.; Shogren, R.L.; Sessa, D.J. Low carbohydrates bread: Formulation, processing and sensory quality. Food Chem. 2006, 99, 686–692. [Google Scholar] [CrossRef]
- Yang, Y.-B.; Jia, G.-Q.; Deng, L.-G.; Qin, L.; Chen, E.-Y.; Cong, X.-J.; Zou, R.-F.; Wang, H.-L.; Zhang, H.-W.; Liu, B.; et al. Genetic variation of yellow pigment and its components in foxtail millet (Setaria italica (L.) P. Beauv.) from different eco-regions in China. J. Integr. Agric. 2017, 16, 2459–2469. [Google Scholar] [CrossRef]
- Helou, C.; Jacolot, P.; Niquet-Leridon, C.; Gadonna-Widehem, P.; Tessier, F.J. Maillard reaction products in bread: A novel semi-quantitative method for evaluating melanoidins in bread. Food Chem. 2016, 190, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Sulek, A.; Kalisz, S.; Sujka, K. Effect of the addition of dried dandelion roots (Taraxacum officinale F. H. Wigg.) on wheat dough and bread properties. Molecules 2021, 26, 7564. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Zhao, Y.; Wang, D.; Wang, W. Influence of antioxidant dietary fiber on dough properties and bread qualities: A review. J. Funct. Foods 2021, 80, 104434. [Google Scholar] [CrossRef]
- Sporin, M.; Avbelj, M.; Kovac, B.; Mozina, S.S. Quality characteristics of wheat flour dough and bread containing grape pomace flour. Food Sci. Technol. Int. 2018, 24, 251–263. [Google Scholar] [CrossRef]
- Fu, J.T.; Chang, Y.H.; Shiau, S.Y. Rheological, antioxidative and sensory properties of dough and Mantou (steamed bread) enriched with lemon fiber. LWT Food Sci. Technol. 2015, 61, 56–62. [Google Scholar] [CrossRef]
- Roman, L.; Cal Edl Gomez, M.; Martinez, M.M. Specific ratio of A-to B-type wheat starch granules improves the quality of gluten-free breads: Optimizing dough viscosity and pickering stabilization. Food Hydrocoll. 2018, 82, 510–518. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Optimization of bread firmness, specific loaf volume and sensory acceptability of bread with soluble fiber and different water levels. J. Cereal Sci. 2016, 70, 186–191. [Google Scholar] [CrossRef]
- Ni, Q.; Ranawana, V.; Hayes, H.E.; Hayward, N.J.; Stead, D.; Raikos, V. Addition of broad bean hull to wheat flour for the development of high-fiber bread: Effects on physical and nutritional properties. Foods 2020, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Riaño, A.M.; Solanilla-Duque, J.F.; Méndez-Arteaga, J.J.; Váquiro-Herrera, H.A. Bioactive potential of Colombian feijoa in physiological ripening stage. J. Saudi Soc. Agric. Sci. 2020, 19, 299–305. [Google Scholar] [CrossRef]
- Wu, G.; Shen, Y.; Qi, Y.; Zhang, H.; Wang, L.; Qian, H.; Qi, X.; Li, Y.; Johnson, S.K. Improvement of in vitro and cellular antioxidant properties of Chinese steamed bread through sorghum addition. LWT Food Sci. Technol. 2018, 91, 77–83. [Google Scholar] [CrossRef]
- Zhu, F.; Sakulnak, R.; Wang, S. Effect of black tea on antioxidant, textural, and sensory properties of Chinese steamed bread. Food Chem. 2016, 194, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Aboshora, W.; Yu, J.; Omar, K.A.; Li, Y.; Hassanin, H.A.M.; Navicha, W.B.; Zhang, L. Preparation of Doum fruit (Hyphaene thebaica) dietary fiber supplemented biscuits: Influence on dough characteristics, biscuits quality, nutritional profile and antioxidant properties. J. Food Sci. Technol. Mysore 2019, 56, 1328–1336. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, Z.; Ameer, K.; Song, C. Physicochemical, antioxidant, microstructural, and sensory characteristics of biscuits as affected by addition of onion residue. J. Food Meas. Charact. 2020, 15, 817–825. [Google Scholar] [CrossRef]
- Aleixandre, A.; Benavent-Gil, Y.; Velickova, E.; Rosell, C.M. Mastication of crisp bread: Role of bread texture and structure on texture perception. Food Res. Intern. 2021, 147, 110477. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Yao, R.; Yan, S.; Wang, Q. Mechanism of lipid metabolism regulation by soluble dietary fibre from micronized and non-micronized powders of lotus root nodes as revealed by their adsorption and activity inhibition of pancreatic lipase. Food Chem. 2020, 305, 125435. [Google Scholar] [CrossRef]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave assisted extraction with three modifications on structural and functional properties of soluble dietary fibers from grapefruit peel. Food Hydrocoll. 2020, 101, 105549. [Google Scholar] [CrossRef]
- Honikel, K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- McRae, M.P.; Facn, D. The benefits of dietary fiber intake on reducing the risk of cancer: An umbrella review of meta-analyses. J. Chiropr. Med. 2018, 17, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Yang, G.; Sun, L.; Song, X.; Chen, Q.; Bao, Y.; Luo, T.; Wang, J. Microstructure, physicochemical properties, and adsorption capacity of deoiled red raspberry pomace and its total dietary fiber. LWT Food Sci. Technol. 2022, 153, 112478. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Chen, Y.; Xie, J.; Liu, S.; Sun, N.; Hu, X.; Yu, Q. Modification of tea residue dietary fiber by high-temperature cooking assisted enzymatic method: Structural, physicochemical and functional properties. LWT Food Sci. Technol. 2021, 145, 111314. [Google Scholar] [CrossRef]
- Mao, T.; Huang, F.; Zhu, X.; Wei, D.; Chen, L. Effects of dietary fiber on glycemic control and insulin sensitivity in patients with type 2 diabetes: A systematic review and meta-analysis. J. Funct. Foods 2021, 82, 104500. [Google Scholar] [CrossRef]
- Chau, C.-F.; Chen, C.-H.; Lin, C.-Y. Insoluble fiber-rich fractions derived from Averrhoa carambola: Hypoglycemic effects determined by in vitro methods. LWT Food Sci. Technol. 2004, 37, 331–335. [Google Scholar] [CrossRef]
- Li, L.; Pan, M.; Pan, S.; Li, W.; Zhong, Y.; Hu, J.; Nie, S. Effects of insoluble and soluble fibers isolated from barley on blood glucose, serum lipids, liver function and caecal short-chain fatty acids in type 2 diabetic and normal rats. Food Chem. Toxicol. 2020, 135, 110937. [Google Scholar] [CrossRef]

| Mineral (Dry Weight) | Value | Physicochemical Properties (Dry Weight) | Value |
|---|---|---|---|
| K(mg/kg) | 2847.47 ± 77.20 | Water-holding capacity (WHC, g/g) | 4.66 ± 0.37 |
| Na(mg/kg) | 388.04 ± 22.06 | Oil–holding capacity (OHC, g/g) | 4.18 ± 0.07 |
| Ca (mg/kg) | 321.41 ± 10.76 | Swelling capacity (SC, mL/g) | 2.09 ± 0.16 |
| Fe (mg/kg) | 84.61 ± 3.66 | Total phenolic content (TPC, mg GAE/g) | 1.63 ± 0.22 |
| Zn(mg/kg) | 80.89 ± 2.40 | Total flavonoid content (TFC, mg RE/g) | 1.15 ± 0.46 |
| Mn(μg/kg) | 873.41 ± 79.02 | ABTS (μmol TE/g) | 3.63 ± 0.17 |
| Cu (μg/kg) | 111.48 ± 5.48 | DPPH (μmol TE/g) | 10.68 ± 0.25 |
| FRAP (μmol TE/g) | 5.40 ± 0.05 | ||
| Bile acid salt adsorption (mg/g, pH = 7) | 61.69 ± 0.28 | ||
| NO2− adsorption (μg/g, pH = 7) | 140.33 ± 3.75 | ||
| NO2− adsorption (μg/g, pH = 2) | 146.31 ± 4.16 | ||
| Cholesterol adsorption (mg/g, pH = 7) | 14.93 ± 0.22 | ||
| Cholesterol adsorption (mg/g, pH = 2) | 7.14 ± 0.14 |
| Samples | Moisture (%) | Total DF (%) | Fat (%) | Ash (%) | Protein (%) | Carbohydrate (%) | Energy Value (kJ/100 g) |
|---|---|---|---|---|---|---|---|
| Control | 34.57 ±0.13 b | 1.46 ± 0.04 e | 14.45 ± 0.02 a | 0.83 ± 0.03 c | 14.06 ± 0.46 b | 36.30 ± 0.26 a | 1378.87 ± 5.06 a |
| FJI 2% | 34.95 ± 0.49 b | 2.23 ± 0.01 d | 14.20 ± 0.01 b | 0.92 ± 0.01 b | 14.81 ± 0.42 b | 35.40 ± 0.66 b | 1364.38 ± 10.25 b |
| FJI 4% | 36.25 ± 0.74 a | 2.96 ± 0.30 c | 13.98 ± 0.05 c | 0.95 ± 0.04 b | 15.85 ± 0.28 a | 33.46 ± 0.26 c | 1351.68 ± 4.53 b |
| FJI 6% | 36.57 ± 0.23 a | 4.07 ± 0.09 b | 13.81 ± 0.05 d | 1.03 ± 0.02 a | 16.25 ± 0.31 a | 32.07 ± 0.64 d | 1328.71 ± 9.37 c |
| FJI 8% | 36.67 ± 0.12 a | 5.10 ± 0.09 a | 13.53 ± 0.06 e | 1.07 ± 0.02 a | 16.59 ± 0.25 a | 31.90 ± 0.25 e | 1321.01 ± 4.39 c |
| Control | FJI 2% | FJI 4% | FJI 6% | FJI 8% | |
|---|---|---|---|---|---|
| Crust color | |||||
| L* | 67.91 ± 1.72 a | 59.16 ± 1.21 b | 54.13 ± 0.42 c | 51.32 ± 1.03 d | 49.63 ± 0.89 d |
| a* | 8.89 ± 0.57 c | 10.25 ± 0.23 b | 10.91 ± 0.57 b | 13.07 ± 0.44 a | 13.55 ± 0.51 a |
| b* | 34.26 ± 0.22 a | 29.23 ± 0.15 b | 28.01 ± 1.59 bc | 30.75 ± 0.11 c | 27.99 ± 1.09 c |
| Crumb color | |||||
| L* | 68.34 ± 1.92 a | 52.43 ± 0.53 b | 49.41 ± 0.63 c | 47.90 ± 1.21 c | 44.53 ± 0.59 d |
| a* | 1.48 ± 0.17 d | 5.84 ± 1.43 c | 10.84 ± 0.46 b | 11.39 ± 0.17 b | 12.81 ± 0.54 a |
| b* | 15.64 ± 1.27 d | 19.38 ± 0.79 c | 26.05 ± 0.60 b | 26.58 ± 0.37 b | 28.87 ± 0.84 a |
| Specific volume (mL/g) | 3.61 ± 0.06 a | 3.02 ± 0.07 b | 2.76 ± 0.05 c | 2.58 ± 0.03 d | 2.45 ± 0.02 e |
| Hardness (g) | 68.86 ± 5.83 e | 158.99 ± 2.71 d | 332.73 ± 22.14 c | 367.78 ± 23.86 b | 415.15 ± 5.52 a |
| Chewiness (g) | 50.63 ± 6.71 e | 92.62 ± 1.89 d | 158.06 ± 3.21 c | 177.38 ± 3.68 b | 191.95 ± 9.97 a |
| Springiness (%) | 96.53 ± 2.44 a | 91.30 ± 0.85 b | 86.20 ± 0.89 c | 81.47 ± 0.15 d | 77.87 ± 2.17 e |
| Resilience (%) | 29.00 ± 1.40 a | 24.00 ± 1.30 b | 23.10 ± 0.30 b | 22.70 ± 0.20 b | 20.30 ± 0.80 c |
| TPC (mg GAE/100 g) | 77.47 ± 2.53 e | 84.08 ± 0.95 d | 92.07 ± 1.66 c | 99.50 ± 2.48 b | 113.01 ± 1.72 a |
| TFC (mg RE/100 g) | 5.55 ± 0.24 d | 6.64 ± 0.39 c | 7.07 ± 0.05 c | 7.78 ± 0.46 b | 9.75 ± 0.19 a |
| ABTS (μmol TE/g) | 0.37 ± 0.03 e | 0.77 ± 0.08 d | 0.95 ± 0.10 c | 1.72 ± 0.08 b | 2.01 ± 0.05 a |
| DPPH (μmol TE/g) | 0.14 ± 0.02 e | 0.40 ± 0.02 d | 0.61 ± 0.05 c | 0.99 ± 0.02 b | 1.28 ± 0.03 a |
| FRAP (μmol TE/g) | 0.10 ± 0.01 e | 0.43 ± 0.01 d | 0.66 ± 0.06 c | 0.93 ± 0.07 b | 1.35 ± 0.03 a |
| 0 Min | 20 Min | 60 Min | 90 Min | 120 Min | |
|---|---|---|---|---|---|
| Control | 170.63 ± 4.76 a | 189.25 ± 1.29 a | 205.42 ± 7.87 a | 230.78 ± 2.59 a | 194.76 ± 5.18 a |
| FJI 2% | 158.13 ± 4.66 b | 175.16 ± 3.45 b | 188.48 ± 8.27 b | 203.63 ± 1.30 b | 200.26 ± 3.95 a |
| FJI 4% | 150.47 ± 7.49 bc | 158.48 ± 9.46 c | 172.94 ± 7.23 c | 187.85 ± 3.94 c | 184.35 ± 9.79 b |
| FJI 6% | 142.59 ± 4.29 c | 154.63 ± 8.48 c | 167.30 ± 7.34 c | 186.19 ± 1.48 c | 179.37 ± 3.44 b |
| FJI 8% | 123.68 ± 3.04 d | 140.29 ± 4.74 d | 160.62 ± 1.29 c | 176.22 ± 6.74 d | 175.92 ± 2.73 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wang, Q.; Sun, Y.; Qing, Z.; Zhang, J.; Chen, Q. Effect of Insoluble Dietary Fiber Extracted from Feijoa (Acca sellowiana (O. Berg) Burret.) Supplementation on Physicochemical and Functional Properties of Wheat Bread. Foods 2023, 12, 2019. https://doi.org/10.3390/foods12102019
Wang D, Wang Q, Sun Y, Qing Z, Zhang J, Chen Q. Effect of Insoluble Dietary Fiber Extracted from Feijoa (Acca sellowiana (O. Berg) Burret.) Supplementation on Physicochemical and Functional Properties of Wheat Bread. Foods. 2023; 12(10):2019. https://doi.org/10.3390/foods12102019
Chicago/Turabian StyleWang, Dan, Qingming Wang, Yunfei Sun, Zilong Qing, Junhui Zhang, and Qiyang Chen. 2023. "Effect of Insoluble Dietary Fiber Extracted from Feijoa (Acca sellowiana (O. Berg) Burret.) Supplementation on Physicochemical and Functional Properties of Wheat Bread" Foods 12, no. 10: 2019. https://doi.org/10.3390/foods12102019
APA StyleWang, D., Wang, Q., Sun, Y., Qing, Z., Zhang, J., & Chen, Q. (2023). Effect of Insoluble Dietary Fiber Extracted from Feijoa (Acca sellowiana (O. Berg) Burret.) Supplementation on Physicochemical and Functional Properties of Wheat Bread. Foods, 12(10), 2019. https://doi.org/10.3390/foods12102019







