Insight into the Interaction of Malondialdehyde with Rabbit Meat Myofibrillar Protein: Fluorescence Quenching and Protein Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of MPs
2.3. Preparation of MDA Solution
2.4. Incubation of MP with MDA
2.5. Measurement of Carbonyl Content of MP
2.6. Measurement of Sulfhydryl Content of MP
2.7. Measurement of Free-Amine Content
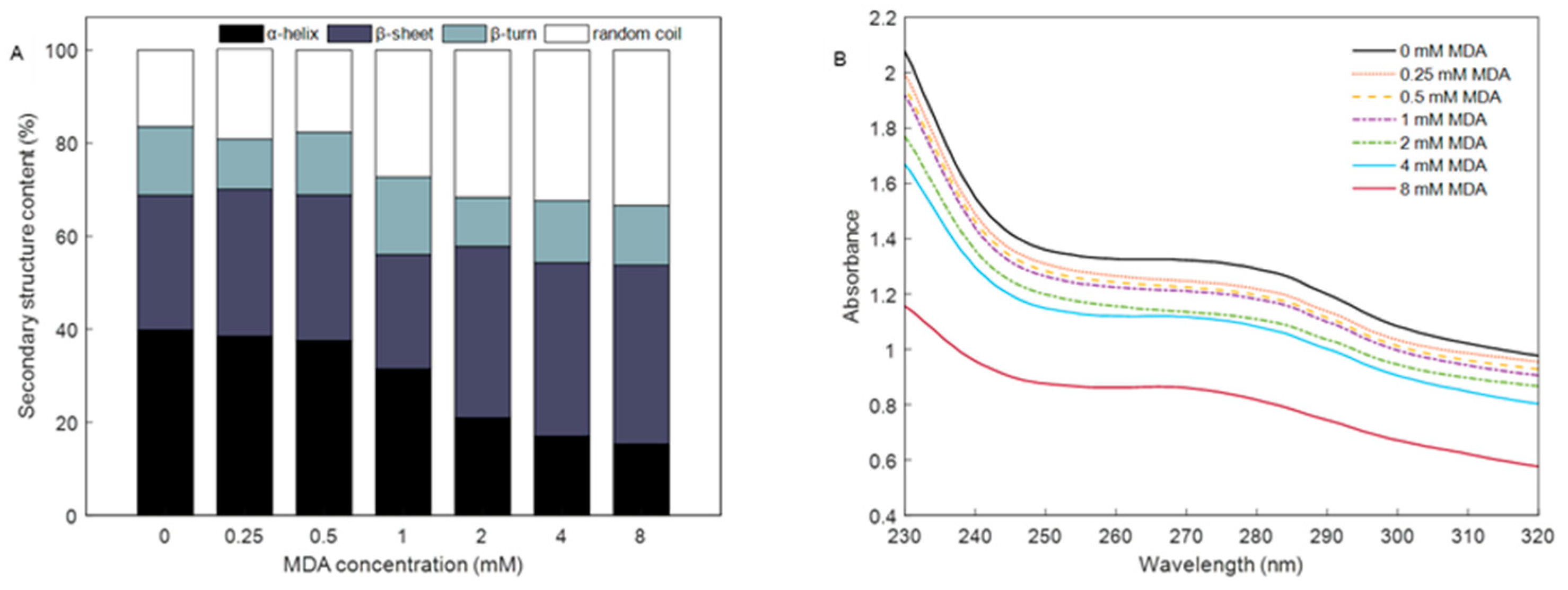
2.8. Secondary Structure of MP
2.9. The Intrinsic Tryptophan Fluorescence of MP
2.10. Determination of UV Absorption Spectra of MPs
2.11. Determination of the Fluorescence Intensity of MDA–MP Adducts
2.12. Surface Hydrophobicity
2.13. Differential Scanning Calorimetry (DSC)
2.14. Measurement of Electrophoresis of MP
2.15. Kinetic Modeling
2.16. Statistical Analysis
3. Results and Discussion
3.1. Changes in Amino-Acid Side Chains
3.1.1. Carbonyl Content
3.1.2. Sulfhydryl Content
3.1.3. Free-Amine Content
3.2. Surface Hydrophobicity
3.3. Fluorescence Intensity of MDA–MP Adducts
3.4. Changes in Secondary Structure
3.5. Absorption Spectra of MP in Soret Band
3.6. Intrinsic Tryptophan Fluorescence
3.7. DSC Analysis
3.8. Cross-Linking
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dalle Zotte, A.; Szendrő, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Z.; Zhang, D.; Li, H.; Wang, Z. Using oxidation kinetic models to predict the quality indices of rabbit meat under different storage temperatures. Meat Sci. 2020, 162, 108042. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Kylli, P.; Puolanne, E.; Kivikari, R.; Heinonen, M. Oxidation of Skeletal Muscle Myofibrillar Proteins in Oil-in-Water Emulsions: Interaction with Lipids and Effect of Selected Phenolic Compounds. J. Agric. Food Chem. 2008, 56, 10933–10940. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, Y.; Chen, L.; Wu, H.; Zhang, Y.; Suo, Z.; Wang, S.; Liang, Y.; Xu, X.; Zhou, G.; et al. Inhibition of Epigallocatechin-3-gallate/Protein Interaction by Methyl-β-cyclodextrin in Myofibrillar Protein Emulsion Gels under Oxidative Stress. J. Agric. Food Chem. 2018, 66, 8094–8103. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2014, 96, 1432–1439. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, L.; Wu, H.; Xu, X.; Zhou, G.; Zhu, B.; Feng, X. (-)-Epigallocatechin-3-gallate-mediated formation of myofibrillar protein emulsion gels under malondialdehyde-induced oxidative stress. Food Chem. 2019, 285, 139–146. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Effect of peroxyl radicals on the structure and gel properties of isolated rabbit meat myofibrillar proteins. Int. J. Food Sci. Technol. 2018, 53, 2687–2696. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, R.; Wang, H.; Hua, C.; Song, S.; Zhou, G.; Zhang, W. Effects of Oxidation in Vitro on Structures and Functions of Myofibrillar Protein from Beef Muscles. J. Agric. Food Chem. 2019, 67, 5866–5873. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.J.; Wu, W. Effects of malondialdehyde-induced protein oxidation on the structural characteristics of rice protein. Int. J. Food Sci. Technol. 2019, 55, 760–768. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Emara, A.M.; Gan, X.; Li, H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019, 288, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, N.; Ahn, B.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhao, X. Structure and property modification of an oligochitosan- glycosylated and crosslinked soybean protein generated by microbial transglutaminase. Food Chem. 2014, 163, 114–119. [Google Scholar] [CrossRef]
- Jia, N.; Zhang, F.; Liu, Q.; Wang, L.; Lin, S.; Liu, D. The beneficial effects of rutin on myofibrillar protein gel properties and related changes in protein conformation. Food Chem. 2019, 301, 125206. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, M.; Qiu, C.; Sun, W. Effect of malondialdehyde modification on the binding of aroma compounds to soy protein isolates. Food Res Int. 2018, 105, 150–158. [Google Scholar] [CrossRef]
- Estévez, M.; Kylli, P.; Puolanne, E.; Kivikari, R.; Heinonen, M. Fluorescence spectroscopy as a novel approach for the assessment of myofibrillar protein oxidation in oil-in-water emulsions. Meat Sci. 2008, 80, 1290–1296. [Google Scholar] [CrossRef]
- Dermesonluoglu, E.; Katsaros, G.; Tsevdou, M.; Giannakourou, M.; Taoukis, P. Kinetic study of quality indices and shelf-life modelling of frozen spinach under dynamic conditions of the cold chain. J. Food Eng. 2015, 148, 13–23. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Investigation and kinetic evaluation of the reactions of hydroxymethylfurfural with amino and thiol groups of amino acids. Food Chem. 2018, 240, 354–360. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Rasco, B.; Sablani, S.S.; Ovissipour, M.; Qu, Z. Kinetics of Quality Changes of Shrimp (Litopenaeus setiferus) During Pasteurization. Food. Bioprocess Technol. 2018, 11, 1027–1038. [Google Scholar] [CrossRef]
- Awasthi, A.; Dhyani, V.; Biswas, B.; Kumar, J.; Bhaskar, T. Production of phenolic compounds using waste coir pith: Estimation of kinetic and thermodynamic parameters. Bioresour. Technol. 2019, 274, 173–179. [Google Scholar] [CrossRef]
- Kou, X.; Li, R.; Zhang, L.; Ramaswamy, H.; Wang, S. Effect of heating rates on thermal destruction kinetics of Escherichia coli ATCC25922 in mashed potato and the associated changes in product color. Food Control 2019, 97, 39–49. [Google Scholar] [CrossRef]
- Arcanjo, N.M.O.; Luna, C.; Madruga, M.S.; Estévez, M. Antioxidant and pro-oxidant actions of resveratrol on human serum albumin in the presence of toxic diabetes metabolites: Glyoxal and methyl-glyoxal. BBA-Gen. Subj. 2018, 1862, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, M.; Fang, Z.; Bhandari, B. Gelation properties of myofibrillar protein under malondialdehyde-induced oxidative stress. J. Sci. Food Agric. 2017, 97, 50–57. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Su, G.; Cui, C.; Sun, W. Gelation of salted myofibrillar protein under malondialdehyde-induced oxidative stress. Food Hydrocoll. 2014, 40, 153–162. [Google Scholar] [CrossRef]
- Estévez, M.; Padilla, P.; Carvalho, L.; Martín, L.; Carrapiso, A.; Delgado, J. Malondialdehyde interferes with the formation and detection of primary carbonyls in oxidized proteins. Redox Biol. 2019, 26, 101277. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Menini, S.; Maineri, E.P.; Patriarca, S.; Odetti, P.; Cottalasso, D.; Marinari, U.M.; Pronzato, M.A. Malondialdehyde, a lipoperoxidation- derived aldehyde, can bring about secondary oxidative damage to proteins. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, B890–B895. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Ávila, F.; Fuentes-Lemus, E.; Fuentealba, D.; López-Alarcón, C. Oxidation of myofibrillar proteins induced by peroxyl radicals: Role of oxidizable amino acids. Food Res. Int. 2019, 126, 108580. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Z.; Lin, H.; Du, S.; Hao, Z.; Lin, H.; Zhu, Z. Effect of malondialdehyde treatment on the IgE binding capacity and conformational structure of shrimp tropomyosin. Food Chem. 2015, 175, 374–380. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mitra, I.; Sarkar, K.; Bardhan, M.; Paul, S.; Basu, S.; Goswami, A.; Saha, A.; Show, B.; Ganguly, T. Fluorescence enhancement via aggregation effect due to microenvironmental alterations in human hemoglobin protein in presence of carbon quantum dots (CQD): Comparative spectroscopic approach. Spectochim. Acta A 2019, 215, 313–326. [Google Scholar] [CrossRef]
- Nyaisaba, B.M.; Hatab, S.; Liu, X.; Chen, Y.; Chen, X.; Miao, W.; Chen, M.; Deng, S. Physicochemical changes of myofibrillar proteins of squid (Argentinus ilex) induced by hydroxyl radical generating system. Food Chem. 2019, 297, 124941. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Meti, M.D.; Xu, H.; Wang, Y.; Lin, J.; Wu, Z.; Han, Q.; Xu, X.; He, Z.; Hong, W.; et al. Binding mechanism of lipase to Ligupurpuroside B extracted from Ku-Ding tea as studied by multi-spectroscopic and molecular docking methods. Int. J. Biol. Macromol. 2018, 120, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Mei, S.; Duan, R.; Geng, F.; Wu, W.; Li, X.; Cheng, L.; Wang, C. How black tea pigment theaflavin dyes chicken eggs: Binding affinity study of theaflavin with ovalbumin. Food Chem. 2020, 303, 125407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ertbjerg, P. On the origin of thaw loss: Relationship between freezing rate and protein denaturation. Food Chem. 2019, 299, 125104. [Google Scholar] [CrossRef]
- Zhou, F.; Sun, W.; Zhao, M. Controlled Formation of Emulsion Gels Stabilized by Salted Myofibrillar Protein under Malondialdehyde (MDA)-Induced Oxidative Stress. J. Agric. Food Chem. 2015, 63, 3766–3777. [Google Scholar] [CrossRef]

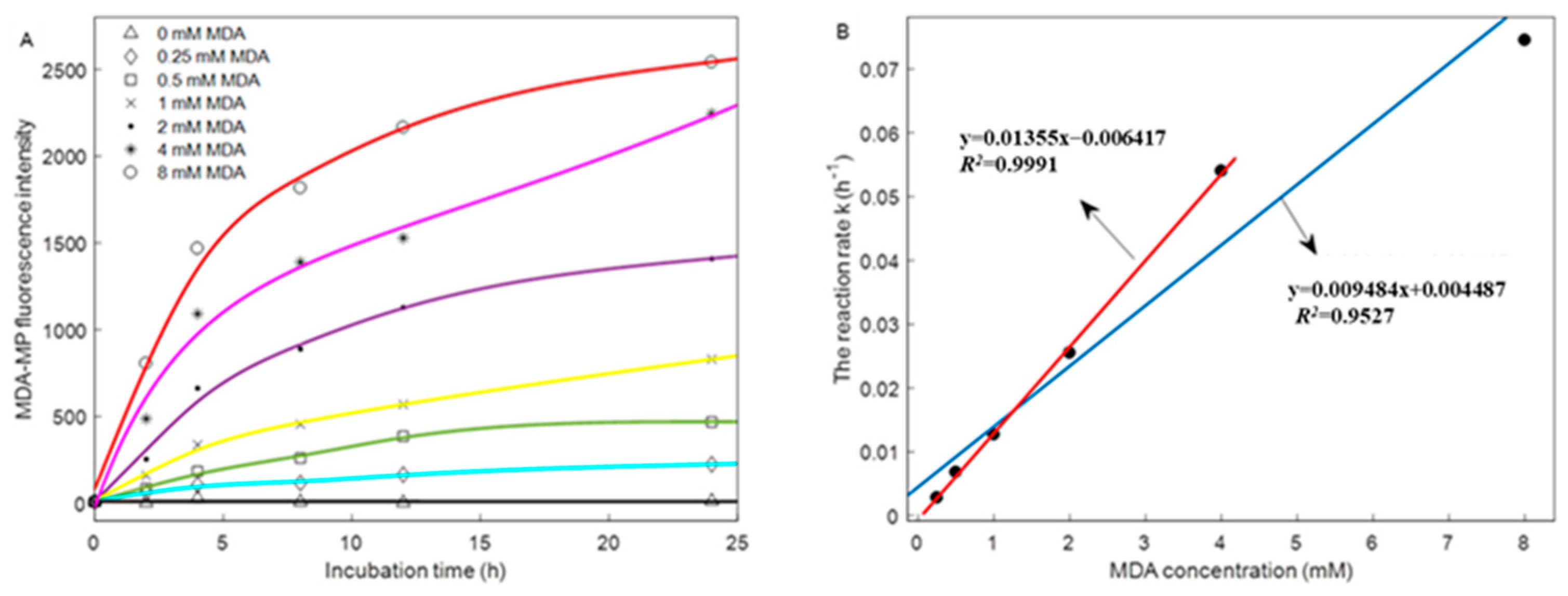


| Kinetic Parameters | Different Concentrations of MDA | |||||
|---|---|---|---|---|---|---|
| 0.25 mM | 0.5 mM | 1 mM | 2 mM | 4 mM | 8 mM | |
| k (h−1) | 0.0028 | 0.0068 | 0.0127 | 0.0255 | 0.054 | 0.0745 |
| R2 | 0.9029 | 0.8959 | 0.9454 | 0.9025 | 0.9672 | 0.9425 |
| MDA Concentration (mM) | Td1 (°C) | ΔH1 (J/g) | Td2 (°C) | ΔH2 (J/g) |
|---|---|---|---|---|
| 0 | 59.107 ± 0.050 a | 0.862 ± 0.040 a | 76.730 ± 0.333 a | 0.430 ± 0.031 a |
| 0.25 | 58.217 ± 0.343 b | 0.323 ± 0.020 b | 72.843 ± 0.125 b | 0.272 ± 0.021 b |
| 0.5 | 57.550 ± 0.075 c | 0.271 ± 0.012 c | 72.817 ± 0.117 b | 0.276 ± 0.013 b |
| 1 | 56.88 ± 0.258 d | 0.074 ± 0.036 d | 71.767 ± 0.009 b | 0.240 ± 0.043 b |
| 2 | - | - | 69.757 ± 1.094 c | 0.172 ± 0.004 c |
| 4 | - | - | 66.003 ± 0.642 d | 0.120 ± 0.016 c |
| 8 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; He, Z.; Wang, Z.; Li, H. Insight into the Interaction of Malondialdehyde with Rabbit Meat Myofibrillar Protein: Fluorescence Quenching and Protein Oxidation. Foods 2023, 12, 2044. https://doi.org/10.3390/foods12102044
Chen X, He Z, Wang Z, Li H. Insight into the Interaction of Malondialdehyde with Rabbit Meat Myofibrillar Protein: Fluorescence Quenching and Protein Oxidation. Foods. 2023; 12(10):2044. https://doi.org/10.3390/foods12102044
Chicago/Turabian StyleChen, Xiaosi, Zhifei He, Zefu Wang, and Hongjun Li. 2023. "Insight into the Interaction of Malondialdehyde with Rabbit Meat Myofibrillar Protein: Fluorescence Quenching and Protein Oxidation" Foods 12, no. 10: 2044. https://doi.org/10.3390/foods12102044




