Piper nigrum Extract: Dietary Supplement for Reducing Mammary Tumor Incidence and Chemotherapy-Induced Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PFPE
2.2. Phytochemical Analysis and Identification of Bioactive Constituents Using Gas Chromatograph-Mass Spectrometer (GC-MS)
2.3. Animals
2.4. Experimental Design
2.4.1. Acute Toxicity
2.4.2. Chemoprevention Study
2.4.3. Chemotherapeutic Study
2.4.4. Chronic Toxicity Study
2.5. Histopathological Study
2.6. Thiobarbituric Acid Reactive Substances (TBARS)
2.7. Cytokine Detection Assay
2.8. Statistical Analysis
3. Results
3.1. GC-MS Analysis Revealed That 41 Compounds Contain PFPE
3.2. PFPE-CH Does Not Induce Acute Toxicity in ICR Mice
3.3. PFPE-CH Reduces Mammary Tumor Rat Incidence
3.4. PFPE-CH Did Not Disrupt the Anticancer Effects of Doxorubicin on Mammary Tumor Rats
3.5. PFPE-CH Increased the Stress Condition, Indicated by the Elevation of TBARS Level
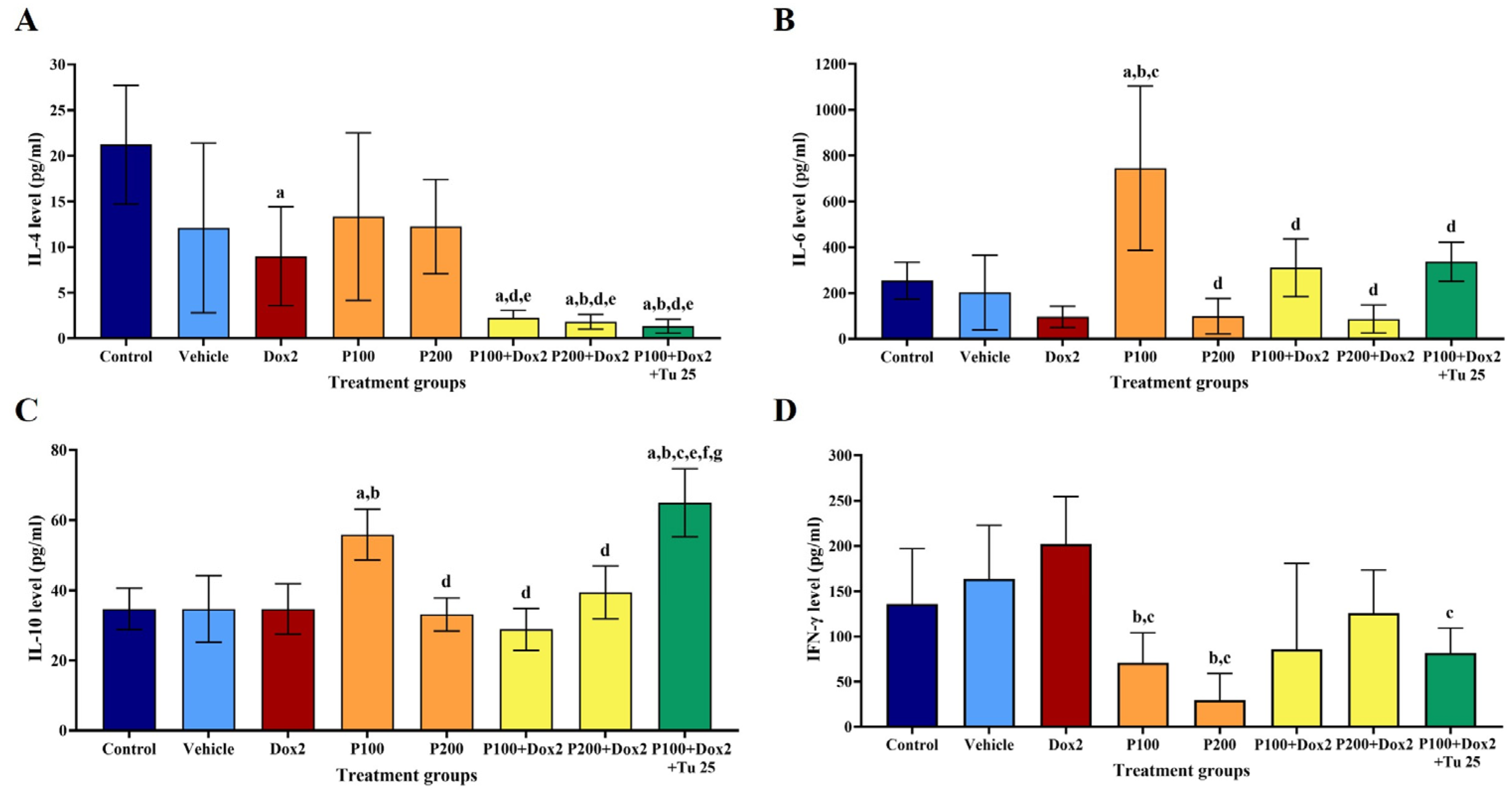
3.6. PFPE-CH Altered the Levels of IL-4, IL-6, IL-10, and IFN-γ
3.7. PFPE-CH Does Not Cause Chronic Toxicity in Sprague Dawley Rat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F.; ESMO Guidelines Committee. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v8–v30. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Barreto, J.N.; McCullough, K.B.; Ice, L.L.; Smith, J.A. Antineoplastic agents and the associated myelosuppressive effects: A review. J. Pharm. Pract. 2014, 27, 440–446. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X. Chemopreventive activity of honokiol against 7, 12—dimethylbenz[a]anthracene-induced mammary cancer in female Sprague Dawley rats. Front. Pharmacol. 2017, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Sreekala, C.; Zhang, Z.; Budhraja, A.; Ding, S.; Son, Y.O.; Wang, X.; Hitron, A.; Hyun-Jung, K.; Wang, L.; et al. Cancer prevention with promising natural products: Mechanisms of action and molecular targets. Anticancer Agents Med. Chem. 2012, 12, 1159–1184. [Google Scholar] [CrossRef] [PubMed]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kumar, S.V.; Rajkapoor, B.; Pravin, N.; Mahendiran, D. Chemopreventive effect of Indigofera linnaei extract against diethylnitrosamine induced hepatocarcinogenesis in rats. J. Appl. Pharm. Sci. 2016, 6, 199–209. [Google Scholar] [CrossRef]
- Vadodkar, A.S.; Suman, S.; Lakshmanaswamy, R.; Damodaran, C. Chemoprevention of breast cancer by dietary compounds. Anticancer Agents Med. Chem. 2012, 12, 1185–1202. [Google Scholar] [CrossRef]
- Shankar, G.M.; Swetha, M.; Keerthana, C.K.; Rayginia, T.P.; Anto, R.J. Cancer chemoprevention: A strategic approach using phytochemicals. Front. Pharmacol. 2022, 12, 809308. [Google Scholar] [CrossRef]
- Kado, K.; Forsyth, A.; Patel, P.R.; Schwartz, J.A. Dietary supplements and natural products in breast cancer trials. Front. Biosci. (Elite Ed.) 2012, 4, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M.; Khan, M.A. Biological role of Piper nigrum L. (Black pepper): A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1945–S1953. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.E.; et al. Phytochemistry of the genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Prashant, A.; Rangaswamy, C.; Yadav, A.K.; Reddy, V.; Sowmya, M.N.; Madhunapantula, S. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int. J. Appl. Basic. Med. Res. 2017, 7, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Grinevicius, V.M.A.S.; Andrade, K.S.; Ourique, F.; Micke, G.A.; Ferreira, S.R.S.; Pedrosa, R.C. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. cultivar Bragantina through cell cycle arrest and apoptosis induction. J. Supercrit. Fluids 2017, 128, 94–101. [Google Scholar] [CrossRef]
- Renju, G.L.; Manoharan, S.; Balakrishnan, S.; Senthil, N. Chemopreventive and antilipidperoxidative potential of Clerodendron inerme (L) Gaertn in 7,12-dimethylbenz(a)anthracene induced skin carcinogenesis in Swiss albino mice. Pak. J. Biol. Sci. 2007, 10, 1465–1470. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Sriwiriyajan, S.; Tedasen, A.; Lailerd, N.; Boonyaphiphat, P.; Nitiruangjarat, A.; Deng, Y.; Graidist, P. Anticancer and cancer prevention effects of piperine-free Piper nigrum extract on N-nitrosomethylurea-induced mammary tumorigenesis in rats. Cancer Prev. Res. 2016, 9, 74–82. [Google Scholar] [CrossRef]
- Deng, Y.; Sriwiriyajan, S.; Tedasen, A.; Hiransai, P.; Graidist, P. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J. Ethnopharmacol. 2016, 188, 87–95. [Google Scholar] [CrossRef]
- Saetang, J.; Tedasen, A.; Sangkhathat, S.; Sangkaew, N.; Dokduang, S.; Prompat, N.; Taraporn, S.; Graidist, P. Low piperine fractional Piper nigrum extract enhanced the antitumor immunity via regulating the Th1/Th2/Treg cell subsets on NMU-induced tumorigenesis rats. Planta Med. 2022, 88, 527–537. [Google Scholar] [CrossRef]
- Saetang, J.; Tedasen, A.; Sangkhathat, S.; Sangkaew, N.; Dokduang, S.; Prompat, N.; Taraporn, S.; Graidist, P. The attenuation effect of low piperine Piper nigrum extract on doxorubicin-induced toxicity of blood chemical and immunological properties in mammary tumour rats. Pharm. Biol. 2022, 60, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Tedasen, A.; Dokduang, S.; Sukpondma, Y.; Lailerd, N.; Madla, S.; Sriwiriyajan, S.; Rattanaburee, T.; Tipmanee, V.; Graidist, P. (−)-Kusunokinin inhibits breast cancer in N-nitrosomethylurea-induced mammary tumor rats. Eur. J. Pharmacol. 2020, 882, 173311. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test. No. 425: Acute Oral Toxicity: Up-and-Down Procedure; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2008. [Google Scholar] [CrossRef]
- Zou, L.; Liu, W.; Yu, L. Beta-elemene induces apoptosis of K562 leukemia cells. Zhonghua Zhong Liu Za Zhi. 2001, 23, 196–198. [Google Scholar] [PubMed]
- Martins, C.D.M.; Nascimento, E.A.D.; de Morais, S.A.; de Oliveira, A.; Chang, R.; Cunha, L.; Martins, M.M.; Martins, C.H.G.; Moraes, T.D.S.; Rodrigues, P.V.; et al. Chemical constituents and evaluation of antimicrobial and cytotoxic activities of Kielmeyera coriacea Mart. & Zucc. Essential oils. Evid. Based Complement. Altern. Med. 2015, 2015, 842047. [Google Scholar] [CrossRef]
- Türkez, H.; Celik, K.; Toğar, B. Effects of copaene, a tricyclic sesquiterpene, on human lymphocytes cells in vitro. Cytotechnology. 2014, 66, 597–603. [Google Scholar] [CrossRef]
- Zhang, Q.; An, R.; Tian, X.; Yang, M.; Li, M.; Lou, J.; Xu, L.; Dong, Z. β-Caryophyllene pretreatment alleviates focal cerebral ischemia-reperfusion injury by activating PI3K/Akt signaling pathway. Neurochem. Res. 2017, 42, 1459–1469. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Stefani, L.M.; Monteiro, S.G. β-caryophyllene reduces atherogenic index and coronary risk index in hypercholesterolemic rats: The involvement of cardiac oxidative damage. Chem. Biol. Interact. 2017, 270, 9–14. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. beta-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef]
- Alvarez-Gonzalez, I.; Madrigal-Bujaidar, E.; Castro-Garcia, S. Antigenotoxic capacity of beta-caryophyllene in mouse, and evaluation of its antioxidant and GST induction activities. J. Toxicol. Sci. 2014, 39, 849–859. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.; Asif, M.; Ahmed, M.; Babu, D.; Hassan, L.E.; Ahamed, M.B.K.; Sandai, D.; Barakat, K.; Siraki, A.; et al. β-Caryophyllene induces apoptosis and inhibits angiogenesis in colorectal cancer models. Int. J. Mol. Sci. 2021, 22, 10550. [Google Scholar] [CrossRef]
- Richmond, J.D.; Agius, B.R.; Wright, B.S.; Haber, W.A.; Moriarity, D.M.; Setzer, W.N. Essential oil compositions and cytotoxic activities of Dendropanax capillaris, Oreopanax nubigenus, and Schefflera rodrigueziana from Monteverde, Costa Rica. Nat. Prod. Commun. 2009, 4, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Flatt, V.D.; Campos, C.R.; Kraemer, M.P.; Bailey, B.A.; Satyal, P.; Setzer, W.N. Compositional variation and bioactivity of the leaf essential oil of Montanoa guatemalensis from Monteverde, Costa Rica: A preliminary investigation. Medicines 2015, 2, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Helichrysum gymnocephalum essential oil: Chemical composition and cytotoxic, antimalarial and antioxidant activities, attribution of the activity origin by correlations. Molecules 2011, 16, 8273–8291. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, E.; Abai, M.R.; Khanavi, M.; Vatandoost, H.; Sedaghat, M.M.; Moridnia, A.; Saber-Navaei, M.; Sanei-Dehkordi, A.; Rafi, F. Chemical composition, larvicidal and repellency properties of Cionura erecta (L.) Griseb. Against malaria vector, anopheles stephensi liston (Diptera: Culicidae). J. Arthropod. Borne Dis. 2014, 8, 147–155. [Google Scholar] [PubMed]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. beta-Bisabolene, a sesquiterpene from the essential oil extract of opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef]
- de SS Quintans, J.; Soares, B.M.; Ferraz, R.P.; Oliveira, A.C.; da Silva, T.B.; Menezes, L.R.; Sampaio, M.F.; Prata, A.P.d.N.; Moraes, M.O.; Pessoa, C. Chemical constituents and anticancer effects of the essential oil from leaves of Xylopia laevigata. Planta medica. 2013, 29, 123–130. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Benelli, G. δ-Cadinene, calarene and δ-4-Carene from Kadsura heteroclita Essential Oil as Novel Larvicides Against Malaria, Dengue and Filariasis Mosquitoes. Comb. Chem. High Throughput Screen 2016, 19, 565–571. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; El-Hefny, M.; Ali, H.M.; Elansary, H.O.; Nasser, R.A.; El-Settawy, A.A.A.; El Shanhorey, N.; Ashmawy, N.A.; Salem, A.Z.M. Antibacterial activity of extracted bioactive molecules of Schinus terebinthifolius ripened fruits against some pathogenic bacteria. Microb. Pathog. 2018, 120, 119–127. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strzadala, L.; Szumny, A. beta-caryophyllene and beta-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Hodaj-Celiku, E.; Tsiftsoglou, O.; Shuka, L.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Antioxidant activity and chemical composition of essential oils of some aromatic and medicinal plants from Albania. Nat. Prod. Commun. 2017, 12, 785–790. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Abdelkafi, S.; Hamdi, N. Essential oil from Artemisia phaeolepis: Chemical composition and antimicrobial activities. J. Oleo Sci. 2013, 62, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. beta-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Elaissi, A.; Rouis, Z.; Mabrouk, S.; Salah, K.B.; Aouni, M.; Khouja, M.L.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Correlation between chemical composition and antibacterial activity of essential oils from fifteen Eucalyptus species growing in the Korbous and Jbel Abderrahman arboreta (North East Tunisia). Molecules 2012, 17, 3044–3057. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Lima, B.; Aragon, L.; Espinar, L.A.; Tapia, A.; Zacchino, S.; Zygadlo, J.; Feresin, G.E.; Lopez, M.L. Essential oil of Azorella cryptantha collected in two different locations from San Juan Province, Argentina: Chemical variability and anti-insect and antimicrobial activities. Chem. Biodivers 2012, 9, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Yap, H.Y.; Muria-Gonzalez, M.J.; Kong, B.H.; Stubbs, K.A.; Tan, C.S.; Ng, S.T.; Tan, N.H.; Solomon, P.S.; Fung, S.Y.; Chooi, Y.H. Heterologous expression of cytotoxic sesquiterpenoids from the medicinal mushroom Lignosus rhinocerotis in yeast. Microb. Cell. Fact. 2017, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.A.; Saleh, A.M.; Alhawsawi, N.L.; Al-Jaber, H.I.; Rizvi, S.A.; Afifi, F.U. Composition, antioxidant, and cytotoxic activities of the essential oils from fresh and air-dried aerial parts of Pallenis spinosa. Chem. Biodivers. 2017, 14, e1700146. [Google Scholar] [CrossRef]
- Cerqueira, F.; Watanadilok, R.; Sonchaeng, P.; Kijjoa, A.; Pinto, M.; Quarles van Ufford, H.; Kroes, B.; Beukelman, C.; Nascimento, M.S. Clionasterol: A potent inhibitor of complement component C1. Planta Med. 2003, 69, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Lee, I.C.; Kim, J.A.; Bae, J.S. Anti-septic effects of pellitorine in HMGB1-induced inflammatory responses in vitro and in vivo. Inflammation 2014, 37, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yasuda, D.; Yamaguchi, I.; Yoshikawa, M. Hepatoprotective amide constituents from the fruit of Piper chaba: Structural requirements, mode of action, and new amides. Bioorg. Med. Chem. 2009, 17, 7313–7323. [Google Scholar] [CrossRef]
- Tu, Y.; Zhong, Y.; Du, H.; Luo, W.; Wen, Y.; Li, Q.; Zhu, C.; Li, Y. Anticholinesterases and antioxidant alkamides from Piper nigrum fruits. Nat. Prod. Res. 2016, 30, 1945–1949. [Google Scholar] [CrossRef]
- Ribeiro, T.S.; Freire-de-Lima, L.; Previato, J.O.; Mendonca-Previato, L.; Heise, N.; de Lima, M.E. Toxic effects of natural piperine and its derivatives on epimastigotes and amastigotes of Trypanosoma cruzi. Bioorg Med. Chem. Lett. 2004, 14, 3555–3558. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.S.; Jeong, C.H.; Petriello, M.C.; Seo, H.G.; Yoo, H.; Hong, K.; Han, S.G. Piperlongumine decreases cell proliferation and the expression of cell cycle-associated proteins by inhibiting Akt pathway in human lung cancer cells. Food Chem. Toxicol. 2018, 111, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sriwiriyajan, S.; Sukpondma, Y.; Srisawat, T.; Madla, S.; Graidist, P. (-)-Kusunokinin and piperloguminine from Piper nigrum: An alternative option to treat breast cancer. Biomed. Pharmacother. 2017, 92, 732–743. [Google Scholar] [CrossRef]
- Mohammad, J.; Dhillon, H.; Chikara, S.; Mamidi, S.; Sreedasyam, A.; Chittem, K.; Orr, M.; Wilkinson, J.C.; Reindl, K.M. Piperlongumine potentiates the effects of gemcitabine in in vitro and in vivo human pancreatic cancer models. Oncotarget 2017, 9, 10457–10469. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, V.; Elangovan, K.; Niranjali Devaraj, S. Targeting hepatocellular carcinoma with piperine by radical-mediated mitochondrial pathway of apoptosis: An in vitro and in vivo study. Food Chem. Toxicol. 2017, 105, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Nawale, S.; Padma Priya, K.; Pranusha, P.; Ganga Raju, M. Data of antihyperlipidaemic activity for methanolic extract of Tagetes patula Linn. flower head along with piperine, as bioavailability enhancer. Data Brief. 2018, 21, 587–597. [Google Scholar] [CrossRef]
- Han, S.Z.; Liu, H.X.; Yang, L.Q.; Cui, L.D.; Xu, Y. Piperine (PP) enhanced mitomycin-C (MMC) therapy of human cervical cancer through suppressing Bcl-2 signaling pathway via inactivating STAT3/NF-kappaB. Biomed. Pharmacother. 2017, 96, 1403–1410. [Google Scholar] [CrossRef]
- Park, I.K. Insecticidal activity of isobutylamides derived from Piper nigrum against adult of two mosquito species, Culex pipiens pallens and Aedes aegypti. Nat. Prod. Res. 2012, 26, 2129–2131. [Google Scholar] [CrossRef]
- Chen, J.J.; Huang, Y.C.; Chen, Y.C.; Huang, Y.T.; Wang, S.W.; Peng, C.Y.; Teng, C.M.; Chen, I.S. Cytotoxic amides from Piper sintenense. Planta Med. 2002, 68, 980–985. [Google Scholar] [CrossRef]
- Park, I.K.; Lee, S.G.; Shin, S.C.; Park, J.D.; Ahn, Y.J. Larvicidal activity of isobutylamides identified in Piper nigrum fruits against three mosquito species. J. Agric. Food Chem. 2002, 50, 1866–1870. [Google Scholar] [CrossRef]
- Zhang, H.; Matsuda, H.; Nakamura, S.; Yoshikawa, M. Effects of amide constituents from pepper on adipogenesis in 3T3-L1 cells. Bioorg. Med. Chem. Lett. 2008, 18, 3272–3277. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Rho, M.-C.; Park, H.R.; Choi, J.-H.; Kang, J.Y.; Lee, J.W.; Kim, K.; Lee, H.S.; Kim, Y.K. Inhibition of diacylglycerol acyltransferase by alkamides isolated from the fruits of Piper longum and Piper nigrum. J. Agric. Food Chem. 2006, 54, 9759–9763. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Xu, Y.; Zhang, Y.F.; Zhang, Y.; Wong, Y.H.; Han, Z.; Yin, Y.; Qian, P.Y. Nontoxic piperamides and their synthetic analogues as novel antifouling reagents. Biofouling. 2014, 30, 473–481. [Google Scholar] [CrossRef]
- Hwang, K.S.; Kim, Y.K.; Park, K.W.; Kim, Y.T. Piperolein B and piperchabamide D isolated from black pepper (Piper nigrum L.) as larvicidal compounds against the diamondback moth (Plutella xylostella). Pest. Manag. Sci. 2017, 73, 1564–1567. [Google Scholar] [CrossRef]
- Okumura, Y.; Narukawa, M.; Iwasaki, Y.; Ishikawa, A.; Matsuda, H.; Yoshikawa, M.; Watanabe, T. Activation of TRPV1 and TRPA1 by black pepper components. Biosci. Biotechnol. Biochem. 2010, 74, 1068–1072. [Google Scholar] [CrossRef]
- Ngo, Q.M.; Tran, P.T.; Tran, M.H.; Kim, J.A.; Rho, S.S.; Lim, C.H.; Kim, J.C.; Woo, M.H.; Choi, J.S.; Lee, J.H.; et al. Alkaloids from Piper nigrum exhibit antiinflammatory activity via activating the Nrf2/HO-1 pathway. Phytother. Res. 2017, 31, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Nicolussi, S.; Viveros-Paredes, J.M.; Gachet, M.S.; Rau, M.; Flores-Soto, M.E.; Blunder, M.; Gertsch, J. Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice. Pharmacol. Res. 2014, 80, 52–65. [Google Scholar] [CrossRef]
- Reynoso-Moreno, I.; Najar-Guerrero, I.; Escareno, N.; Flores-Soto, M.E.; Gertsch, J.; Viveros-Paredes, J.M. An endocannabinoid uptake inhibitor from black pepper exerts pronounced anti-inflammatory effects in mice. J. Agric. Food Chem. 2017, 65, 9435–9442. [Google Scholar] [CrossRef]
- Mad-Adam, N.; Rattanaburee, T.; Tanawattanasuntorn, T.; Graidist, P. Effects of trans-(±)-kusunokinin on chemosensitive and chemoresistant ovarian cancer cells. Oncol. Lett. 2022, 23, 59. [Google Scholar] [CrossRef]
- Tanawattanasuntorn, T.; Rattanaburee, T.; Thongpanchang, T.; Graidist, P. Trans-(±)-kusunokinin binding to AKR1B1 inhibits oxidative stress and proteins involved in migration in aggressive breast cancer. Antioxidants 2022, 11, 2347. [Google Scholar] [CrossRef]
- Rattanaburee, T.; Sermmai, P.; Tangthana-umrung, K.; Thongpanchang, T.; Graidist, P. Anticancer activity of (±)-kusunokinin derivatives towards cholangiocarcinoma cells. Molecules. 2022, 27, 8291. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, K.U.; Terezan, A.P.; Martins, D.C.; Freitas, T.G.; Fernandes, J.B.; Da Silva, M.F.D.G.F.; Vieira, P.C.; Pagnocca, F.C.; Bueno, O.C. Evaluation of the toxicity of Virola sebifera crude extracts, fractions and isolated compounds on the nest of leaf-cutting ants. Psyche 2012, 2012, 785424. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Mondal, A.K.; Banerjee, D. Traditional phytotherapeutic uses in Purba Medinipur, West Bengal, India. Int. J. Pharm. Sci. Res. 2017, 8, 3904–3910. [Google Scholar]
- Reddy, B.S.; Maeura, Y. Tumor promotion of dietary fat in azoxymethane-induced colon carcinogenesis in female F 344 rats. J. Natl. Cancer Inst. 1984, 72, 745–750. [Google Scholar]
- Cohen, L.A.; Thompson, D.O.; Maeura, Y.; Choi, K.; Blank, M.E.; Rose, D.P. Dietary fat and mammary cancer. Promoting effects of different dietary fats on N-nitrosomethylurea- induced rat mammary tumorigenesis. J. Natl. Cancer Inst. 1986, 77, 33–42. [Google Scholar] [PubMed]
- Law, K.S.; Azman, N.; Omar, E.A.; Musa, M.Y.; Yusoff, N.M.; Sulaiman, S.A.; Hussain, N.H. The effects of virgin coconut oil (VCO) as supplementation on quality of life (QOL) among breast cancer patients. Lipids Health Dis. 2014, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Isidorow, W.; Witkowski, S.; Iwaniuk, P.; Zambrzycka, M.; Swiecicka, I. Royal jelly aliphatic acids contribute to antimicrobial activity of honey. J. Apic. Sci. 2018, 62, 111–123. [Google Scholar] [CrossRef]
- Kunat-Budzyńska, M.; Rysiak, A.; Wiater, A.; Grąz, M.; Andrejko, M.; Budzyński, M.; Bryś, M.S.; Sudziński, M.; Tomczyk, M.; Gancarz, M.; et al. Chemical composition and antimicrobial activity of new honey varietals. Int. J. Environ. Res. Public Health 2023, 20, 2458. [Google Scholar] [CrossRef]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic. Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive effect of curcumin against chemotherapy-induced side-effects. Front. Pharm. 2018, 9, 1374. [Google Scholar] [CrossRef]
- Morris, R.M.; Mortimer, T.O.; O’Neill, K.L. Cytokines: Can cancer get the message? Cancers 2022, 14, 2178. [Google Scholar] [CrossRef] [PubMed]
- Gaggianesi, M.; Turdo, A.; Chinnici, A.; Lipari, E.; Apuzzo, T.; Benfante, A.; Sperduti, I.; Di Franco, S.; Meraviglia, S.; Lo Presti, E.; et al. IL4 primes the dynamics of breast cancer progression via DUSP4 inhibition. Cancer Res. 2017, 77, 3268–3279. [Google Scholar] [CrossRef] [PubMed]
- Joimel, U.; Gest, C.; Soria, J.; Pritchard, L.L.; Alexandre, J.; Laurent, M.; Blot, E.; Cazin, L.; Vannier, J.P.; Varin, R.; et al. Stimulation of angiogenesis resulting from cooperation between macrophages and MDA-MB-231 breast cancer cells: Proposed molecular mechanism and effect of tetrathiomolybdate. BMC Cancer 2010, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Shiri, S.; Alizadeh, A.M.; Baradaran, B.; Farhanghi, B.; Shanehbandi, D.; Khodayari, S.; Khodayari, H.; Tavassoli, A. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac. J. Cancer Prev. 2015, 16, 3917–3922. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Akinbami, A.; Popoola, A.; Adediran, A.; Dosunmu, A.; Oshinaike, O.; Adebola, P.; Ajibola, S. Full blood count pattern of pre-chemotherapy breast cancer patients in Lagos, Nigeria. Casp. J. Intern. Med. 2013, 4, 574–579. [Google Scholar]
- Semler, O.; Partsch, C.J.; Das, A.M.; Prechtl, A.; Grasemann, C. Cross-sectional analysis: Clinical presentation of children with persistently low ALP levels. J. Pediatr. Endocrinol. Metab. 2021, 34, 1559–1566. [Google Scholar] [CrossRef]
- Aspartate Aminotransferase (AST) Test. Available online: https://www.healthlinkbc.ca/tests-treatments-medications/medical-tests/aspartate-aminotransferase-ast (accessed on 9 March 2023).
- Martinel Lamas, D.J.; Nicoud, M.B.; Sterle, H.A.; Carabajal, E.; Tesan, F.; Perazzo, J.C.; Cremaschi, G.A.; Rivera, E.S.; Medina, V.A. Selective cytoprotective effect of histamine on doxorubicin-induced hepatic and cardiac toxicity in animal models. Cell Death Discov. 2015, 1, 15059. [Google Scholar] [CrossRef]
- Zhu, S.; Waguespack, M.; Barker, S.A.; Li, S. Doxorubicin directs the accumulation of interleukin-12 induced IFN gamma into tumors for enhancing STAT1 dependent antitumor effect. Clin. Cancer Res. 2007, 13, 4252–4260. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta 2014, 1845, 84–89. [Google Scholar] [CrossRef]
- Mödinger, Y.; Knaub, K.; Dharsono, T.; Wacker, R.; Meyrat, R.; Land, M.H.; Petraglia, A.L.; Schön, C. Enhanced oral bioavailability of β-caryophyllene in healthy subjects using the VESIsorb® formulation technology, a novel self-emulsifying drug delivery system (SEDDS). Molecules 2022, 27, 2860. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, M.; Gupta, E.D.; Ghosh, P.C. Piperine loaded PEG-PLGA nanoparticles: Preparation, characterization and targeted delivery for adjuvant breast cancer chemotherapy. J. Drug. Deliv. Sci. Technol. 2015, 29, 269–282. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential microwave-ultrasound-assisted extraction for isolation of piperine from black pepper (Piper nigrum L.). Food Sci. Biotechnol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Hirsch, H.A.; Struhl, K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009, 139, 693–706. [Google Scholar] [CrossRef]
- Sullivan, N.J.; Sasser, A.K.; Axel, A.E.; Vesuna, F.; Raman, V.; Ramirez, N.; Oberyszyn, T.M.; Hall, B.M. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009, 28, 2940–2947. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Ostermann, M.; Joannidis, M. Acute kidney injury 2016: Diagnosis and diagnostic workup. Crit. Care 2016, 20, 299. [Google Scholar] [CrossRef]

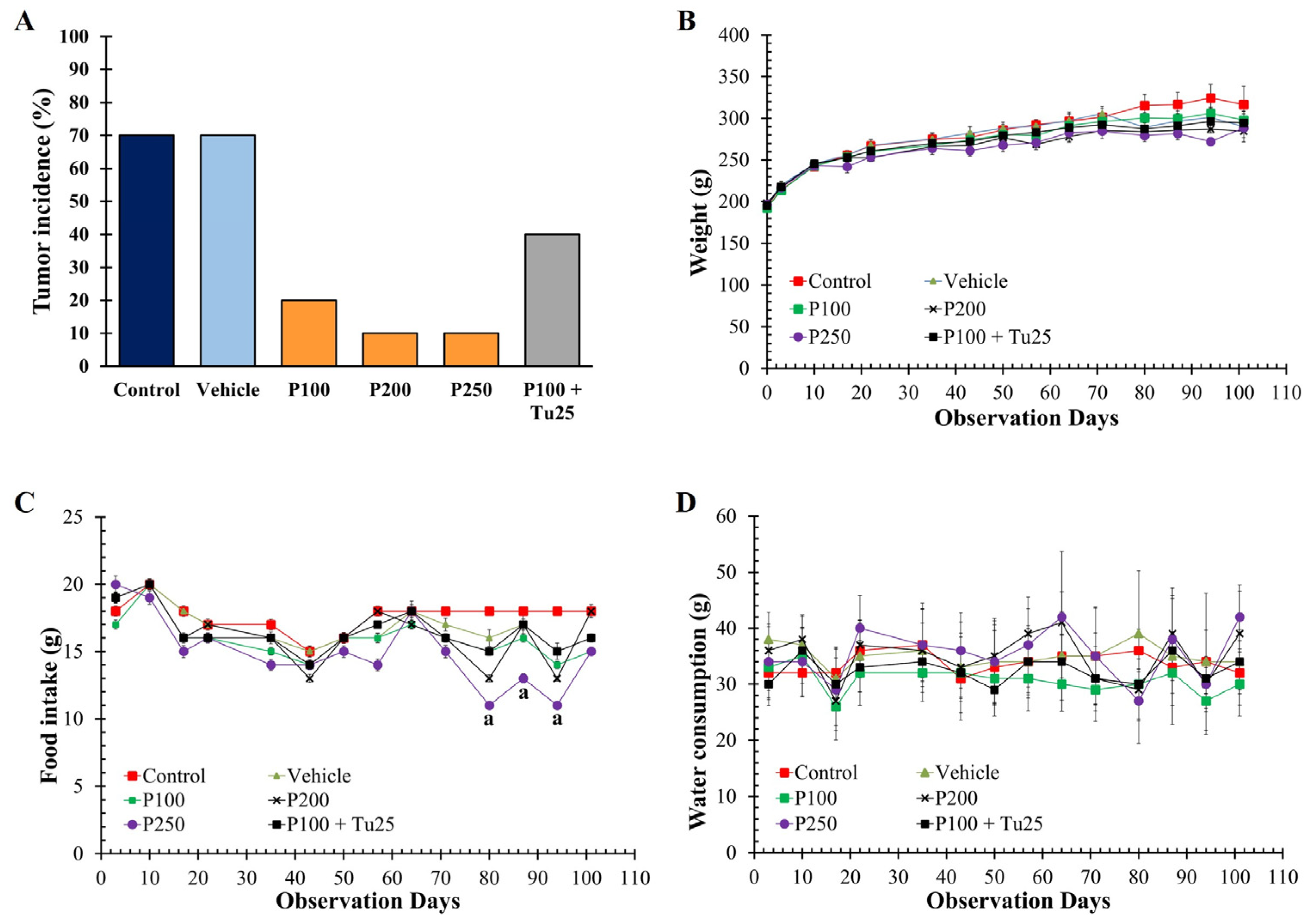
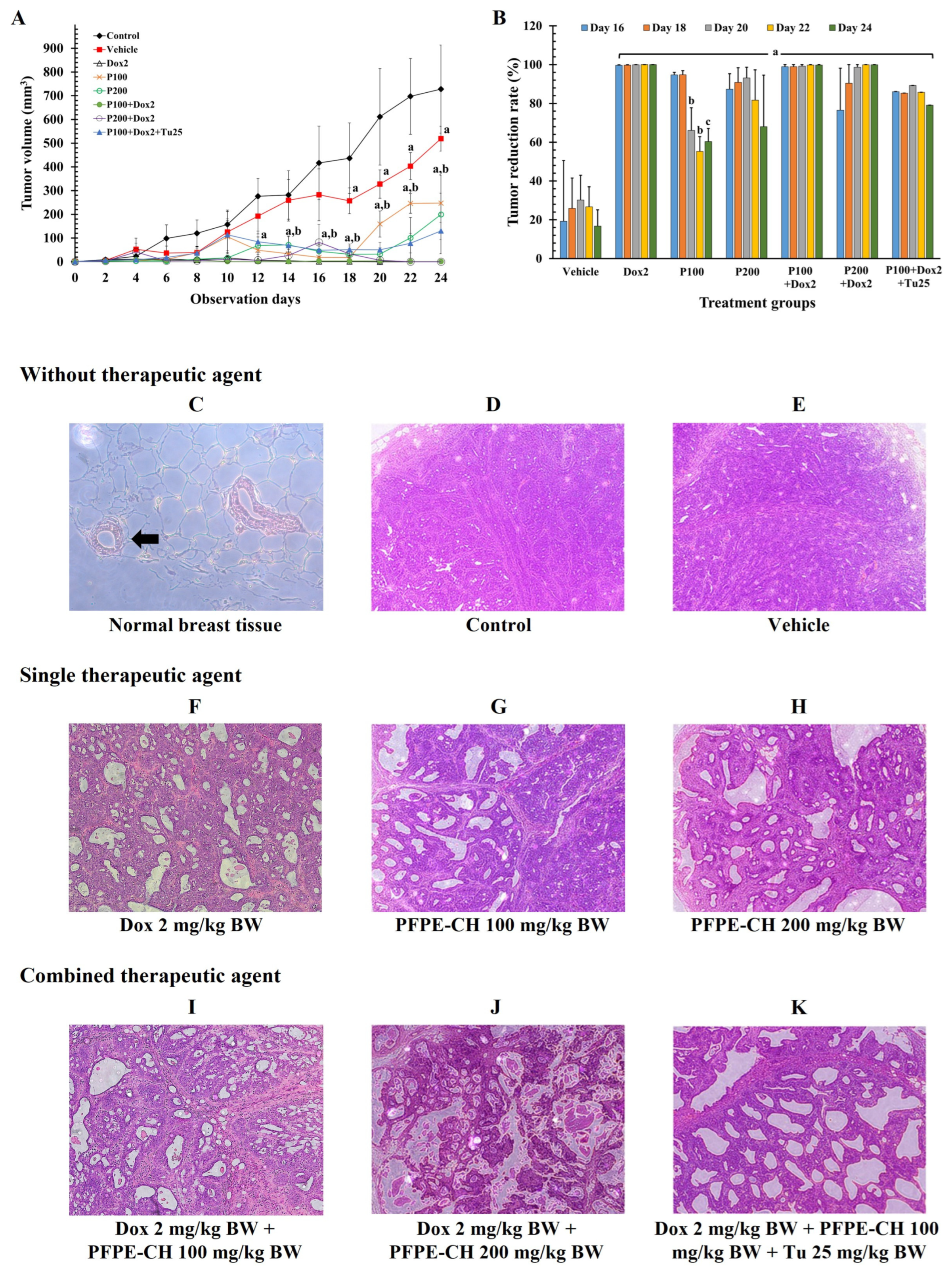
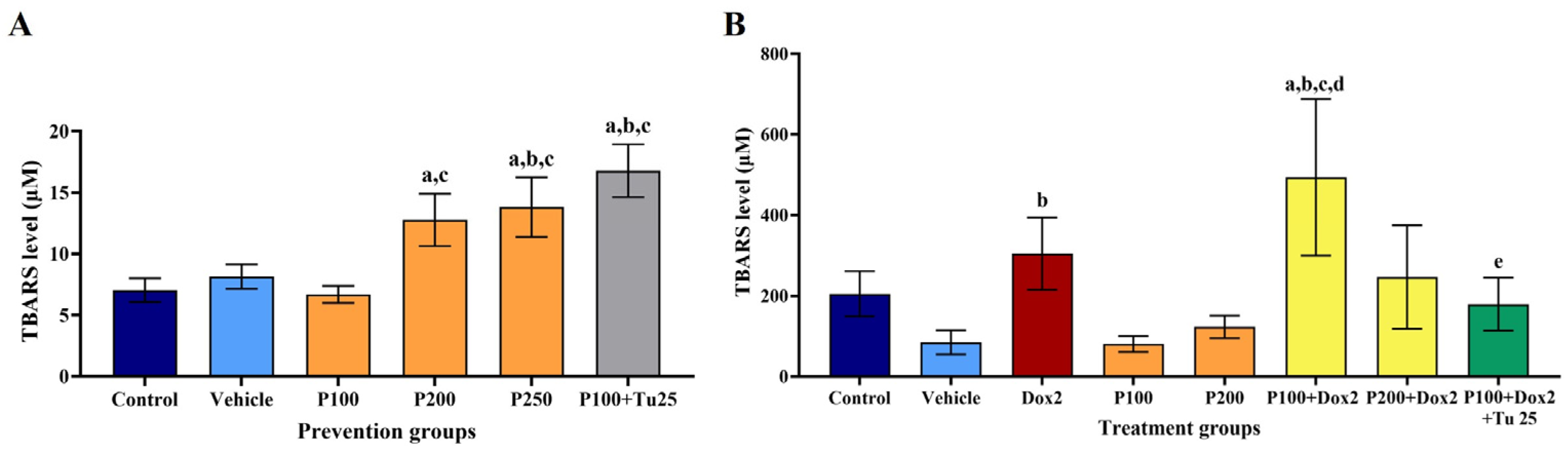
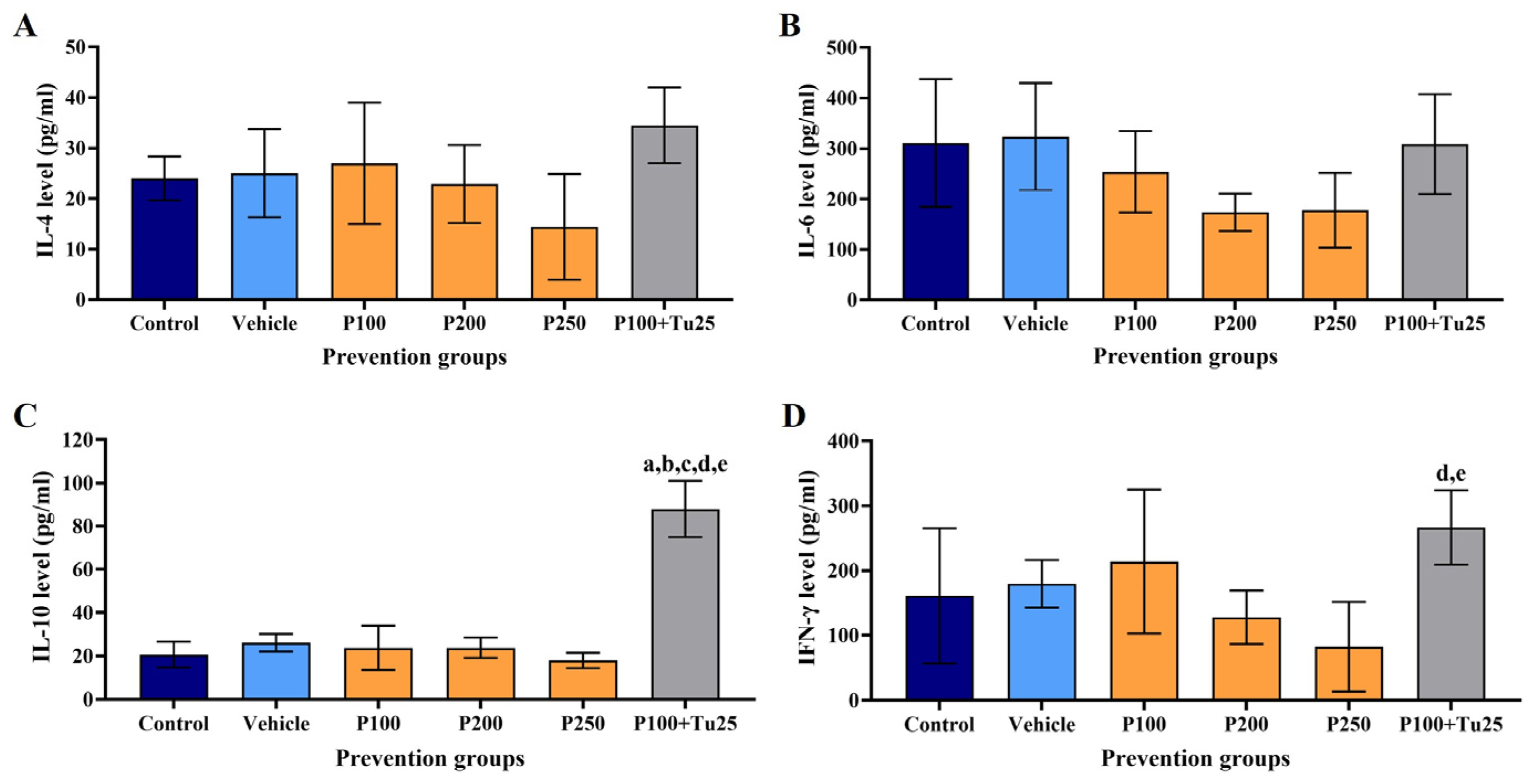
| Parameters | Control | Vehicle | PFPE-CH 100 | PFPE-CH 200 | PFPE-CH 250 | PFPE-CH 100+ Tu 25 |
|---|---|---|---|---|---|---|
| Body weight (g) | 307.90 ± 8.34 | 305.40 ± 8.37 | 302.40 ± 6.77 | 297.00 ± 6.50 | 289.86 ± 10.68 | 302.60 ± 3.70 |
| Organ weight/body weight ratio | ||||||
| Heart | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.28 ± 0.00 | 0.31 ± 0.01 | 0.33 ± 0.01 | 0.29 ± 0.01 |
| Kidney | 0.63 ± 0.02 | 0.62 ± 0.02 | 0.61 ± 0.02 | 0.64 ± 0.01 | 0.64 ± 0.02 | 0.67 ± 0.02 |
| Liver | 3.45 ± 0.03 | 3.58 ± 0.19 | 3.33 ± 0.11 | 3.73 ± 0.12 | 4.08 ± 0.10 | 3.52 ± 0.12 |
| Lung | 0.43 ± 0.01 | 0.42 ± 0.01 | 0.40 ± 0.01 | 0.45 ± 0.01 | 0.46 ± 0.02 | 0.41 ± 0.01 |
| Spleen | 0.18 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.19 ± 0.01 |
| Stomach | 0.42 ± 0.02 | 0.47 ± 0.03 | 0.45 ± 0.02 | 0.51 ± 0.02 | 0.51 ± 0.01 | 0.46 ± 0.02 |
| Hematologic values | ||||||
| White blood cells (×103/µL) | 3.38 ± 0.22 | 3.51 ± 0.16 | 3.54 ± 0.27 | 3.33 ± 0.25 | 3.01 ± 0.35 | 3.81 ± 0.23 |
| Neutrophil (%) | 30.80 ± 1.18 | 37.80 ± 3.65 | 31.0 ± 2.45 | 29.0 ± 3.21 | 46.42 ± 4.09 a | 32.60 ± 3.23 |
| Lymphocyte (%) | 67.80 ± 1.24 | 61.60 ± 3.83 | 69.60 ± 3.11 | 67.14 ± 5.56 | 53.57 ± 4.09 a | 67.20 ± 3.27 |
| Monocyte (%) | 0.90 ± 0.50 | 0.20 ± 0.13 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Eosinophil (%) | 0.50 ± 0.27 | 0.40 ± 0.27 | 0.00 ± 0.00 | 0.57 ± 0.57 | 0.00 ± 0.00 | 0.20 ± 0.20 |
| Basophil (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Red blood cell (×106/µL) | 7.77 ± 0.27 | 7.89 ± 0.22 | 7.35 ± 0.23 | 7.44 ± 0.16 | 6.91 ± 0.41 a,b | 7.58 ± 0.17 |
| MCV (fL) | 54.40 ± 0.56 | 52.20 ± 0.47 a | 53.30 ± 0.45 | 52.71 ± 0.61 a | 53.86 ± 0.77 | 53.10 ± 0.43 |
| MCH (pg) | 19.11 ± 0.35 | 18.90 ± 0.23 | 19.30 ± 0.21 | 18.67 ± 0.21 | 20.86 ± 0.55 | 19.37 ± 0.46 |
| MCHC (g/dL) | 34.10 ± 1.73 | 36.60 ± 0.64 | 36.50 ± 0.37 | 33.57 ± 1.97 | 38.43 ± 1.13 | 33.20 ± 2.22 |
| Hemoglobin (g/dL) | 15.09 ± 0.60 | 14.93 ± 0.36 | 14.19 ± 0.45 | 14.30 ± 0.37 | 14.23 ± 0.67 | 14.79 ± 0.13 |
| Hematocrit (%) | 41.70 ± 1.73 | 40.70 ± 1.16 | 38.50 ± 1.20 | 38.86 ± 0.91 | 36.86 ± 1.97 a | 39.60 ± 0.81 |
| Platelet (×103/µL) | 748.60 ± 33.60 | 754.70 ± 26.39 | 750.20 ± 25.79 | 783.29 ± 46.05 | 748.43 ± 25.53 | 795.40 ± 19.84 |
| Clinical blood chemistry values | ||||||
| BUN (mg/dL) | 26.43 ± 1.45 | 28.03 ± 2.05 | 31.53 ± 1.43 | 31.71 ± 2.09 a | 34.61 ± 2.29 a,b | 31.89 ± 2.67 |
| Creatinine (mg/dL) | 0.66 ± 0.04 | 0.77 ± 0.03 | 0.85 ± 0.04 | 0.66 ± 0.05 | 0.83 ± 0.05 a | 0.73 ± 0.07 |
| Total protein (g/dL) | 6.36 ± 0.16 | 6.58 ± 0.19 | 6.56 ± 0.16 | 6.53 ± 0.14 | 6.27 ± 0.14 | 6.46 ± 0.16 |
| Albumin (g/dL) | 4.16 ± 0.11 | 4.30 ± 0.09 | 4.29 ± 0.06 | 4.24 ± 0.10 | 4.23 ± 0.11 | 4.25 ± 0.08 |
| Total bilirubin (mg/dL) | 0.35 ± 0.06 | 0.31 ± 0.07 | 0.39 ± 0.06 | 0.45 ± 0.14 | 0.53 ± 0.12 | 0.52 ± 0.09 |
| SGOT (U/L) | 101.60 ± 5.95 | 104.90 ± 6.20 | 94.20 ± 6.72 | 84.14 ± 7.80 a,b | 99.14 ± 5.98 | 105.90 ± 7.10 |
| SGPT (U/L) | 44.44 ± 2.56 | 44.10 ± 1.47 | 41.10 ± 2.02 | 43.00 ± 1.88 | 40.29 ± 1.76 | 38.90 ± 2.10 |
| Alkaline phosphatase (U/L) | 66.63 ± 5.68 | 70.80 ± 7.63 | 80.88 ± 4.15 | 68.50 ± 4.19 | 39.71 ± 5.32 a | 74.28 ± 10.02 |
| Parameters | Control | Vehicle | Doxorubicin | PFPE100 | PFPE200 | Dox+ PFPE100 | Dox+ PFPE200 | Dox+ PFPE100+ Tumeric25 |
|---|---|---|---|---|---|---|---|---|
| Body weight (g) | 283.50 ± 8.71 | 255.33 ± 3.20 | 251.40 ± 5.82 | 277.67 ± 7.52 | 258.83 ± 3.79 | 249.33 ± 9.05 | 265.50 ± 9.38 | 249.00 ± 14.13 |
| Organ weight/body weight ratio | ||||||||
| Heart | 0.33 ± 0.01 | 0.33 ± 0.01 | 0.33 ± 0.02 | 0.31 ± 0.01 | 0.33 ± 0.01 | 0.29 ± 0.01 | 0.31 ± 0.01 | 0.32 ± 0.02 |
| Kidney | 0.60 ± 0.02 | 0.64 ± 0.01 | 0.67 ± 0.02 | 0.62 ± 0.01 | 0.64 ± 0.01 | 0.69 ± 0.01 | 0.69 ± 0.03 | 0.67 ± 0.04 |
| Liver | 2.80 ± 0.12 | 3.07 ± 0.09 | 3.69 ± 0.11 a | 3.21 ± 0.07 | 3.28 ± 0.05 | 4.27 ± 0.16 a | 4.16 ± 0.09 a | 3.55 ± 0.10 |
| Lung | 0.37 ± 0.01 | 0.41 ± 0.01 | 0.39 ± 0.01 | 0.38 ± 0.02 | 0.45 ± 0.04 | 0.40 ± 0.02 | 0.39 ± 0.01 | 0.38 ± 0.02 |
| Spleen | 0.27 ± 0.01 | 0.29 ± 0.02 | 0.17 ± 0.05 a | 0.27 ± 0.01 | 0.25 ± 0.00 | 0.27 ± 0.05 | 0.20 ± 0.04 | 0.12 ± 0.02 a |
| Stomach | 0.46 ± 0.01 | 0.50 ± 0.01 | 0.42 ± 0.03 | 0.46 ± 0.01 | 0.52 ± 0.02 | 0.52 ± 0.03 | 0.55 ± 0.05 | 0.45 ± 0.01 |
| Hematologic values | ||||||||
| White blood cells (×103/µL) | 3.72 ± 0.16 | 4.40 ± 0.54 | 1.23 ± 0.41 a,b | 3.33 ± 0.16 b,c | 3.22 ± 0.14 b,c | 2.13 ± 1.05 a,b | 1.50 ± 1.53 a,b | 1.08 ± 0.37 a,b |
| Neutrophil (%) | 28.00 ± 2.79 | 42.83 ± 4.85 | 50.40 ± 9.53 a | 45.20 ± 8.65 | 36.17 ± 8.57 | 55.17 ± 6.28 a | 60.67 ± 7.60 a | 51.33 ± 4.32 a |
| Lymphocyte (%) | 71.83 ± 2.56 | 59.00 ± 5.84 | 49.20 ± 9.71 | 47.17 ± 8.14 | 60.67 ± 8.89 | 37.83 ± 6.55 a,b | 43.60 ± 6.82 | 47.33 ± 4.32 |
| Monocyte (%) | 0.17 ± 0.18 | 0.83 ± 0.29 | 0.00 ± 0.00 | 0.83 ± 0.41 | 0.33 ± 0.37 | 0.80 ± 0.37 | 0.67 ± 0.42 | 1.33 ± 0.71 |
| Eosinophil (%) | 0.83 ± 0.40 | 0.67 ± 0.42 | 0.00 ± 0.00 | 1.50 ± 0.81 | 2.17 ± 0.48 a,b,c | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.25 ± 0.25 |
| Basophil (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Red blood cell (×106/µL) | 7.13 ± 0.35 | 7.92 ± 0.32 | 5.17 ± 1.13 a,b | 7.40 ± 0.25 c | 7.41 ± 0.35 c | 5.53 ± 1.13 b | 5.55 ± 0.20 a,b | 4.62 ± 1.13 a,b |
| Hemoglobin (g/dL) | 13.82 ± 0.55 | 14.38 ± 0.43 | 9.72 ± 2.00 a,b | 13.97 ± 0.19 | 13.97 ± 0.63 | 10.47 ± 2.21 a,b | 10.68 ± 0.41 a,b | 9.62 ± 1.37 a,b |
| Hematocrit (%) | 36.50 ± 1.71 | 40.17 ± 1.48 | 26.00 ± 3.11 b | 37.00 ± 1.29 | 37.17 ± 1.70 | 31.33 ± 5.02 | 27.00 ± 1.24 b | 26.67 ± 3.76 b |
| MCV (fL) | 51.17 ± 0.48 | 50.83 ± 0.31 | 49.00 ± 0.58 a,b | 50.33 ± 0.42 | 50.17 ± 0.31 | 50.33 ± 1.20 | 50.25 ± 0.63 | 49.00 ± 1.08 |
| MCH (pg) | 19.00 ± 0.37 | 17.83 ± 0.31 a | 18.67 ± 0.33 | 18.50 ± 0.56 | 18.33 ± 0.33 | 18.33 ± 0.33 | 18.75 ± 0.25 | 18.75 ± 0.63 |
| MCHC (g/dL) | 37.50 ± 0.76 | 35.00 ± 0.58 a | 37.67 ± 0.33 b | 36.83 ± 1.08 | 36.67 ± 0.42 | 37.00 ± 0.58 | 37.75 ± 0.75 | 38.00 ± 1.08 |
| Platelet (×103/µL) | 689.33 ± 44.85 | 497.67 ± 73.36 | 70.66 ± 512.47 a,b | 515.00 ± 67.45 c | 640.50 ± 51.83 c | 216.50 ± 95.50 a,b | 298.75 ± 46.69 a,b | 118.33 ± 38.40 a,b |
| Clinical blood chemistry values | ||||||||
| BUN (mg/dL) | 22.83 ± 1.25 | 21.17 ± 1.38 | 34.00 ± 7.64 b | 20.67 ± 1.48 c | 19.83 ± 0.87 c | 17.67 ± 3.28 c | 15.75 ± 1.18 c | 20.00 ± 5.00 |
| Creatinine (mg/dL) | 0.66 ± 0.10 | 0.63 ± 0.04 | 0.51 ± 0.16 | 0.68 ± 0.06 | 0.67 ± 0.04 | 0.58 ± 0.05 | 0.49 ± 0.04 | 0.54 ± 0.06 |
| Total protein (g/dL) | 6.18 ± 0.19 | 6.05 ± 0.20 | 4.37 ± 0.43 a | 6.00 ± 0.14 c | 6.03 ± 0.15 c | 4.70 ± 0.35 a | 4.55 ± 0.17 a | 4.88 ± 0.21 a |
| Albumin (g/dL) | 3.52 ± 0.10 | 3.63 ± 0.09 | 2.73 ± 0.37 a | 3.67 ± 0.08 | 3.73 ± 0.07 | 2.40 ± 0.21 a | 2.80 ± 0.12 a | 2.95 ± 0.12 a |
| Total bilirubin (mg/dL) | 0.30 ± 0.07 | 0.30 ± 0.04 | 0.42 ± 0.07 | 0.26 ± 0.04 | 0.48 ± 0.04 a,b | 0.50 ± 0.07 | 0.38 ± 0.07 | 0.42 ± 0.06 |
| SGOT (U/L) | 124.00 ± 15.14 | 149.33 ± 9.50 | 147.67 ± 38.05 | 161.00 ± 23.32 | 148.83 ± 10.07 | 109.00 ± 47.90 | 119.50 ± 27.64 | 124.75 ± 15.90 |
| SGPT (U/L) | 51.83 ± 5.07 | 50.33 ± 2.62 | 34.00 ± 10.41 a | 58.67 ± 6.16 | 50.17 ± 3.24 | 36.00 ± 6.00 | 42.00 ± 5.12 | 37.75 ± 7.26 |
| ALP (U/L) | 117.50 ± 9.13 | 216.50 ± 29.18 a | 30.67 ± 13.97 a,b | 164.00 ± 11.81 | 112.20 ± 13.69 b | 114.50 ± 19.78 b | 59.53 ± 12.86 b | 66.00 ± 9.54 b |
| Parameters | Control | Vehicle | PFPE43 | PFPE86 | PFPE108 | PFPE43 + Tumeric11 |
|---|---|---|---|---|---|---|
| Body weight (g) | 313.30 ± 6.05 | 312.80 ± 6.35 | 314.44 ± 4.18 | 313.12 ± 4.18 | 309.71 ± 2.70 | 316.35 ± 5.03 |
| Organ weight/body weight ratio | ||||||
| Heart | 0.30 ± 0.01 | 0.32 ± 0.01 | 0.31 ± 0.00 | 0.32 ± 0.01 | 0.35 ± 0.01 | 0.32 ± 0.00 |
| Kidney | 0.60 ± 0.01 | 0.59 ± 0.01 | 0.59 ± 0.01 | 0.60 ± 0.02 | 0.59 ± 0.01 | 0.61 ± 0.01 |
| Liver | 2.86 ± 0.07 | 2.83 ± 0.07 | 2.90 ± 0.04 | 3.11 ± 0.03 | 3.17 ± 0.05 | 2.96 ± 0.04 |
| Lung | 0.42 ± 0.02 | 0.42 ± 0.03 | 0.38 ± 0.00 | 0.39 ± 0.01 | 0.44 ± 0.01 | 0.41 ± 0.02 |
| Spleen | 0.24 ± 0.01 | 0.24 ± 0.01 | 0.25 ± 0.00 | 0.26 ± 0.01 | 0.24 ± 0.01 | 0.25 ± 0.00 |
| Stomach | 0.49 ± 0.01 | 0.47 ± 0.01 | 0.49 ± 0.01 | 0.52 ± 0.01 | 0.54 ± 0.01 | 0.49 ± 0.01 |
| Hematologic values | ||||||
| White blood cells (×103/µL) | 4.24 ± 0.16 | 3.34 ± 0.40 | 2.79 ± 0.11 a | 2.95 ± 0.19 a | 2.81 ± 0.20 a | 3.36 ± 0.26 |
| Neutrophil (%) | 19.90 ± 1.57 | 27.60 ± 2.83 | 28.35 ± 3.14 a | 29.65 ± 1.79 a | 31.50 ± 3.36 a | 25.58 ± 1.23 |
| Lymphocyte (%) | 72.40 ± 2.11 | 64.10 ± 2.89 | 68.59 ± 3.04 | 62.00 ± 1.78 | 64.79 ± 3.31 | 67.79 ± 1.69 |
| Monocyte (%) | 6.80 ± 0.79 | 6.70 ± 0.66 | 1.29 ± 0.20 a,b | 5.59 ± 0.52 | 1.79 ± 0.21 a,b | 5.21 ± 0.77 |
| Eosinophil (%) | 1.90 ± 0.72 | 1.60 ± 0.41 | 1.76 ± 0.13 | 3.35 ± 0.52 a,b | 2.00 ± 0.18 | 1.95 ± 0.28 |
| Basophil (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Red blood cell (×106/µL) | 7.79 ± 0.22 | 7.07 ± 0.50 | 7.84 ± 0.09 | 6.69 ± 0.20 | 6.97 ± 0.25 | 7.10 ± 0.27 |
| Hemoglobin (g/dL) | 14.34 ± 0.45 | 13.49 ± 0.63 | 14.73 ± 0.15 | 13.04 ± 0.39 | 13.60 ± 0.44 | 13.58 ± 0.48 |
| Hematocrit (%) | 40.70 ± 1.11 | 37.90 ± 2.06 | 40.06 ± 0.57 | 35.88 ± 1.07 | 36.86 ± 1.20 | 37.21 ± 1.45 |
| MCV (fL) | 52.50 ± 0.39 | 54.20 ± 1.74 | 51.18 ± 0.22 | 53.76 ± 0.24 | 53.43 ± 0.47 | 52.32 ± 0.15 |
| MCH (pg) | 17.90 ± 0.17 | 18.80 ± 0.69 | 18.35 ± 0.11 | 19.06 ± 0.16 | 19.21 ± 0.19 | 18.74 ± 0.15 |
| MCHC (g/dL) | 34.20 ± 0.48 | 34.70 ± 0.50 | 35.94 ± 0.22 | 35.35 ± 0.23 | 35.79 ± 0.32 | 35.37 ± 0.50 |
| Platelet (×103/µL) | 781.60 ± 40.07 | 635.30 ± 40.19 | 721.18 ± 21.74 | 647.12 ± 24.82 | 791.07 ± 43.22 | 671.47 ± 52.17 |
| Clinical blood chemistry values | ||||||
| BUN (mg/dL) | 21.00 ± 0.73 | 21.10 ± 0.91 | 22.35 ± 0.24 | 23.41 ± 0.52 | 26.14 ± 0.73 | 24.11 ± 0.59 |
| Creatinine (mg/dL) | 0.61 ± 0.03 | 0.60 ± 0.02 | 0.67 ± 0.02 | 0.73 ± 0.02 | 0.54 ± 0.03 | 0.47 ± 0.02 a,b |
| Total protein (g/dL) | 6.59 ± 0.11 | 6.49 ± 0.11 | 6.54 ± 0.07 | 6.28 ± 0.07 | 6.42 ± 0.07 | 6.49 ± 0.06 |
| Albumin (g/dL) | 3.72 ± 0.07 | 3.87 ± 0.06 | 3.74 ± 0.03 | 3.78 ± 0.03 | 3.69 ± 0.03 | 3.81 ± 0.03 |
| Total bilirubin (mg/dL) | 0.33 ± 0.06 | 0.26 ± 0.02 | 0.30 ± 0.05 | 0.26 ± 0.04 | 0.14 ± 0.05 | 0.25 ± 0.03 |
| SGOT (U/L) | 157.80 ± 5.83 | 142.40 ± 8.13 | 165.65 ± 2.80 | 140.59 ± 8.91 | 108.21 ± 3.82 a,b | 118.32 ± 6.44 |
| SGPT (U/L) | 63.70 ± 3.82 | 61.10 ± 4.30 | 60.06 ± 3.17 | 64.41 ± 4.15 | 66.43 ± 2.58 | 60.32 ± 3.63 |
| ALP (U/L) | 98.60 ± 10.88 | 119.10 ± 32.05 | 102.06 ± 3.00 | 105.65 ± 5.89 | 142.07 ± 7.22 a | 113.74 ± 7.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mad-adam, N.; Madla, S.; Lailerd, N.; Hiransai, P.; Graidist, P. Piper nigrum Extract: Dietary Supplement for Reducing Mammary Tumor Incidence and Chemotherapy-Induced Toxicity. Foods 2023, 12, 2053. https://doi.org/10.3390/foods12102053
Mad-adam N, Madla S, Lailerd N, Hiransai P, Graidist P. Piper nigrum Extract: Dietary Supplement for Reducing Mammary Tumor Incidence and Chemotherapy-Induced Toxicity. Foods. 2023; 12(10):2053. https://doi.org/10.3390/foods12102053
Chicago/Turabian StyleMad-adam, Nadeeya, Siribhon Madla, Narissara Lailerd, Poonsit Hiransai, and Potchanapond Graidist. 2023. "Piper nigrum Extract: Dietary Supplement for Reducing Mammary Tumor Incidence and Chemotherapy-Induced Toxicity" Foods 12, no. 10: 2053. https://doi.org/10.3390/foods12102053





