Use of Inverse Method to Determine Thermophysical Properties of Minimally Processed Carrots during Chilling under Natural Convection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
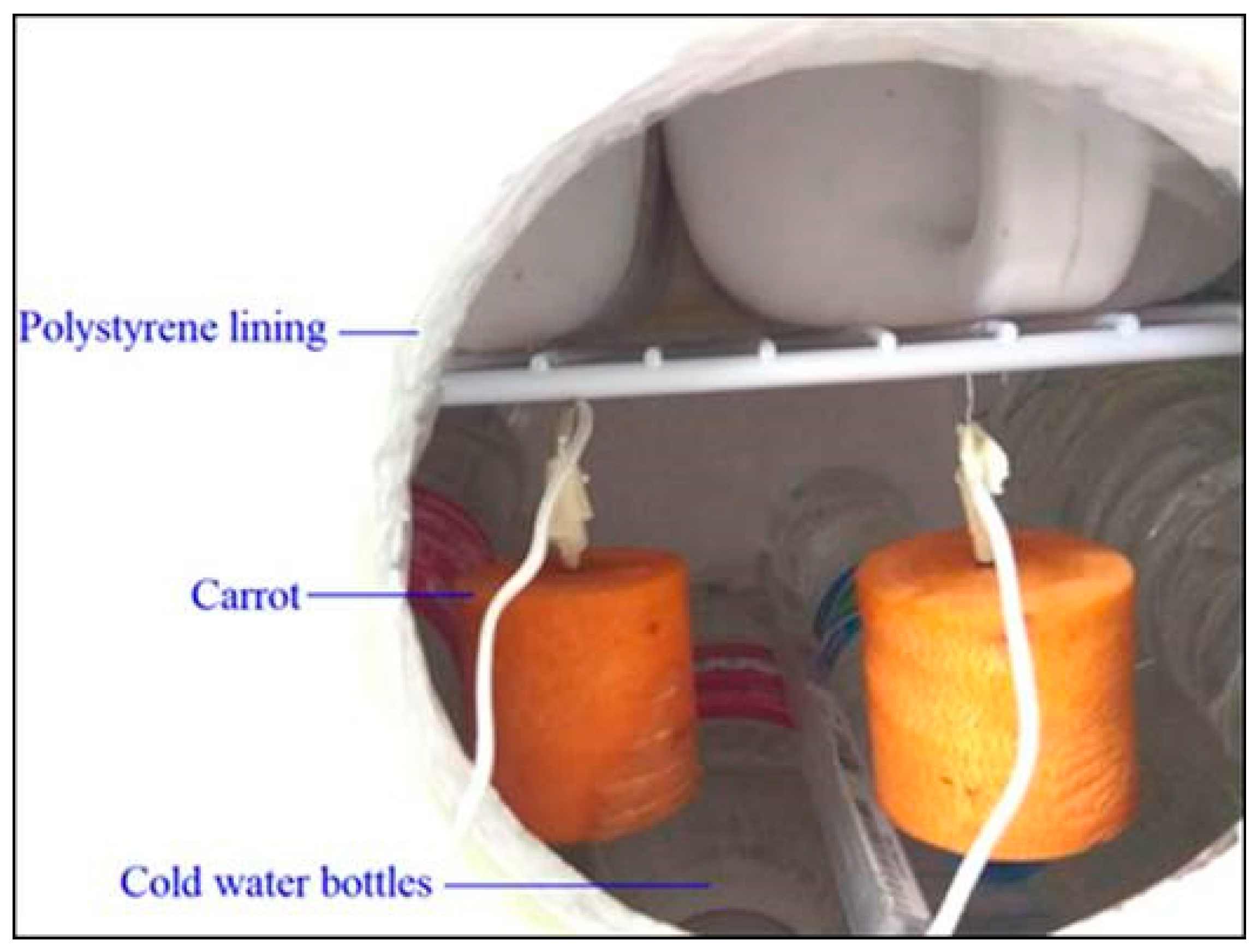
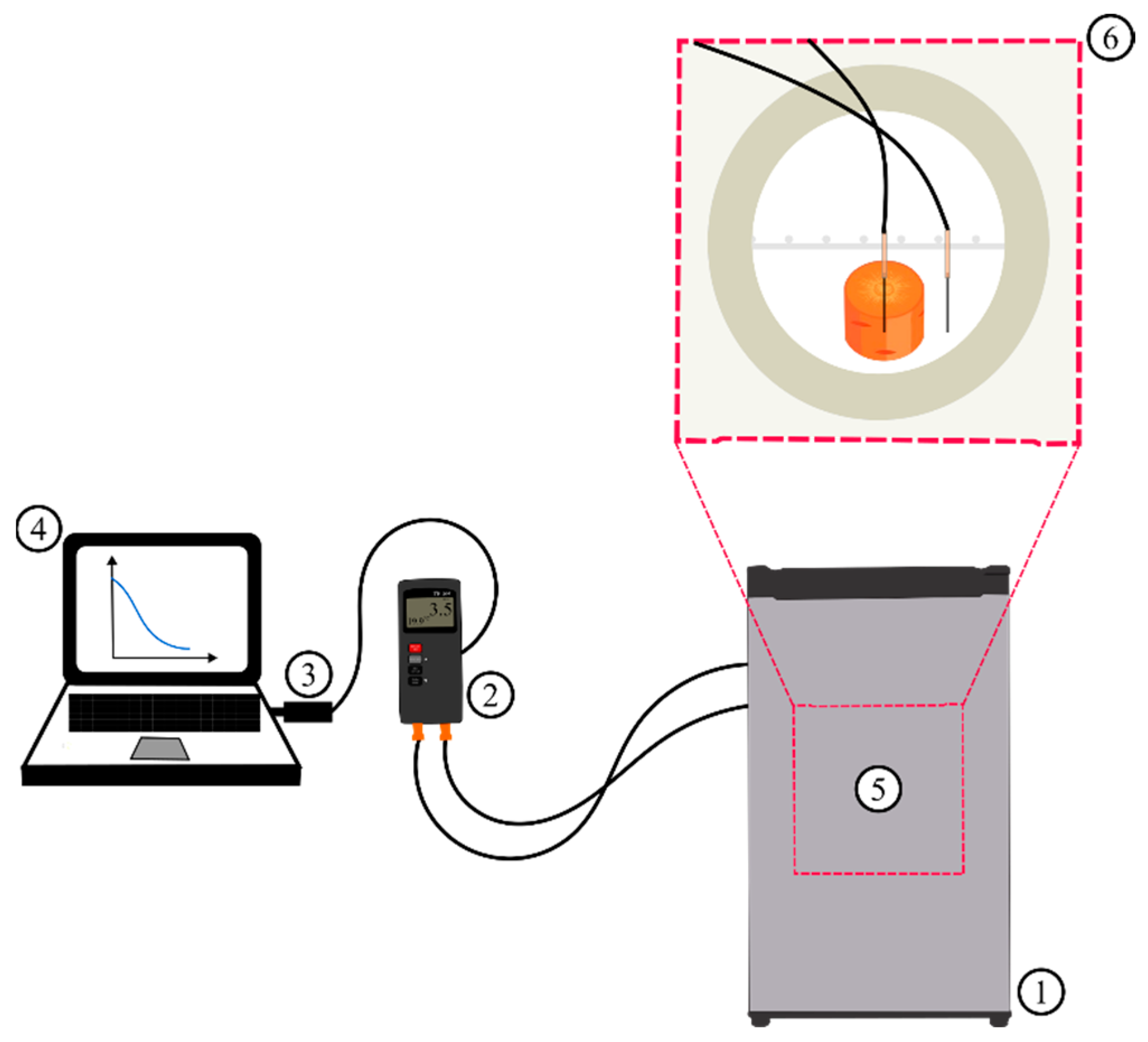
2.2. Chilling Experiment
2.3. Governing Equation
2.3.1. Initial Condition and Radial and Axial Symmetry
2.3.2. Boundary Condition
2.3.3. Analytical Solution of the Heat Conduction Equation
2.4. Proposed Heat Transfer Model for Chilling
2.4.1. Direct Problem: Solver Development
2.4.2. Inverse Problem: Simultaneous Determination of Optimal Values of α and
3. Results
3.1. Thermophysical Properties and Parameters
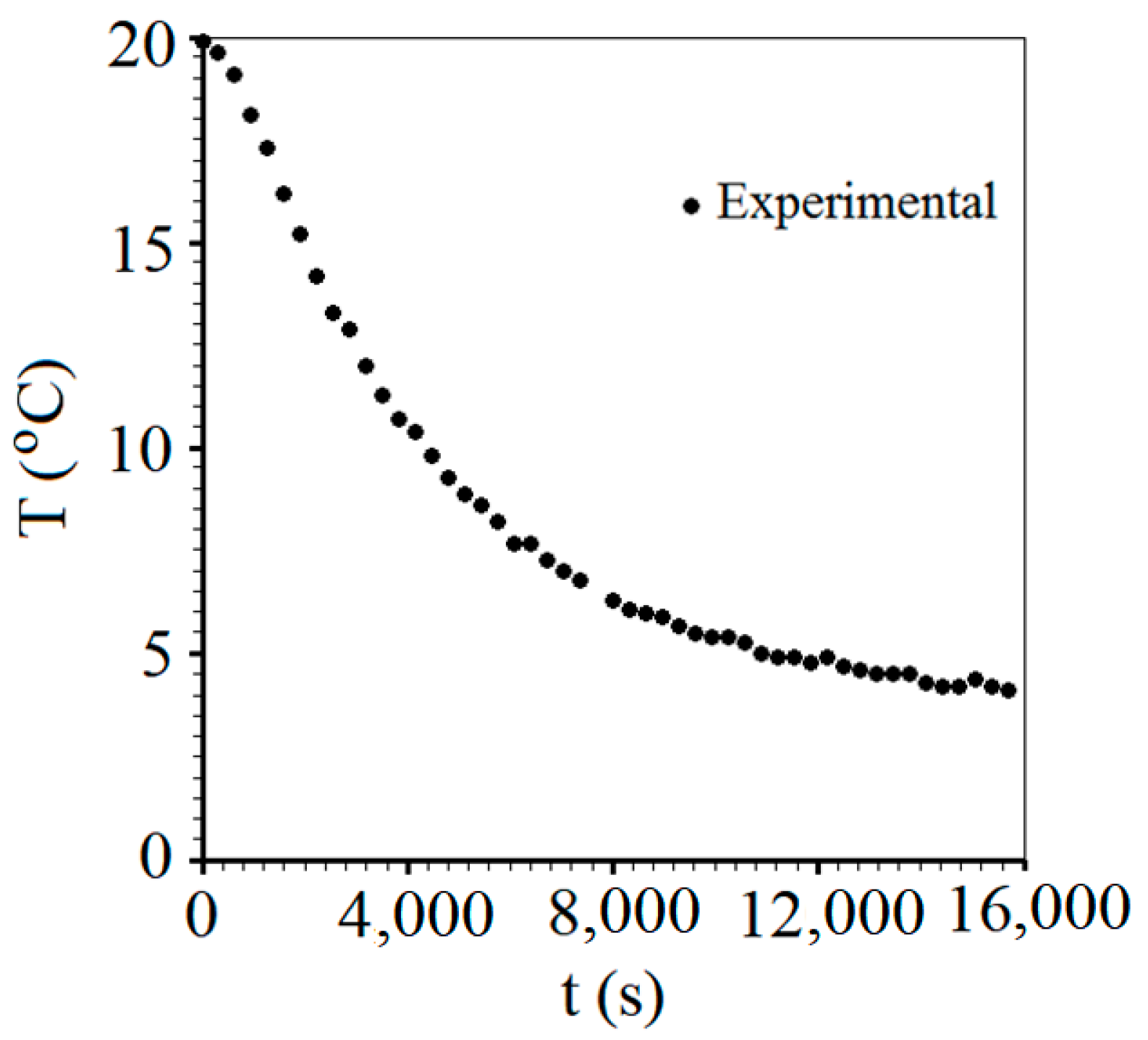
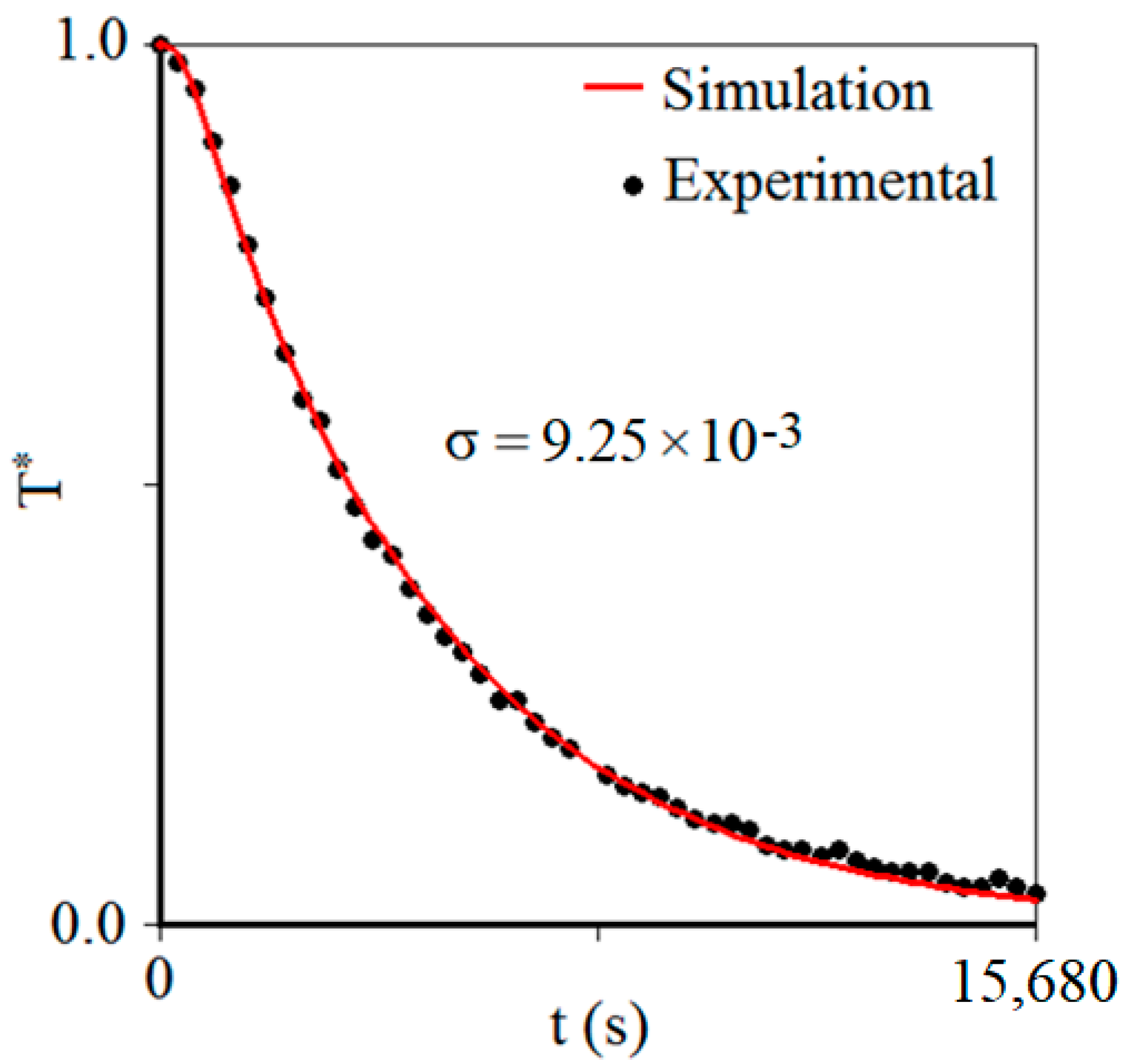
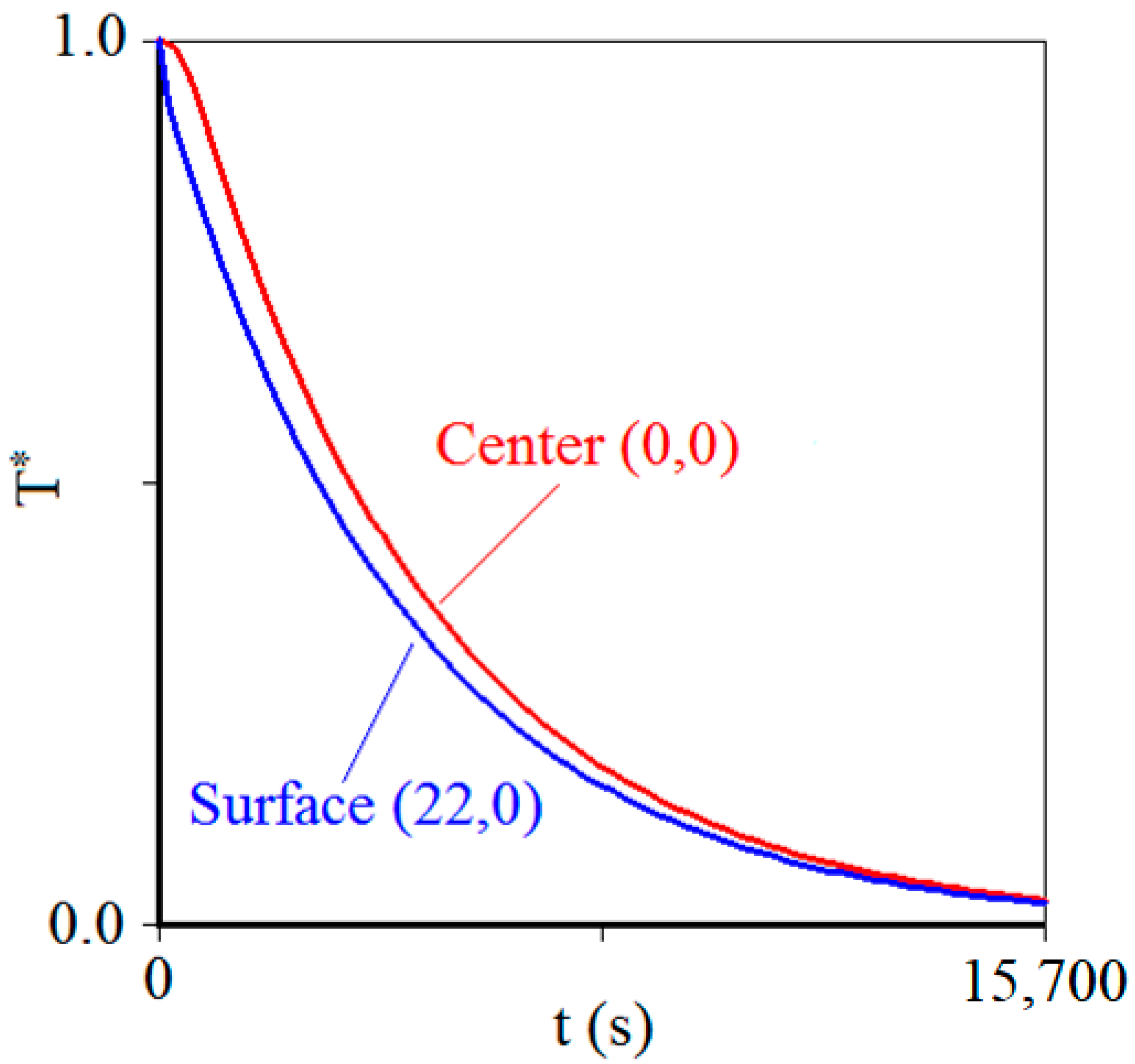
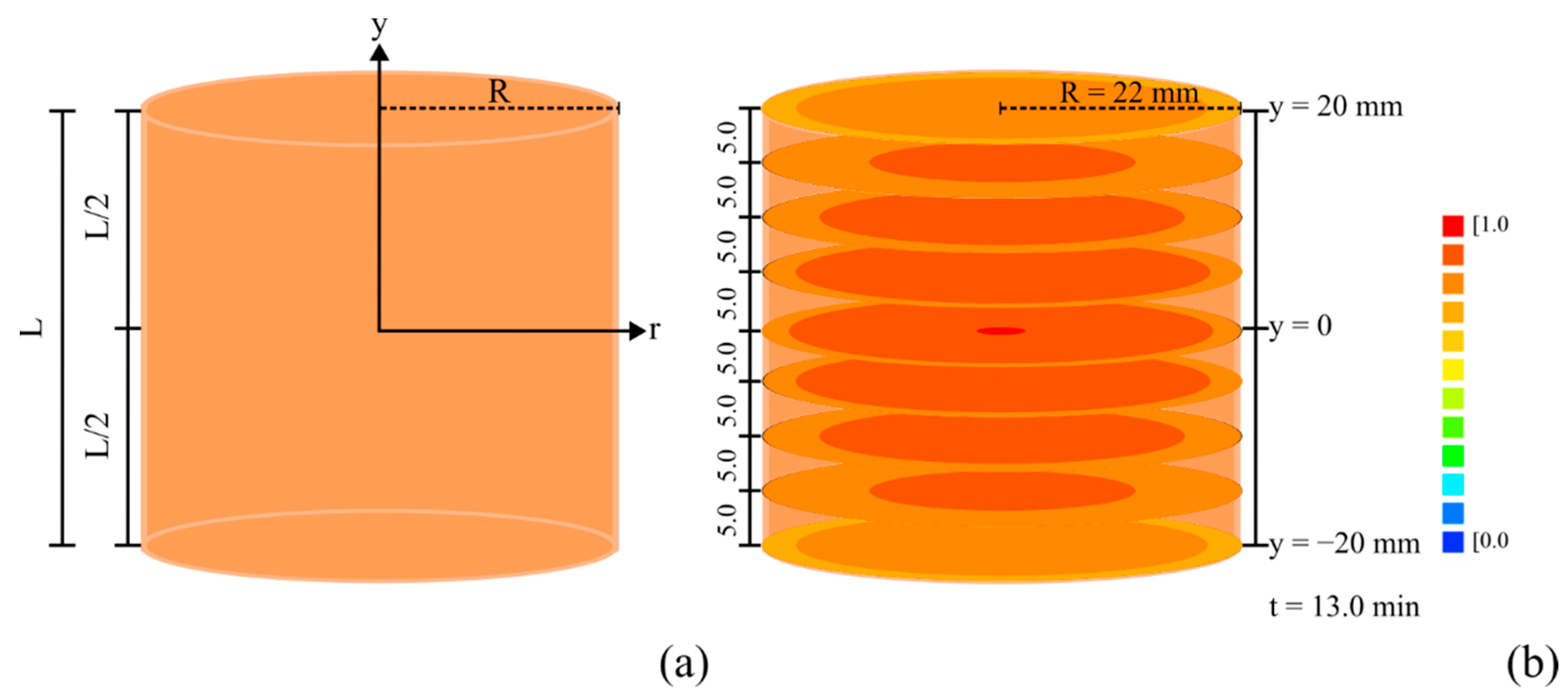
3.2. Product Chilling Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| An; Am | Coefficients of the analytical solution |
| a1; a2 | Fitting parameters |
| a10; a20 | Initial values of the fitting parameters |
| Bi1; Bi2 | Biot numbers for heat transfer (dimensionless) |
| Cp | Specific heat (J kg−1 K−1) |
| hH | Heat transfer coefficient (W m−2 K−1) |
| J | Bessel function |
| k | Thermal conductivity (W m−1 K−1) |
| L | Height of the cylinder (m) |
| M−1 | Covariance matrix |
| R | Radius of the finite cylinder (m) |
| R2 | Determination coefficient (dimensionless) |
| r | Radial position in cylindrical coordinates (m) |
| t | Time (s) |
| T | Temperature (K) |
| Teq | Equilibrium temperature (K) |
| T0 | Initial temperature (K) |
| Tsim | Temperature simulated by the solver (K) |
| Texp | Experimental value of temperature (K) |
| y | Axial position in cylindrical coordinates (m) |
| Greek symbols | |
| α | Thermal diffusivity (m2 s−1) |
| Δa1; Δa2 | Corrections of the fitting parameters |
| χ2 | Chi-square or objective function |
| μn, μm | Roots of the characteristic equations |
| ρ | Density (kg m−3) |
| σT | Uncertainty of experimental temperature (K) |
References
- FAOSTAT Statistics Division, Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data (accessed on 1 March 2023).
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of Volatiles, Phenolics, Sugars, Antioxidant Vitamins, and Sensory Quality of Different Colored Carrot Varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Czepa, A.; Hofmann, T. Quantitative Studies and Sensory Analyses on the Influence of Cultivar, Spatial Tissue Distribution, and Industrial Processing on the Bitter Off-Taste of Carrots (Daucus carota L.) and Carrot Products. J. Agric. Food Chem. 2004, 52, 4508–4514. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tian, C.; Luo, J.; Zhou, R.; Sun, X.; Ma, J. Influence of technical processing units on polyphenols and antioxidant capacity of carrot (Daucus carrot L.) juice. Food Chem. 2013, 141, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and Their Health Benefits—Review Article. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Skoczylas, Ł.; Tabaszewska, M.; Smoleń, S.; Słupski, J.; Liszka-Skoczylas, M.; Barański, R. Carrots (Daucus carota L.) Biofortified with Iodine and Selenium as a Raw Material for the Production of Juice with Additional Nutritional Functions. Agronomy 2020, 10, 1360. [Google Scholar] [CrossRef]
- Hammaz, F.; Charles, F.; Kopec, R.E.; Halimi, C.; Fgaier, S.; Aarrouf, J.; Urban, L.; Borel, P. Temperature and storage time increase provitamin A carotenoid concentrations and bioaccessibility in post-harvest carrots. Food Chem. 2021, 338, 128004. [Google Scholar] [CrossRef]
- Aubert, C.; Bruaut, M.; Chalot, G. Spatial distribution of sugars, organic acids, vitamin C, carotenoids, tocopherols, 6-methoxymellein, polyacetylenic compounds, polyphenols and terpenes in two orange Nantes type carrots (Daucus carota L.). J. Food Compos. Anal. 2022, 108, 104421. [Google Scholar] [CrossRef]
- Testa, R.; Schifani, G.; Migliore, G. Understanding Consumers’ Convenience Orientation. An Exploratory Study of Fresh-Cut Fruit in Italy. Sustainability 2021, 13, 1027. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Hussain, S. Effect of postharvest oxalic acid application on enzymatic browning and quality of lotus (Nelumbo nucifera Gaertn.) root slices. Food Chem. 2020, 312, 126051. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Li, D.; Li, L.; Luo, Z. Effect of high carbon dioxide treatment on reactive oxygen species accumulation and antioxidant capacity in fresh-cut pear fruit during storage. Sci. Hortic. 2021, 281, 109925. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Yi, Y.; Hou, W.; Wang, L.; Ai, Y.; Wang, H.; Min, T. Regulations and mechanisms of 1-methylcyclopropene treatment on browning and quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root slices. Postharvest Biol. Technol. 2022, 185, 111782. [Google Scholar] [CrossRef]
- Poubol, J.; Izumi, H. Shelf Life and Microbial Quality of Fresh-cut Mango Cubes Stored in High CO2 Atmospheres. J. Food Sci. 2005, 70, M69–M74. [Google Scholar] [CrossRef]
- Min, T.; Xie, J.; Zheng, M.; Yi, Y.; Hou, W.; Wang, L.; Ai, Y.; Wang, H. The effect of different temperatures on browning incidence and phenol compound metabolism in fresh-cut lotus (Nelumbo nucifera G.) root. Postharvest Biol. Technol. 2017, 123, 69–76. [Google Scholar] [CrossRef]
- Allong, R.; Wickham, L.; Mohammed, M. The effect of cultivar, fruit ripeness, storage temperature and duration on quality of fresh-cut mango. Acta Hortic. 2000, 509, 487–494. [Google Scholar] [CrossRef]
- Rattanapanone, N.; Lee, Y.; Wu, T.; Watada, A.E. Quality and Microbial Changes of Fresh-cut Mango Cubes Held in Controlled Atmosphere. Hortscience 2001, 36, 1091–1095. [Google Scholar] [CrossRef]
- Izumi, H.; Nagatoma, T.; Tanaka, C.; Kanlayanarat, S. Physiology and quality of fresh-cut mango is affected by low O2 controlled atmosphere storage, maturity and storage temperature. Acta Hortic. 2003, 600, 833–838. [Google Scholar] [CrossRef]
- Poubol, J.; Izumi, H. Physiology and Microbiological Quality of Fresh-cut Mango Cubes as Affected by High-O2 Controlled Atmospheres. J. Food Sci. 2006, 70, m286–m291. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Lu, Y.; Yang, Q.; Li, Y.; Deng, X.; Liu, Y.; Du, X.; Qiao, L.; Zheng, J. Effect of high oxygen pretreatment of whole tuber on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 301, 125287. [Google Scholar] [CrossRef]
- Kakiomenou, K.; Tassou, C.; Nychas, G. Microbiological, physicochemical and organoleptic changes of shredded carrots stored under modified storage. Int. J. Food Sci. Technol. 1996, 31, 359–366. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Xu, Y.; Wu, J.; Xiao, G.; Zhang, Y.; Liu, Z. Effect of dimethyl dicarbonate as disinfectant on the quality of fresh-cut carrot (Daucus carota L.). J. Food Process. Preserv. 2013, 37, 751–758. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kusznierewicz, B.; Leszczyńska, T.; Borczak, B. The Effect of Package Type on Selected Parameters of Nutritional Quality of the Chilled Stored Red Sauerkraut. J. Food Process. Preserv. 2017, 41, e13105. [Google Scholar] [CrossRef]
- Da Silva, W.P.; Mata, M.E.R.M.C.; E Silva, C.D.P.S.; Guedes, M.A.; Lima, A.G.B. Determinação da difusividade e da energia de ativação para feijão macassar (Vigna unguiculata (L.) Walp.), variedade sempre-verde, com base no comportamento da secagem. Eng. Agrícola 2008, 28, 325–333. [Google Scholar] [CrossRef]
- da Silva, W.P.; Aires, J.E.D.F.; de Castro, D.S.; Silva, C.M.D.P.D.S.E.; Gomes, J.P. Numerical description of guava osmotic dehydration including shrinkage and variable effective mass diffusivity. LWT-Food Sci. Technol. 2014, 59, 859–866. [Google Scholar] [CrossRef]
- da Silva, W.P.; e Silva, C.M.; Lins, M.A.; Gomes, J.P. Osmotic dehydration of pineapple (Ananas comosus) pieces in cubical shape described by diffusion models. LWT-Food Sci. Technol. 2013, 55, 1–8. [Google Scholar] [CrossRef]
- Da Silva, W.P.; E Silva, C.M.; Lins, M.A. Determination of expressions for the thermal diffusivity of canned foodstuffs by the inverse method and numerical simulations of heat penetration. Int. J. Food Sci. Technol. 2011, 46, 811–818. [Google Scholar] [CrossRef]
- da Silva, W.P.; e Silva, C.M.D.P.S.; Farias, V.S.D.O.; e Silva, D.D.P.S. Calculation of the convective heat transfer coefficient and cooling kinetics of an individual fig fruit. Heat Mass Transf. 2010, 46, 371–380. [Google Scholar] [CrossRef]
- Mendonça, S.L.; Filho, C.R.; da Silva, Z. Transient conduction in spherical fruits: Method to estimate the thermal conductivity and volumetric thermal capacity. J. Food Eng. 2005, 67, 261–266. [Google Scholar] [CrossRef]
- Baïri, A.; Laraqi, N.; de María, J.G. Determination of thermal diffusivity of foods using 1D Fourier cylindrical solution. J. Food Eng. 2007, 78, 669–675. [Google Scholar] [CrossRef]
- Bock, V.; Nilsson, O.; Blumm, J.; Fricke, J. Thermal properties of carbon aerogels. J. Non-Cryst. Solids 1995, 185, 233–239. [Google Scholar] [CrossRef]
- Murakami, E.G. Thermal Processing Affects Properties of Commercial Shrimp and Scallops. J. Food Sci. 1994, 59, 237–241. [Google Scholar] [CrossRef]
- Murakami, E.G. The thermal properties of potatoes and carrots as affected by thermal processing. J. Food Process. Eng. 1997, 20, 415–432. [Google Scholar] [CrossRef]
- Kumcuoglu, S.; Tavman, S. Thermal diffusivity determination of pizza and puff pastry doughs at freezing temperatures. J. Food Process. Preserv. 2007, 31, 41–51. [Google Scholar] [CrossRef]
- Monde, M.; Mitsutake, Y. A new estimation method of thermal diffusivity using analytical inverse solution for one-dimensional heat conduction. Int. J. Heat Mass Transf. 2001, 44, 3169–3177. [Google Scholar] [CrossRef]
- Markowski, M.; Bialobrzewski, I.; Cierach, M.; Paulo, A. Determination of thermal diffusivity of Lyoner type sausages during water bath cooking and cooling. J. Food Eng. 2004, 65, 591–598. [Google Scholar] [CrossRef]
- Ukrainczyk, N. Thermal diffusivity estimation using numerical inverse solution for 1D heat conduction. Int. J. Heat Mass Transf. 2009, 52, 5675–5681. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Greiby, I.; Mishra, D.K.; Dolan, K.D. Rapid Inverse Method to Measure Thermal Diffusivity of Low-Moisture Foods. J. Food Sci. 2017, 82, 420–428. [Google Scholar] [CrossRef]
- da Silva, W.P.; de Medeiros, M.S.; Gomes, J.P.; e Silva, C.M.D.P.S. Improvement of methodology for determining local thermal diffusivity and heating time of green coconut pulp during its pasteurization. J. Food Eng. 2020, 285, 110104. [Google Scholar] [CrossRef]
- Fan, T.-H.; Pang, H.-Q.; Zhong, W.-R.; Zhang, S.-N.; Wu, X. Experiment and Inverse Analysis to Estimate SiO2 Aerogel Composite’s Thermophysical Properties by the Surface’s Temperature Response. Int. J. Thermophys. 2022, 43, 79. [Google Scholar] [CrossRef]
- Rabie, M.A.; Soliman, A.Z.; Diaconeasa, Z.S.; Constantin, B. Effect of Pasteurization and Shelf Life on the Physicochemical Properties of Physalis (Physalis peruviana L.) Juice. J. Food Process. Preserv. 2015, 39, 1051–1060. [Google Scholar] [CrossRef]
- Saeeduddin, M.; Abid, M.; Jabbar, S.; Wu, T.; Yuan, Q.; Riaz, A.; Hu, B.; Zhou, L.; Zeng, X. Nutritional, microbial and physicochemical changes in pear juice under ultrasound and commercial pasteurization during storage. J. Food Process. Preserv. 2017, 41, e13237. [Google Scholar] [CrossRef]
- Debbarma, T.; Thangalakshmi, S.; Tadakod, M.; Singh, R.; Singh, A. Comparative analysis of ohmic and conventional heat-treated carrot juice. J. Food Process. Preserv. 2021, 45, e15687. [Google Scholar] [CrossRef]
- da Silva, W.P.; da Silva, P.; de Souto, L.M.; Junior, A.F.D.S.; Ferreira, J.P.D.L.; Gomes, J.P.; Queiroz, A.J.D.M. Determination of constant and variable thermal diffusivity of cashew pulp during heating: Experimentation, optimizations and simulations. Case Stud. Therm. Eng. 2022, 39, 102428. [Google Scholar] [CrossRef]
- Da Silva, W.P.; E Silva, C.M.D.P.S. Calculation of the convective heat transfer coefficient and thermal diffusivity of cucumbers using numerical simulation and the inverse method. J. Food Sci. Technol. 2014, 51, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.P.; e Silva, C.M.D.; de Souto, L.M.; Moreira, I.D.S.; da Silva, E.C.O. Mathematical model for determining thermal properties of whole bananas with peel during the cooling process. J. Food Eng. 2018, 227, 11–17. [Google Scholar] [CrossRef]
- Santos, N.C.; Silva, W.P.; Gomes, J.P.; Barros, S.L.; Silva, C.M.D.P.S.E.; Santiago, M.; Júnior, A.F.S. Determination of thermal properties (and their uncertainties) during the cooling of apples (Malus communis). J. Food Process. Eng. 2022, 45, 14013. [Google Scholar] [CrossRef]
- da Silva, W.P.; e Silva, C.M.; Gama, F.J. An improved technique for determining transport parameters in cooling processes. J. Food Eng. 2012, 111, 394–402. [Google Scholar] [CrossRef]
- Erdoğdu, F.; Linke, M.; Praeger, U.; Geyer, M.; Schlüter, O. Experimental determination of thermal conductivity and thermal diffusivity of whole green (unripe) and yellow (ripe) Cavendish bananas under cooling conditions. J. Food Eng. 2014, 128, 46–52. [Google Scholar] [CrossRef]
- Incropera, F.P.; Dewitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 6th ed.; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Erdoğdu, F. A review on simultaneous determination of thermal diffusivity and heat transfer coefficient. J. Food Eng. 2008, 86, 453–459. [Google Scholar] [CrossRef]
- Mari, J.; Mari, M.; Ferreira, M.; Conceição, W.; Andrade, C. A simple method to estimate the thermal diffusivity of foods. J. Food Process. Eng. 2018, 41, e12821. [Google Scholar] [CrossRef]
- Chang, S.; Toledo, R. Simultaneous Determination of Thermal Diffusivity and Heat Transfer Coefficient during Sterilization of Carrot Dices in a Packed Bed. J. Food Sci. 1990, 55, 199–205. [Google Scholar] [CrossRef]
- Luikov, A. Analytical Heat Diffusion Theory; Academic Press, Inc. Ltd.: London, UK, 1968. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Levenberg, K. A method for the solution of certain non-linear problems in least squares. Q. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Pereira, J.C.A.; da Silva, W.P.; da Silva, R.C.; e Silva, C.M.D.P.; Gomes, J.P. Use of empirical and diffusion models in the description of the process of water absorption by rice. Eng. Comput. 2022, 39, 1556–1574. [Google Scholar] [CrossRef]
- Fikiin, K. Authentic form and origin of a popular predictive equation for specific heat capacity of unfrozen foods. Int. J. Refrig. 2021, 126, 291–293. [Google Scholar] [CrossRef]
- Riedel, L. Measurement of Thermal Diffusivity on Foodstuffs Rich in Water. Kaltetech.-Klim. 1969, 21, 315–320. [Google Scholar]
- Sweat, V.E. Experimental values of thermal conductivity of selected fruits and vegetables. J. Food Sci. 1974, 39, 1080–1083. [Google Scholar] [CrossRef]
- Silva, W.P.; E Silva, C.M.D.P.S.; Gomes, J.P.; Santos, N.C.; Queiroz, A.J.M.; De Figuiredo, R.M.F. Calculation of the Thermal Properties (and Their Uncertainties) of Strawberry During Its Cooling Under Natural Convection. J. Agric. Sci. 2019, 11, 114. [Google Scholar] [CrossRef]
- Da Silva, W.P.; E Silva, C.M.D.P.S.; De Souto, L.M.; Gomes, J.P.; Queiroz, A.J.M.; De Figueiredo, R.M.F. Thermal properties determination of a cylindrical product during its cooling: Two-dimensional numerical model and uncertainty. Int. J. Food Prop. 2019, 22, 343–354. [Google Scholar] [CrossRef]
- Silva, W.P.; Silva, C.M.D.P.S.; Nascimento, P.L.; Carmo, J.E.F.; Silva, D.D.P.S. Influence of the geometry on the numerical simulation of the cooling kinetics of cucumbers. Span. J. Agric. Res. 2011, 9, 242. [Google Scholar] [CrossRef]
- da Silva, M.M.; de Lima, A.B.; Gomes, J.P.; da Silva, W.P.; de Queiroz, R.A.; de Lima, W.P.B. The Cooling and Freezing of Parallelepiped-Shaped Solid: Foundations and Application to Food Product. Diffus. Found. 2018, 20, 78–91. [Google Scholar]
- Brischetto, S. Encyclopedia of Thermal Stresses, 1st ed.; Spring: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]

| Properties | Carrot | Reference |
|---|---|---|
| α (m2 s−1) | (1.43 ± 0.18) × 10−7 | Proposed model |
| k (W m−1 K−1) | 0.56 ± 0.07 | Proposed model |
| Cp (J kg−1 K−1) | 3918 | Fikiin [58] |
| ρ (kg m−3) | 1003 | Proposed model |
| Parameters | ||
| hH (W m−2 K−1) | 6.92 ± 0.20 | Proposed model |
| Bi1 (−) | 0.271 | Proposed model |
| Bi2 (−) | 0.246 | Proposed model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, W.P.; Souto, L.M.d.; Ferreira, J.P.d.L.; Gomes, J.P.; Lima, A.G.B.d.; Queiroz, A.J.d.M.; Figueirêdo, R.M.F.d.; Santos, D.d.C.; Santana, M.d.F.S.d.; Santos, F.S.d.; et al. Use of Inverse Method to Determine Thermophysical Properties of Minimally Processed Carrots during Chilling under Natural Convection. Foods 2023, 12, 2084. https://doi.org/10.3390/foods12102084
da Silva WP, Souto LMd, Ferreira JPdL, Gomes JP, Lima AGBd, Queiroz AJdM, Figueirêdo RMFd, Santos DdC, Santana MdFSd, Santos FSd, et al. Use of Inverse Method to Determine Thermophysical Properties of Minimally Processed Carrots during Chilling under Natural Convection. Foods. 2023; 12(10):2084. https://doi.org/10.3390/foods12102084
Chicago/Turabian Styleda Silva, Wilton Pereira, Leidjane Matos de Souto, João Paulo de Lima Ferreira, Josivanda Palmeira Gomes, Antonio Gilson Barbosa de Lima, Alexandre José de Melo Queiroz, Rossana Maria Feitosa de Figueirêdo, Dyego da Costa Santos, Maristela de Fátima Simplicio de Santana, Francislaine Suelia dos Santos, and et al. 2023. "Use of Inverse Method to Determine Thermophysical Properties of Minimally Processed Carrots during Chilling under Natural Convection" Foods 12, no. 10: 2084. https://doi.org/10.3390/foods12102084






