Research Progress of Quinoa Seeds (Chenopodium quinoa Wild.): Nutritional Components, Technological Treatment, and Application
Abstract
1. Introduction
2. Nutritional Composition of Quinoa
2.1. Protein
2.1.1. Functional Properties
2.1.2. Extraction Methods
2.2. Carbohydrates
2.2.1. Starch
2.2.2. Dietary Fiber
2.3. Lipids
2.4. Phytochemicals
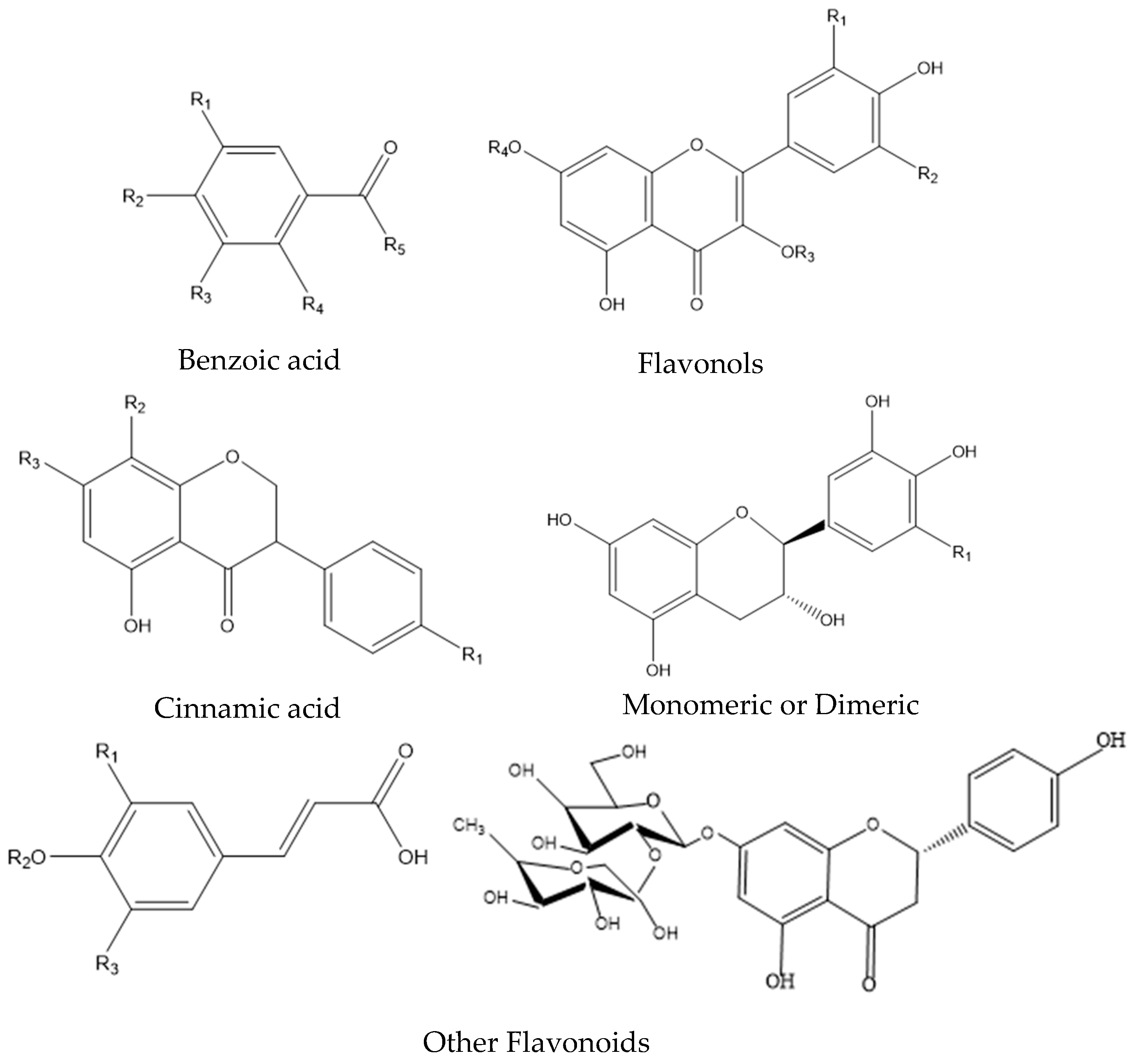
2.4.1. Polyphenolic Compounds
2.4.2. Tocopherols
2.4.3. Phytosterols
2.4.4. Saponins
3. Technological Approaches in Improving the Potential of Quinoa Seeds
3.1. Thermal Treatment
3.1.1. Extrusion
3.1.2. Drying
3.1.3. Heating under Pressure
3.2. Nonthermal Treatment
3.2.1. High Hydrostatic Pressure (HHP)
3.2.2. Atmospheric Pressure Cold Plasma (ACP)
3.2.3. Sonication
4. Various Applications
4.1. Bakery Food
4.2. Meat Analogues
4.3. Plant Milk
4.4. Fermented Beverages
4.5. Delivering Hydrophobic Bioactive Agents
4.6. Edible Films
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Aziz, A.; Akram, N.A.; Ashraf, M. Influence of natural and synthetic vitamin C (ascorbic acid) on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol. Biochem. 2018, 123, 192–203. [Google Scholar] [CrossRef]
- Pedrali, D.; Giupponi, L.; De la Peña-Armada, R.; Villanueva-Suárez, M.; Mateos-Aparicio, I. The quinoa variety influences the nutritional and antioxidant profile rather than the geographic factors. Food Chem. 2023, 402, 133531. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, R.; Yuan, W. Composition and secondary structure of proteins isolated from six different quinoa varieties from China. J. Cereal Sci. 2020, 95, 103036. [Google Scholar] [CrossRef]
- Wang, S.; Opassathavorn, A.; Zhu, F. Influence of Quinoa Flour on Quality Characteristics of Cookie, Bread and Chinese Steamed Bread. J. Texture Stud. 2015, 46, 281–292. [Google Scholar] [CrossRef]
- Sayas-Barberá, E.; Valero-Asencio, M.M.; Rodríguez-Vera, C.N.; Fernández-López, J.; Haros, C.M.; Pérez-Álvarez, J.; Viuda-Martos, M. Effect of Different Black Quinoa Fractions (Seed, Flour and Wet-Milling Coproducts) upon Quality of Meat Patties during Freezing Storage. Foods 2021, 10, 3080. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; Paredes-Gónzalez, X. Nutraceutical perspectives of quinoa: Biological properties and functional applications. FAO CIRAD State Art Rep. Quinoa World 2013, 286–299. [Google Scholar]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydr. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Tao, S.-Y.; Wu, Y.-X.; An, T.; Lv, B.-H.; Liu, J.-X.; Liu, Y.-T.; Jiang, G.-J. Quinoa Reduces High-Fat Diet-Induced Obesity in Mice via Potential Microbiota-Gut-Brain-Liver Interaction Mechanisms. Microbiol. Spectr. 2022, 10, e0032922. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Sant’Ana, H.M.P.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Vilcacundo, R.; Barrio, D.; Carpio, C.; García-Ruiz, A.; Rúales, J.; Hernández-Ledesma, B.; Carrillo, W. Digestibility of Quinoa (Chenopodium quinoa Willd.) Protein Concentrate and Its Potential to Inhibit Lipid Peroxidation in the Zebrafish Larvae Model. Plant Foods Hum. Nutr. 2017, 72, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.-M.; Mattila, P.H. Flavonoids and other phenolic compounds in An-dean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crop. Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Lingiardi, N.; Galante, M.; de Sanctis, M.; Spelzini, D. Are quinoa proteins a promising alternative to be applied in plant-based emulsion gel formulation? Food Chem. 2022, 394, 133485. [Google Scholar] [CrossRef]
- Wu, G.; Morris, C.F.; Murphy, K.M. Evaluation of Texture Differences among Varieties of Cooked Quinoa. J. Food Sci. 2014, 79, S2337–S2345. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.A.-M.; Serna, L.A. Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Food Sci. Tech. 2011, 31, 225–230. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Srichuwong, S.; Reuhs, B.L.; Hamaker, B.R. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem. 2015, 167, 490–496. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Functional components and anti-nutritional factors ingluten-free grains: A focus on quinoa seeds. Foods 2021, 10, 351. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C.F.R. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Schlag, S.; Götz, S.; Rüttler, F.; Schmöckel, S.M.; Vetter, W. Quantitation of 20 Phytosterols in 34 Different Accessions of Quinoa (Chenopodium quinoa). J. Agric. Food Chem. 2022, 70, 9856–9864. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Aluwi, N.A.; Saunders, S.R.; Ganjyal, G.M.; Medina-Meza, I.G. Metabolic fingerprinting unveils quinoa oil as a source of bioactive phytochemicals. Food Chem. 2019, 286, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Iafelice, G.; Verardo, V.; Marconi, E.; Caboni, M.F. Influence of pearling process on phenolic and saponin content in quinoa (Chenopodium quinoa Willd). Food Chem. 2014, 157, 174–178. [Google Scholar] [CrossRef]
- Elsohaimy, S.; Refaay, T.; Zaytoun, M. Physicochemical and functional properties of quinoa protein isolate. Ann. Agric. Sci. 2015, 60, 297–305. [Google Scholar] [CrossRef]
- Ye, D.; Sun, L.; Zou, B.; Zhang, Q.; Tan, W.; Che, W. Non-destructive prediction of protein content in wheat using NIRS. Spectrochim. Acta Part A 2018, 189, 463–472. [Google Scholar] [CrossRef]
- Rahnotra, G.; Gelroth, J.; Glaser, B.; Lorenz, K.; Jhonson, D. Composition and nutritional quality of quinoa. Cereal Chem. 1993, 63, 471–475. [Google Scholar]
- Ruales, J.; Nair, B.M. Nutritional quality of the protein in quinoa (Chenopodium quinoa, Willd) seeds. Plant Foods Hum. Nutr. 1992, 42, 1–11. [Google Scholar] [CrossRef]
- Song, H.; Fu, Q.; Huang, K.; Zou, Z.; Chen, L.; Chen, H.; Ge, S.; Wang, J.; Guan, X. Digestion characteristics of quinoa, barley and mungbean proteins and the effects of their simulated gastrointestinal digests on CCK secretion in enteroendo-crine STC-1 cells. Food Funct. 2022, 13, 6233–6243. [Google Scholar] [CrossRef]
- Cerdán-Leal, M.A.; López-Alarcón, C.A.; Ortiz-Basurto, R.I.; Luna-Solano, G.; Jiménez-Fernández, M. Influence of heat denaturation and freezing–lyophilization on physicochemical and functional properties of quinoa protein isolate. Cereal Chem. 2020, 97, 373–381. [Google Scholar] [CrossRef]
- Ruiz, G.A.; Xiao, W.; van Boekel, M.; Minor, M.; Stieger, M. Effect of extraction pH on heat-induced aggregation, gelation and microstructure of protein isolate from quinoa (Chenopodium quinoa Willd). Food Chem. 2016, 209, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, P.; Chen, Z.; Zhou, X.; Wang, T. Complexation of rice proteins and whey protein isolates by structural interactions to prepare soluble protein composites. LWT—Food Sci. Technol. 2019, 101, 207–213. [Google Scholar] [CrossRef]
- Aussenac, T.; Rhazi, L.; Branlard, G. Molecular Weight Distribution of Polymeric Proteins in Wheat Grains: The Rheologically Active Polymers. Foods 2020, 9, 1675. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Preparation and characterization of chia seed protein isolate–chia seed gum complex coacervates. Food Hydrocoll. 2016, 52, 554–563. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, X.; Li, Y. Drying methods affect physicochemical and functional properties of quinoa protein isolate. Food Chem. 2020, 339, 127823. [Google Scholar] [CrossRef]
- López, D.N.; Galante, M.; Raimundo, G.; Spelzini, D.; Boeris, V. Functional properties of amaranth, quinoa and chia proteins and the biological activities of their hydrolyzates. Food Res. Int. 2019, 116, 419–429. [Google Scholar] [CrossRef]
- Sánchez-Reséndiz, A.I.; Escalante-Aburto, A.; Andía-Ayme, V.; Chuck-Hernández, C. Structural prope rties, functional evaluation, and in vitro protein digestibility of black and yellow quinoa (Chenopodium petiolare) protein isolates. CyTA—J. Food 2019, 17, 864–872. [Google Scholar] [CrossRef]
- López-Castejón, M.L.; Bengoechea, C.; Díaz-Franco, J.; Carrera, C. Interfacial and emulsifying properties of quinoa protein concentrates. Food Biophys. 2020, 15, 122–132. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Zannini, E.; Arendt, E.K. Modifying the Cold Gelation Properties of Quinoa Protein Isolate: Influence of Heat-Denaturation pH in the Alkaline Range. Plant Foods Hum. Nutr. 2015, 70, 250–256. [Google Scholar] [CrossRef]
- Föste, M.; Elgeti, D.; Brunner, A.-K.; Jekle, M.; Becker, T. Isolation of quinoa protein by milling fractionation and solvent extraction. Food Bioprod. Process. 2015, 96, 20–26. [Google Scholar] [CrossRef]
- Guerreo-Ochoa, M.R.; Pedreschi, R.; Chirinos, R. Optimised methodology for the extraction of protein from quinoa (Chenopodium quinoa Willd.). Int. J. Food Sci. Tech. 2015, 50, 1815–1822. [Google Scholar] [CrossRef]
- Ruiz, G.A.; Arts, A.; Minor, M.; Schutyser, M. A Hybrid Dry and Aqueous Fractionation Method to Obtain Protein-Rich Fractions from Quinoa (Chenopodium quinoa Willd). Food Bioprocess Technol. 2016, 9, 1502–1510. [Google Scholar] [CrossRef]
- Opazo-Navarrete, M.; Schutyser, M.A.I.; Boom, R.M.; Janssen, A.E.M. Effect of pre-treatment on in vitro gastric digestion of quinoa protein (Chenopodium quinoa Willd.) obtained by wet and dry fractionation. Int. J. Food Sci. Nutr. 2018, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional Value and Use of the Andean Crops Quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Guan, X.; Cao, H.; Li, L.; Yu, J.; Liu, H. Characterization of Saponins from Differently Colored Quinoa Cultivars and Their In Vitro Gastrointestinal Digestion and Fermentation Properties. J. Agric. Food Chem. 2022, 70, 1810–1818. [Google Scholar] [CrossRef]
- James, L.E.A. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar]
- Tang, H.; Watanabe, K.; Mitsunaga, T. Characterization of storage starches from quinoa, barley and adzuki seeds. Carbohydr. Polym. 2002, 49, 13–22. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhu, F. Physicochemical properties of quinoa starch. Carbohydr. Polym. 2016, 137, 328–338. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Arendt, E.K. In vitro starch digestibility and predicted glycaemic indexes of buck-wheat, oat, quinoa, sorghum, teff and commercial gluten-free bread. J. Cereal Sci. 2013, 58, 431–436. [Google Scholar] [CrossRef]
- Valdez-Arana, J.-D.; Steffolani, M.E.; Repo-Carrasco-Valencia, R.; Pérez, G.T.; Condezo-Hoyos, L. Physicochemical and functional properties of isolated starch and their correlation with flour from the Andean Peruvian quinoa varieties. Int. J. Biol. Macromol. 2020, 147, 997–1007. [Google Scholar] [CrossRef]
- Wu, G.; Morris, C.F.; Murphy, K.M. Quinoa Starch Characteristics and Their Correlations with the Texture Profile Analysis (TPA) of Cooked Quinoa. J. Food Sci. 2017, 82, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, F. Quinoa starch: Structure, properties, and applications. Carbohydr. Polym. 2018, 181, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Yin, L.; Dong, J.; Shen, R.; Zhu, Y. Physicochemical characteristics and in vitro digestibility of starches from colored quinoa (Chenopodium quinoa) varieties. J. Food Sci. 2022, 87, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Srichuwong, S.; Curti, D.; Austin, S.; King, R.; Lamothe, L.; Gloria-Hernandez, H. Physicochemical properties and starch digestibility of whole grain sorghums, millet, quinoa and amaranth flours, as affected by starch and non-starch constituents. Food Chem. 2017, 233, 1–10. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Ji, J.; Wen, Y.; Li, H.; Wang, J.; Sun, B. Endogenous protein and lipid facilitate the digestion process of starch in cooked quinoa flours. Food Hydrocoll. 2023, 134, 108099. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, M.; Bai, T.; Chen, D.; Zhang, Q.; Lin, D.; Liu, Y.; Liu, A.; Huang, Z.; Qin, W. Comparative study on the structure, physicochemical, and functional properties of dietary fiber extracts from quinoa and wheat. LWT—Food Sci. Technol. 2021, 149, 111816. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Tan, B.; Zhang, W. Dietary fibre extracted from different types of whole grains and beans: A comparative study. Int. J. Food Sci. Tech. 2020, 55, 2188–2196. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Mai, P.; Hao, Y.; Wang, Z.; Wang, J. Quinoa bran soluble dietary fiber ameliorates dextran sodium sulfate induced ulcerative colitis in BALB/c mice by maintaining intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2022, 216, 75–85. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Fanali, C.; Beccaria, M.; Salivo, S.; Tranchida, P.; Tripodo, G.; Farnetti, S.; Dugo, L.; Dugo, P.; Mondello, L. Non-polar lipids characterization of quinoa (Chenopodium quinoa) seed by comprehensive two-dimensional gas chromatography with flame ionization/mass spectrometry detection and non-aqueous reversed-phase liquid chromatography with atmospheric pressure chemical ionization mass spectrometry detection. J. Sep. Sci. 2015, 38, 3151–3160. [Google Scholar]
- Przybylski, R.; Chauhan, G.; Eskin, N. Characterization of quinoa (Chenopodium quinoa) lipids. Food Chem. 1994, 51, 187–192. [Google Scholar] [CrossRef]
- Ryan, E.; Galvin, K.; O’connor, T.P.; Maguire, A.R.; O’brien, N.M. Phytosterol, Squalene, Tocopherol Content and Fatty Acid Profile of Selected Seeds, Grains, and Legumes. Plant Foods Hum. Nutr. 2007, 62, 85–91. [Google Scholar] [CrossRef]
- Murphy, K.S.; Matanguihan, J. Quinoa: Improvement and Sustainable Production; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M.; Valdivia, W.; Bustamante, L.; Graf, F.; Obal, I. Protein and antioxidant compo-sition of quinoa (Chenopodium quinoa Willd.) sprout from seeds submitted to water stress, salinity and light conditions. Dustrial Crop. Prod. 2017, 107, 558–564. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Liu, R.; Tsao, R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shao, Y.; Chen, X.; Li, X. Release of characteristic phenolics of quinoa based on extrusion technique. Food Chem. 2020, 374, 128780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, Y.; Li, W.; Huang, K.; Li, S.; Cao, H.; Guan, X. Microwaving released more polyphenols from black quinoa grains with hypoglycemic effects compared with traditional cooking methods. J. Sci. Food Agric. 2022, 102, 5948–5956. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B. Quinoa (Chenopodium quinoa Willd.) as source of bioactive compounds: A review. Bioact. Com-Pounds Health Dis. 2019, 2, 27–47. [Google Scholar] [CrossRef]
- Przygoda, K.; Wejnerowska, G. Extraction of tocopherol-enriched oils from Quinoa seeds by supercritical fluid extraction. Ind. Crop. Prod. 2015, 63, 41–47. [Google Scholar] [CrossRef]
- Miranda, M.; Barbosa, R.G.; Trigo, M.; Uribe, E.; Vega-Gálvez, A.; Aubourg, S.P. Enhancement of the rancidity stability in a marine-oil model by addition of a saponin-free quinoa (Chenopodium quinoa Willd.) ethanol extract. Eur. J. Lipid. Sci. Tech. 2017, 119, 1600291. [Google Scholar] [CrossRef]
- Trigo, M.; Rodríguez, A.; Dovale, G.; Pastén, A.; Vega-Gálvez, A.; Aubourg, S.P. The effect of glazing based on saponin-free quinoa (Chenopodium quinoa) extract on the lipid quality of frozen fatty fish. LWT—Food Sci. Technol. 2018, 98, 231–236. [Google Scholar] [CrossRef]
- El Hazzam, K.; Hafsa, J.; Sobeh, M.; Mhada, M.; Taourirte, M.; EL Kacimi, K.; Yasri, A. An Insight into Saponins from Quinoa (Chenopodium quinoa Willd): A Review. Molecules 2020, 25, 1059. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, X.; Shi, Z.; Ren, G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophages cells. J. Food Sci. 2014, 79, H1018–H1023. [Google Scholar] [CrossRef]
- Huang, R.; Huang, K.; Guan, X.; Li, S.; Cao, H.; Zhang, Y.; Lao, X.; Bao, Y.; Wang, J. Effect of defatting and extruding treatment on the physicochemical and storage properties of quinoa (Chenopodium quinoa Wild) flour. LWT—Food Sci. Technol. 2021, 147, 111612. [Google Scholar] [CrossRef]
- Kowalski, R.J.; Medina-Meza, I.G.; Thapa, B.B.; Murphy, K.M.; Ganjyal, G.M. Extrusion processing characteristics of quinoa (Chenopodium quinoa Willd.) var. Cherry Vanilla. J. Cereal Sci. 2016, 70, 91–98. [Google Scholar] [CrossRef]
- Cao, H.; Sun, R.; Liu, Y.; Wang, X.; Guan, X.; Huang, K.; Zhang, Y. Appropriate microwave improved the texture proper-ties of quinoa due to starch gelatinization from the destructed cyptomere structure. Food Chem. X 2022, 14, 100347. [Google Scholar] [CrossRef]
- Romano, N.; Ureta, M.M.; Guerrero-Sánchez, M.; Gómez-Zavaglia, A. Nutritional and technological properties of a quinoa (Chenopodium quinoa Willd.) spray-dried powdered extract. Food Res. Int. 2020, 129, 108884. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, A.; Shen, R.; Qu, L. Effect of processing on the contents of amino acids and fatty acids, and glucose release from the starch of quinoa. Food Sci. Nutr. 2020, 8, 4877–4887. [Google Scholar] [CrossRef]
- Wu, L.-G.; Wang, A.; Shen, R.; Qu, L. Effect of heating under pressure treatment on the antioxidant of quinoa. Int. J. Food Eng. 2021, 17, 795–804. [Google Scholar] [CrossRef]
- Sharma, S.; Kataria, A.; Singh, B. Effect of thermal processing on the bioactive compounds, antioxidative, antinutritional and functional characteristics of quinoa (Chenopodium quinoa). LWT—Food Sci. Technol. 2022, 160, 113256. [Google Scholar] [CrossRef]
- Okur, I.; Ozel, B.; Oztop, M.H.; Alpas, H. Effect of high hydrostatic pressure in physicochemical properties and in vitro digestibility of cornstarch by nuclear magnetic resonance relaxometry. J. Food Process. Eng. 2019, 42, e13168. [Google Scholar] [CrossRef]
- Okur, I.; Sezer, P.; Oztop, M.H.; Alpas, H. Recent advances in gelatinisation and retrogradation of starch by high hydrostatic pressure. Int. J. Food Sci. Tech. 2021, 56, 4367–4375. [Google Scholar] [CrossRef]
- Zare, L.; Mollakhalili-Meybodi, N.; Fallahzadeh, H.; Arab, M. Effect of atmospheric pressure cold plasma (ACP) treatment on the technological characteristics of quinoa flour. LWT—Food Sci. Technol. 2022, 155, 112898. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification of quinoa seed protein isolates (QPIs) by variable time sonification for improving its physicochemical and functional characteristics. Ultrason. Sonochem. 2019, 58, 104700. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, M.; Bhandari, B.; Chen, J. Instant quinoa prepared by different cooking methods and infrared-assisted freeze drying: Effects of variables on the physicochemical properties. Food Chem. 2022, 370, 131091. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Effect of high pressure on rheological and thermal properties of quinoa and maize starches. Food Chem. 2018, 241, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Thomas, L. Changes in structural, functional and antioxidant properties induced by high pressure on quinoa flour. J. Food Meas. Charact. 2020, 14, 401–410. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F.; Mo, G.; Hemar, Y. Supramolecular structure of high hydrostatic pressure treated quinoa and maize starches. Food Hydrocoll. 2019, 92, 276–284. [Google Scholar] [CrossRef]
- Unal, K.; Alpas, H.; Oztop, M.H. Modification of quinoa starch by high hydrostatic pressure and ultrasonication and as-sessment of its physicochemical properties. ACS Food Sci. Technol. 2023, 3, 31–40. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kadam, D.; Annapure, U.S. Cold Plasma: An Alternative Technology for the Starch Modification. Food Biophys. 2017, 12, 129–139. [Google Scholar] [CrossRef]
- Szwengiel, A.; Lewandowicz, G.; Górecki, A.R.; Błaszczak, W. The effect of high hydrostatic pressure treatment on the molecular structure of starches with different amylose content. Food Chem. 2018, 240, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Smith, B. The functional modification of legume proteins by ultrasonication: A review. Trends Food Sci. Technol. 2020, 98, 107–116. [Google Scholar] [CrossRef]
- Cao, H.; Sun, R.; Shi, J.; Li, M.; Guan, X.; Liu, J.; Huang, K.; Zhang, Y. Effect of ultrasonic on the structure and quality char-acteristics of quinoa protein oxidation aggregates. Ultrason. Sonochem. 2021, 77, 105685. [Google Scholar] [CrossRef]
- Luo, L.; Yang, Z.; Wang, H.; Ashokkumar, M.; Hemar, Y. Impacts of sonication and high hydrostatic pressure on the structural and physicochemical properties of quinoa protein isolate dispersions at acidic, neutral and alkaline pHs. Ultrason. Sonochem. 2022, 91, 106232. [Google Scholar] [CrossRef]
- Qin, X.-S.; Luo, Z.-G.; Peng, X.-C. Fabrication and Characterization of Quinoa Protein Nanoparticle-Stabilized Food-Grade Pickering Emulsions with Ultrasound Treatment: Interfacial Adsorption/Arrangement Properties. J. Agric. Food Chem. 2018, 66, 4449–4457. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, Z.; Ma, W.; Yu, P.; Li, T.; Wang, L. Assemble behavior of ultrasound-induced quinoa protein nanoparticles and their roles on rheological properties and stability of high internal phase emulsions. Food Hydrocoll. 2021, 117, 106748. [Google Scholar] [CrossRef]
- Zeng, T.; Wu, Z.-L.; Zhu, J.-Y.; Yin, S.-W.; Tang, C.-H.; Wu, L.-Y.; Yang, X.-Q. Development of antioxidant Pickering high internal phase emulsions (HIPEs) stabilized by protein/polysaccharide hybrid particles as potential alternative for PHOs. Food Chem. 2017, 231, 122–130. [Google Scholar] [CrossRef]
- Jia, M.; Yu, Q.; Chen, J.; He, Z.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Physical quality and in vitro starch digestibility of biscuits as affected by addition of soluble dietary fiber from defatted rice bran. Food Hydrocoll. 2020, 99, 105349. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Ballester-Sánchez, J.; Yalcin, E.; Fernández-Espinar, M.T.; Haros, C.M. Rheological and thermal properties of royal quinoa and wheat flour blends for breadmaking. Eur. Food Res. Technol. 2019, 245, 1571–1582. [Google Scholar] [CrossRef]
- Brito, I.L.; de Souza, E.L.; Felex, S.S.S.; Madruga, M.S.; Yamashita, F.; Magnani, M. Nutritional and sensory characteristics of gluten-free quinoa (Chenopodium quinoa Willd)-based cookies development using an experimental mixture design. J. Food Sci. Technol. 2015, 52, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.M.R.; Suuronen, J.-P.; Deegan, K.C.; Serimaa, R.; Tuorila, H.; Jouppila, K. Physical and sensory characteristics of corn-based extruded snacks containing amaranth, quinoa and kañiwa flour. LWT—Food Sci. Technol. 2015, 64, 1047–1056. [Google Scholar] [CrossRef]
- Lorenz, K. Quinoa (Chenopodium quinoa) Starch—Physico-chemical Properties and Functional Characteristics. Starch-Stärke 1990, 42, 81–86. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food microbiol 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Yeşil, S.; Levent, H. The influence of fermented buckwheat, quinoa and amaranth flour on gluten-free bread quality. LWT—Food Sci. Technol. 2022, 160, 113301. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Gaide, I.; Basinskiene, L. Effect of lactic acid fermentation on quinoa characteristics and quality of quinoa-wheat composite bread. Foods 2021, 10, 171. [Google Scholar] [CrossRef]
- Bozdogan, N.; Kumcuoglu, S.; Tavman, S. Investigation of the effects of using quinoa flour on gluten-free cake batters and cake properties. J. Food Sci. Technol. 2019, 56, 683–694. [Google Scholar] [CrossRef]
- Aprodu, I.; Banu, I. Effect of starch and dairy proteins on the gluten free bread formulation based on quinoa. J. Food Meas. Charact. 2021, 15, 2264–2274. [Google Scholar] [CrossRef]
- Azizi, S.; Azizi, M.H.; Moogouei, R.; Rajaei, P. The effect of Quinoa flour and enzymes on the quality of gluten-free bread. Food Sci. Nutr. 2020, 8, 2373–2382. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Pérez-Alvarez, J.A. Quinoa and chia products as ingredients for healthier processed meat products: Technological strategies for their application and effects on the final product. Curr. Opin. Food Sci. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Shokry, A.M. The usage of quinoa flour as a potential ingredient in production of meat burger with functional properties. Middle East J. Appl. Sci. 2016, 6, 1128–1137. [Google Scholar]
- Bağdatli, A. The influence of quinoa (Chenopodium quinoa Willd.) flour on the pshycochmical, textural and sensorial properties of beef meatball. Ital. J. Food Sci. 2018, 30, 280–288. [Google Scholar]
- Özer, C.O.; Seçen, S.M. Effects of quinoa flour on lipid and protein oxidation in raw and cooked beef burger during long term frozen storage. Food Sci. Technol. 2018, 38, 221–227. [Google Scholar] [CrossRef]
- Felix, M.; Camacho-Ocaña, Z.; López-Castejón, M.L.; Ruiz-Domínguez, M. Rheological properties of quinoa-based gels. An alternative for vegan diets. Food Hydrocoll. 2021, 120, 106827. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, Y.-J.; Lim, J.-G.; Jeon, J.-H.; Yoon, K.-S. Effect of Quinoa (Chenopodium quinoa Willd.) Starch and Seeds on the Physicochemical and Textural and Sensory Properties of Chicken Meatballs during Frozen Storage. Foods 2021, 10, 1601. [Google Scholar] [CrossRef]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In vitro study of the effect of quinoa and quinoa polysaccharides on human gut microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Shen, S.; Fang, Z. Cereal grain-based functional beverages: From cereal grain bioactive phytochemicals to beverage processing technologies, health benefits and product features. Crit. Rev. Food Sci. Nutr. 2022, 62, 2404–2431. [Google Scholar] [CrossRef] [PubMed]
- Pineli, L.d.L.d.O.; Botelho, R.B.A.; Zandonadi, R.P.; Solorzano, J.L.; de Oliveira, G.T.; Reis, C.E.G.; Teixeira, D.d.S. Low gly-cemic index and increased protein content in a novel quinoa milk. LWT—Food Sci. Technol. 2015, 63, 1261–1267. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E.; Bogdan, P.; Pielech-Przybylska, K.; Michalowska, D. Suitability of unmalted quinoa for beer production. J. Sci. Food Agric. 2018, 98, 5027–5036. [Google Scholar] [CrossRef]
- Pino-Ramos, L.L.; Peña-Martínez, P.A.; Laurie, V.F. Quinoa protein extract: An effective alternative for the fining of wine phenolics. J. Sci. Food Agric. 2022, 102, 6320–6327. [Google Scholar] [CrossRef]
- Liu, K.; Zha, X.-Q.; Li, Q.-M.; Pan, L.-H.; Luo, J.-P. Hydrophobic interaction and hydrogen bonding driving the self-assembling of quinoa protein and flavonoids. Food Hydrocoll. 2021, 118, 106807. [Google Scholar] [CrossRef]
- Li, G.; Xu, X.; Zhu, F. Physicochemical properties of dodecenyl succinic anhydride (DDSA) modified quinoa starch. Food Chem. 2019, 300, 125201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Du, C.; Zhao, N.; Jiang, W.; Yu, X.; Du, S.-K. Preparation and characterization of quinoa starch nanoparticles as quercetin carriers. Food Chem. 2022, 369, 130895. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef] [PubMed]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin in Pickering emulsions stabilized using octenyl succinic anhydride (OSA) modified quinoa, maize, and potato starch nanoparticles. Food Chem. 2023, 405, 134790. [Google Scholar] [CrossRef]
- Luo, Y.; Ni, F.; Guo, M.; Liu, J.; Chen, H.; Zhang, S.; Li, Y.; Chen, G.; Wang, G. Quinoa starch microspheres for drug delivery: Preparation and their characteristics. Food Sci. Tech. 2022, 42, 126421. [Google Scholar] [CrossRef]
- Abugoch, L.E.; Tapia, C.; Villamán, M.C.; Yazdani-Pedram, M.; Díaz-Dosque, M. Characterization of quinoa protein–chitosan blend edible films. Food Hydrocoll. 2011, 25, 879–886. [Google Scholar] [CrossRef]
- Pająk, P.; Przetaczek-Rożnowska, I.; Juszczak, L. Development and physicochemical, thermal and mechanical properties of edible films based on pumpkin, lentil and quinoa starches. Int. J. Biol. Macromol. 2019, 138, 441–449. [Google Scholar] [CrossRef]

| Nutritional Components | Content | Unit | Origin | Reference |
|---|---|---|---|---|
| Protein | 11.3–14.7 | g/100 g | Peru | [13] |
| 11.6–13.7 | g/100 g | Europe | [14] | |
| 14.7–18.9 | g/100 g | China | ||
| Starch | 55.6–63.0 | g/100 g | China | |
| 53.2–61.3 | g/100 g | Peru | [15] | |
| 53.2–73.4 | g/100 g | U.S.A | [16] | |
| Dietary fiber | 13.66–16.0 | g/100 g | Peru | [17] |
| 7.7–15.9 | g/100 g | Spain and the Andean | [3,18] | |
| 12.71–18.59 | g/100 g | Europe | [14] | |
| Lipids | 4.0–6.9 | g/100 g | Peru | [13] |
| 4.9–6.5 | g/100 g | Europe | [14] | |
| 4.11–7.09 | g/100 g | China | ||
| Phenolic compounds | 30.3–59.7 | mg/100 g | Peru | [13] |
| 46.7–68.2 | mg/100 g | Canada | [1] | |
| 75.3–87.58 | mg/100 g | Europe | [14] | |
| 51.5–141.95 | mg/100 g | China | ||
| 66–202 | mg/100 g | Africa, Egypt | [19] | |
| Flavonoids | 36.2–72.6 | mg/100 g | Peru | [13] |
| 127–288.8 | mg/100 g | Africa, Egypt | [19] | |
| 175–400 | mg/100 g | China | [19] | |
| 89.7–93.45 | mg/100 g | Europe, Serbia | [19] | |
| Tocopherols | 37.5–59.8 | µg/g | South America | [20] |
| 971–1764 | µg/100 g | Peru | [21] | |
| Phytosterols | 120–180 | mg/100 g | N.A. | [22] |
| 28.7–67.7 | mg/g | U.S.A, South America | [23] | |
| Saponins | 244.3 | mg/100 g | Europe | [24] |
| 2.76–4.12 | mg/100 g | Africa, Egypt | [19] | |
| 15.50 | mg/100 g | China | [19] |
| Composition | Quinoa | Wheat | Maize | Oats | Rye | Barley | Buckwheat | Rice |
|---|---|---|---|---|---|---|---|---|
| Protein (g/100 g) | 11.3–18.9 | 10–18 | 7.8–11.2 | 12.1–14.1 | 10.8–12.7 | 10.8–13.6 | 11–17 | 7–8 |
| Starch (g/100 g) | 53.2–73.4 | 60–75 | 66–78 | 41–53 | 63–66 | 56.7–64.3 | 60–70 | 70–80 |
| Dietary fiber (g/100 g) | 7.7–18.59 | 1.14 | 1.4–2.2 | 8.8–13.4 | 1.5–2.0 | 3.5–5.4 | 3.4–6.5 | 0.2–0.9 |
| Lipids (g/100 g) | 4.0–7.09 | 2–2.5 | 4.1–12.3 | 4.4–7.2 | 1.7–2.1 | 2.4–3.4 | 2–3 | 1.3–1.8 |
| Phenolics (mg/100 g) | 30.3–202 | 46–134 | 60–460 | 320 | 136 | 45–135 | 70.4–124 | 48–467 |
| Flavonoids (mg/100 g) | 36.2–288 | 102 | 260 | 380 | 116.7 | 62–300.8 | 387 | 13.49–169.22 |
| Tocopherol (µg/100 g) | 0.4–1700 | 0–340 | 669–1300 | 721 | 68.4–290 | 747 | 0.1–8.51 | 70–190 |
| Phytosterols (mg/100 g) | 0.3–180 | 70–92 | 43.6 | 35–49 | 95.5 | 50.4 | 60 | 13.62–52.71 |
| Saponins (mg/100 g) | 2–244 | - | - | 0.02–0.05 | - | - | - | - |
| Potential Treatment Techniques for Quinoa Seeds and Their Products | Mechanism Involved | Characteristics of the Treated Quinoa | Reference | |
|---|---|---|---|---|
| Thermal treatment | Extrusion | Heat, mechanical energy, and pressure provided by screw extrusion; the starch in quinoa seeds gelatinizes and the protein denatures, thus changing the structure and nutritional characteristics of quinoa seeds. | (1) Moderate expansion; (2) The original structures of the saponins are destroyed into smaller fragments; (3) Formation of protein/starch–lipid complexes is induced; (4) Degradation of phenolic compounds; (5) Improved protein digestibility due to denaturation. | [75,76] |
| Drying | Removes most of the free water in quinoa seeds by the action of high thermal energy. | (1) Improved stability of quinoa seeds during storage; (2) Enhanced antioxidant compounds retention and improved antioxidative properties; (3) Increased swelling. | [77,78] | |
| Heating under pressure | High temperatures and high pressure provided by high-pressure-resistant equipment changes the structure and nutritional characteristics of quinoa seeds. | (1) Higher levels of active phytochemicals and antioxidants can be retained; (2) Quinoa seed products have better rehydration capacity. | [79,80,81] | |
| Non-thermal treatment | High hydrostatic pressure (HHP) | Water or other fluid as a medium to transfer 100–1000 MPa of high pressure, acting on quinoa seeds, so that some large molecular substances in quinoa seeds are changed. | (1) Nutrient loss and chemical composition changes are minimal; (2) Higher pressure induces complete starch gelatinization, though the retrogradation and textural properties of starch gels were little affected. | [82,83] |
| Atmospheric pressure cold plasma (ACP) | Cold plasma flow is applied to the surface of quinoa seeds to achieve sterilization. | (1) Wide sterilization range; (2) Improving thermal stability and reduced gel strength. | [84] | |
| Sonication | Due to the cavitation effect, high shear forces, microjets, and shock waves are generated, which leads to the expansion of some protein structures in quinoa seeds and the disintegration of large aggregates. | Improved functional properties of quinoa seed protein. | [85] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, H.; Xue, S.; Sun, Q.; Shi, J.; Zhang, D.; Wang, D.; Wei, J. Research Progress of Quinoa Seeds (Chenopodium quinoa Wild.): Nutritional Components, Technological Treatment, and Application. Foods 2023, 12, 2087. https://doi.org/10.3390/foods12102087
Mu H, Xue S, Sun Q, Shi J, Zhang D, Wang D, Wei J. Research Progress of Quinoa Seeds (Chenopodium quinoa Wild.): Nutritional Components, Technological Treatment, and Application. Foods. 2023; 12(10):2087. https://doi.org/10.3390/foods12102087
Chicago/Turabian StyleMu, Hongyan, Sophia Xue, Qingrui Sun, John Shi, Danyang Zhang, Deda Wang, and Jianteng Wei. 2023. "Research Progress of Quinoa Seeds (Chenopodium quinoa Wild.): Nutritional Components, Technological Treatment, and Application" Foods 12, no. 10: 2087. https://doi.org/10.3390/foods12102087
APA StyleMu, H., Xue, S., Sun, Q., Shi, J., Zhang, D., Wang, D., & Wei, J. (2023). Research Progress of Quinoa Seeds (Chenopodium quinoa Wild.): Nutritional Components, Technological Treatment, and Application. Foods, 12(10), 2087. https://doi.org/10.3390/foods12102087







