Yak Milk: Nutritional Value, Functional Activity, and Current Applications
Abstract
:1. Introduction
2. Nutritional Components of Yak Milk
2.1. Substance Composition of Yak Milk
2.2. Yak Milk Protein
2.2.1. Distribution of Nitrogen in Yak Milk
2.2.2. Protein Composition of Yak Milk
2.2.3. Amino Acid Composition of Proteins in Yak Milk
2.3. Fat
2.4. Minerals
2.5. Vitamins
2.6. Other Nutritional Components
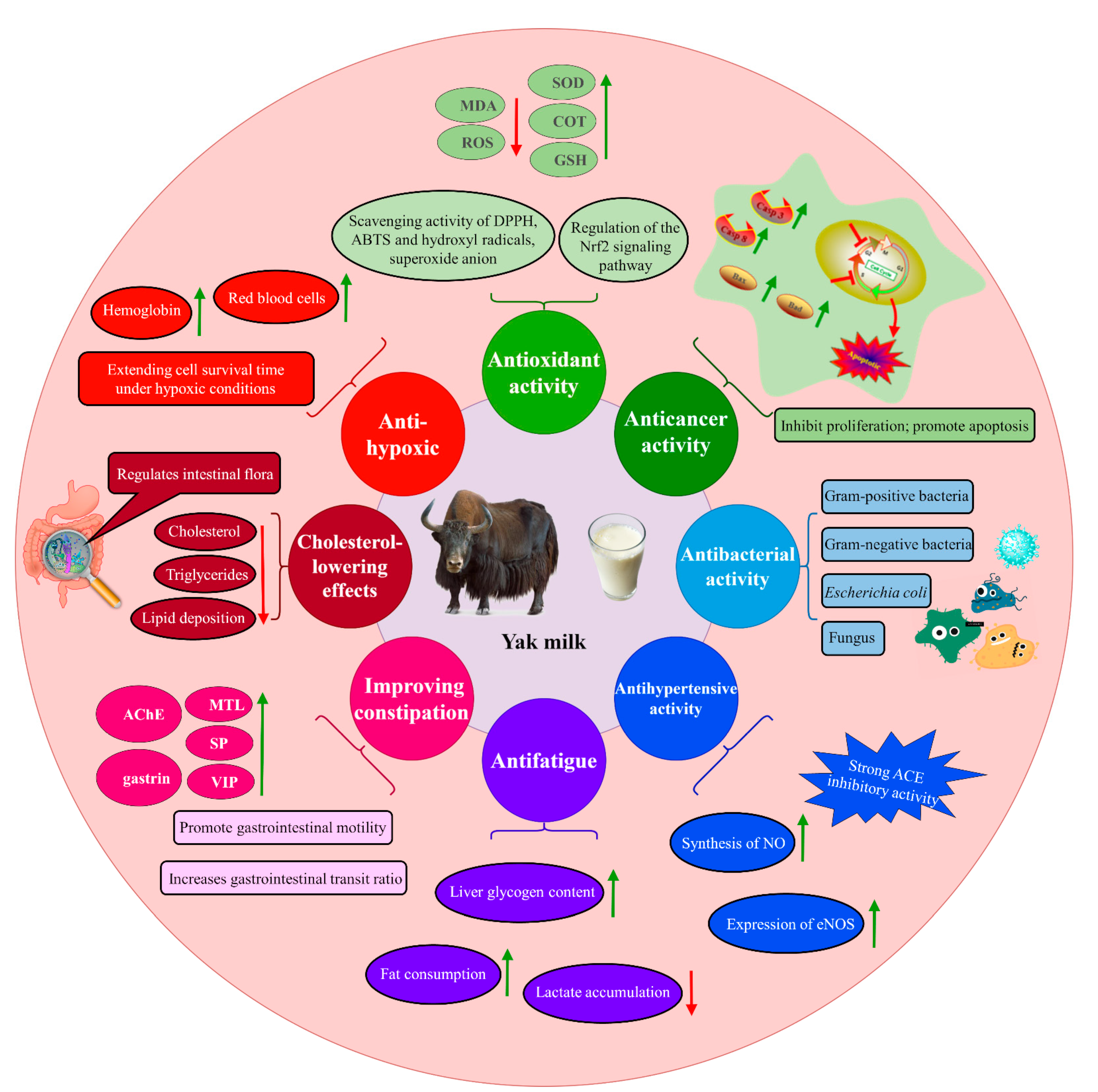
3. Functional Properties of Yak Milk
3.1. Antioxidant Activity
3.2. Anticancer Activity
3.3. Antibacterial Activity
3.4. Antihypertensive Activity
3.5. Antifatigue
3.6. Improving Constipation
3.7. Cholesterol-Lowering Effects
3.8. Anti-Hypoxic
3.9. Other Functions
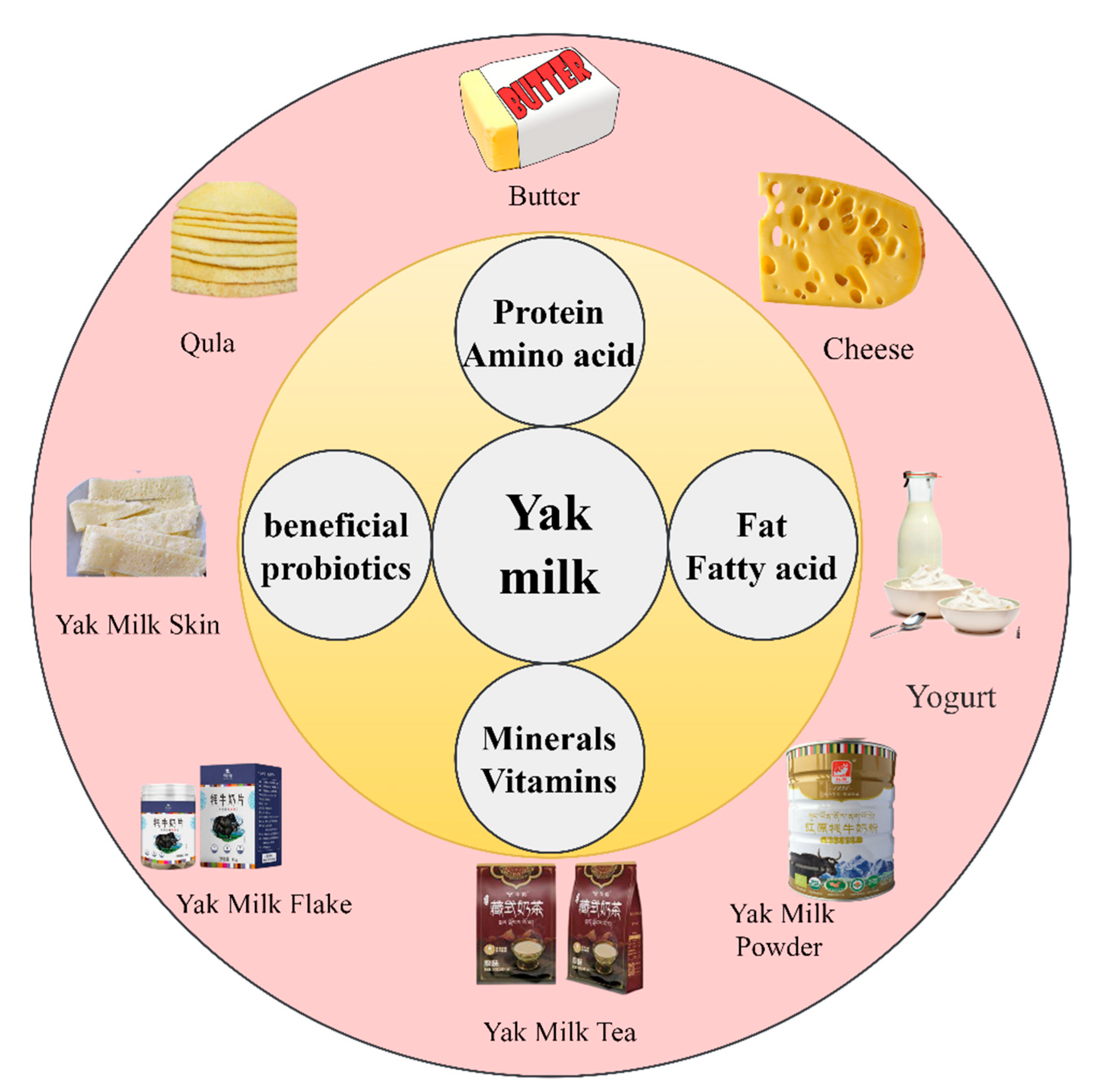
4. Yak Milk Products
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Liu, H.; Wen, P.; Zhang, H.; Guo, H.; Ren, F. The composition, size and hydration of yak casein micelles. Int. Dairy J. 2013, 31, 107–110. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Wang, J.; Elzo, M.A.; Yang, X.; Lai, S. Genetic diversities of MT-ND1 and MT-ND2 genes are associated with high-altitude adaptation in yak. Mitochondrial DNA Part A 2018, 29, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.S.; Long, R.J.; Guo, X.S.; Irvine, J.; Ding, L.M.; Ding, L.L.; Shang, Z.H. Blood mineral status of grazing Tibetan sheep in the Northeast of the Qinghai–Tibetan Plateau. Livest. Sci. 2011, 136, 102–107. [Google Scholar] [CrossRef]
- Fan, Q.; Wanapat, M.; Yan, T.; Hou, F. Altitude influences microbial diversity and herbage fermentation in the rumen of yaks. BMC Microbiol. 2020, 20, 370. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.B.; Jianlin, H.; Wang, G.; Rege, J.E.O.; Hanotte, O. Assessment of cattle genetic introgression into domestic yak populations using mitochondrial and microsatellite DNA markers. Anim. Genet. 2010, 41, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, Q.; Ma, H.; Wang, L.; Yang, Y.; Luo, W.; Qiu, Q. Genome-wide variation within and between wild and domestic yak. Mol. Ecol. Resour. 2014, 14, 794–801. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Li, Q.; Wang, J.; Cheng, J.; Xue, J.; Shi, J. The Chemical Composition and Nitrogen Distribution of Chinese Yak (Maiwa) Milk. Int. J. Mol. Sci. 2011, 12, 4885–4895. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, Z.; Zhao, S.; Xiao, Y. Classification of ecological types of the Chinese yak. Acta Ecol. Sin. 2006, 26, 2068–2072. [Google Scholar] [CrossRef]
- Guo, X.; Long, R.; Kreuzer, M.; Ding, L.; Shang, Z.; Zhang, Y.; Yang, Y.; Cui, G. Importance of Functional Ingredients in Yak Milk-Derived Food on Health of Tibetan Nomads Living Under High-Altitude Stress: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 292–302. [Google Scholar] [CrossRef]
- Singh, T.P.; Arora, S.; Sarkar, M. Yak milk and milk products: Functional, bioactive constituents and therapeutic potential. Int. Dairy J. 2023, 142, 105637. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Dong, A.; Wang, J.; Li, Q.; He, S.; Maubois, J.-L. Protein composition of yak milk. Dairy Sci. Technol. 2010, 90, 111–117. [Google Scholar] [CrossRef]
- Liu, H.N.; Ren, F.Z.; Jiang, L.; Ma, Z.L.; Qiao, H.J.; Zeng, S.S.; Gan, B.Z.; Guo, H.Y. Short communication: Fatty acid profile of yak milk from the Qinghai-Tibetan Plateau in different seasons and for different parities. J. Dairy Sci. 2011, 94, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Barsila, S.R. Effect of parity in different grazing seasons on milk yield and composition of cattle × yak hybrids in the Himalayan alpines. J. Appl. Anim. Res. 2019, 47, 591–596. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Yu, S.; Cao, S. Quantitative proteomic analysis of whey proteins in the colostrum and mature milk of yak (Bos grunniens). J. Sci. Food Agric. 2015, 95, 592–597. [Google Scholar] [CrossRef]
- Zhi-yun, H.U.; Qi, L.; Wei-bing, Z.; Min, Y.; Na, C.U.I.; Wei-dong, G.A.O.; Peng-cheng, W.E.N. Influence of Parities and Lactation Days on Nutritional Quality of Yak Colostrum. J. Food Sci. Technol. 2013, 31, 38–42. [Google Scholar] [CrossRef]
- Ma, Y.; He, S.; Park, Y.W. Yak Milk. In Handbook of Milk of Non-Bovine Mammals; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 481–513. [Google Scholar]
- Li, H.; He, S.; Liu, T.; Ma, Y. Research development in physical and chemical characters of yak milk. China Dairy Ind. 2009, 37, 35–40. [Google Scholar]
- Feagan, J.T. Factors affecting protein composition of milk and their significance to dairy processing. Aust. J. Dairy Technol. 1979, 60, 167–177. [Google Scholar]
- DePeters, E.J.; Ferguson, J.D. Nonprotein Nitrogen and Protein Distribution in the Milk of Cows. J. Dairy Sci. 1992, 75, 3192–3209. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Michaelidou, A.M. Factors influencing nutritional and health profile of milk and milk products. Small Rumin. Res. 2008, 79, 42–50. [Google Scholar] [CrossRef]
- Li, H. Characteristics of Yak Milk and its Properties of Casein Micelle Structure. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2011. [Google Scholar]
- Rafiq, S.; Huma, N.; Pasha, I.; Sameen, A.; Mukhtar, O.; Khan, M.I. Chemical Composition, Nitrogen Fractions and Amino Acids Profile of Milk from Different Animal Species. Asian-Australas. J. Anim. Sci. 2016, 29, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.M.; Chourasia, R.; Chiring Phukon, L.; Singh, S.P.; Kumar Rai, A. Characterization of ACE inhibitory and antioxidant peptides in yak and cow milk hard chhurpi cheese of the Sikkim Himalayan region. Food Chem. X 2022, 13, 100231. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ren, F.; Li, Y.; Nan, Q.; Song, J. Comparison of the lymphoproliferation activity of yak milk casein hydrolysates hydrolyzed by microbial-derived and animal-derived proteinase. J. Food Biochem. 2007, 31, 289–299. [Google Scholar] [CrossRef]
- Kulyar, M.F.-e.-A.; Yao, W.; Ding, Y.; Li, K.; Zhang, L.; Li, A.; Waqas, M.; Huachun, P.; Quan, M.; Zeng, Z.; et al. Bioactive potential of yak’s milk and its products; pathophysiological and molecular role as an immune booster in antibiotic resistance. Food Biosci. 2021, 39, 100838. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Kreuzer, M.; Guo, X.; Mi, J.; Gou, Y.; Shang, Z.; Zhang, Y.; Zhou, J.; Wang, H.; et al. Seasonal variations in the fatty acid profile of milk from yaks grazing on the Qinghai-Tibetan plateau. J. Dairy Res. 2013, 80, 410–417. [Google Scholar] [CrossRef]
- He, S.; Ma, Y.; Wang, J.; Li, Q.; Yang, X.; Tang, S.; Li, H. Milk fat chemical composition of yak breeds in China. J. Food Compos. Anal. 2011, 24, 223–230. [Google Scholar] [CrossRef]
- Wang, F.; Chen, M.; Luo, R.; Huang, G.; Wu, X.; Zheng, N.; Zhang, Y.; Wang, J. Fatty acid profiles of milk from Holstein cows, Jersey cows, buffalos, yaks, humans, goats, camels, and donkeys based on gas chromatography–mass spectrometry. J. Dairy Sci. 2022, 105, 1687–1700. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Z.-W.; Song, J.-H.; Pang, R.-P.; Ren, F.-Z. Lipid Composition of Different Breeds of Milk Fat Globules by Confocal Raman Microscopy. Guang Pu Xue Yu Guang Pu Fen Xi 2016, 36, 125–129. [Google Scholar]
- Cui, G.X.; Yuan, F.; Degen, A.A.; Liu, S.M.; Zhou, J.W.; Shang, Z.H.; Ding, L.M.; Mi, J.D.; Wei, X.H.; Long, R.J. Composition of the milk of yaks raised at different altitudes on the Qinghai–Tibetan Plateau. Int. Dairy J. 2016, 59, 29–35. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, N.; Wang, J.; Yang, Y. Comparative milk fatty acid analysis of different dairy species. Int. J. Dairy Technol. 2018, 71, 544–550. [Google Scholar] [CrossRef]
- Yang, L.; Yang, C.; Chi, F.; Gu, X.; Zhu, Y. A Survey of the Vitamin and Mineral Content in Milk from Yaks Raised at Different Altitudes. Int. J. Food Sci. 2021, 2021, 1855149. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E.E. Consumption of cow’s milk as a cause of iron deficiency in infants and toddlers. Nutr. Rev. 2011, 69, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Haijun, C.; Bozhong, G. Study on content determination of vitamin A and E in white yak′ s milk by HPLC. J. Gansu Agric. Univ. 2007, 42, 108–111. [Google Scholar]
- Qu, S.; Barrett-Wilt, G.; Fonseca, L.M.; Rankin, S.A. A profile of sphingolipids and related compounds tentatively identified in yak milk. J. Dairy Sci. 2016, 99, 5083–5092. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Srivastava, G.; Deepak, D.; Singh, M.P.V.V. Isolation, structure elucidation and DFT study on two novel oligosaccharides from yak milk. J. Mol. Struct. 2016, 1117, 69–78. [Google Scholar] [CrossRef]
- Zheng, J.; Du, M.; Jiang, W.; Zhang, J.; Shen, W.; Ma, X.; Liang, Z.; Shen, J.; Wu, X.; Ding, X. In Vitro Probiotic Characteristics and Whole Genome Sequence Analysis of Lactobacillus Strains Isolated from Cattle-Yak Milk. Biology 2022, 11, 44. [Google Scholar] [CrossRef]
- Li, W.; Gao, L.e.; Huang, W.; Ma, Y.; Muhammad, I.; Hanif, A.; Ding, Z.; Guo, X. Antioxidant properties of lactic acid bacteria isolated from traditional fermented yak milk and their probiotic effects on the oxidative senescence of Caenorhabditis elegans. Food Funct. 2022, 13, 3690–3703. [Google Scholar] [CrossRef]
- Nag, D.; Goel, A.; Padwad, Y.; Singh, D. In Vitro Characterisation Revealed Himalayan Dairy Kluyveromyces marxianus PCH397 as Potential Probiotic with Therapeutic Properties. Probiotics Antimicrob. Proteins 2022, 1–13. [Google Scholar] [CrossRef]
- Mendonça, J.D.; Guimarães, R.D.; Zorgetto-Pinheiro, V.A.; Fernandes, C.D.; Marcelino, G.; Bogo, D.; Freitas, K.D.; Hiane, P.A.; de Pádua Melo, E.S.; Vilela, M.L.; et al. Natural Antioxidant Evaluation: A Review of Detection Methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, V.A.; Zamboni, P.; Mahajan, T.R. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, M.; Zhao, B.; Yang, F. Isolation of antioxidant peptides from yak casein hydrolysate. RSC Adv. 2020, 10, 19844–19851. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; He, X.; Chen, T.; Liu, J.; Luo, Z.; Sun, S.; Qin, D.; Huang, W.; Tang, Y.; Liu, C.; et al. Peptides Isolated from Yak Milk Residue Exert Antioxidant Effects through Nrf2 Signal Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 9426314. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wang, L.; Zhang, J.; Ke, W.; Zhou, J.; Zhu, J.; Guo, X.; Long, R. Characterization of antioxidant properties of lactic acid bacteria isolated from spontaneously fermented yak milk in the Tibetan Plateau. J. Funct. Foods 2017, 35, 481–488. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Identification of Anticancer Peptides from Bovine Milk Proteins and Their Potential Roles in Management of Cancer: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 123–138. [Google Scholar] [CrossRef]
- Salzano, A.; Neglia, G.; D’Onofrio, N.; Balestrieri, M.L.; Limone, A.; Cotticelli, A.; Marrone, R.; Anastasio, A.; D’Occhio, M.J.; Campanile, G. Green feed increases antioxidant and antineoplastic activity of buffalo milk: A globally significant livestock. Food Chem. 2021, 344, 128669. [Google Scholar] [CrossRef]
- Gu, H.; Liang, L.; Zhu, Z.; Mao, X. Preparation and identification of anti-breast cancer cells peptides released from yak milk casein. Front. Nutr. 2022, 9, 997514. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, H.; Jangra, M.; Kaur, L.; Jaswal, P.; Dureja, C.; Nandanwar, H.; Chaudhuri, S.R.; Raje, M.; Mishra, S.; et al. Lactic acid bacteria isolated from yak milk show probiotic potential. Appl. Microbiol. Biotechnol. 2017, 101, 7635–7652. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Wang, S.; Yi, H.; Han, X.; Fan, R.; Zhang, Y. Physicochemical characterization and antitumour activity of exopolysaccharides produced by Lactobacillus casei SB27 from yak milk. Carbohydr. Polym. 2017, 171, 307–315. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Li, J.; Fu, F.; Zheng, Y.; Zhang, L. Molecular characterization, expression and functional analysis of yak IFITM3 gene. Int. J. Biol. Macromol. 2021, 184, 349–357. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Luo, X.; Guan, J.; Zhang, X.; Zhang, L.; Xiang, Y. Molecular characterization, expression and anti-tumor function analysis of yak IFITM2 gene. Int. J. Biol. Macromol. 2022, 209, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Yang, F.; Hu, Z.; Liu, J.; Wu, Q.; Luo, Y.; Yang, L.; Han, S.; Luo, F. Peptide T8 isolated from yak milk residue ameliorates H2O2-induced oxidative stress through Nrf2 signaling pathway in HUVEC cells. Food Biosci. 2021, 44, 101408. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Cheng, X.; Wang, X.; Wu, S.-J. Free-radical-scavenging and anti-inflammatory effect of yak milk casein before and after enzymatic hydrolysis. Food Chem. 2011, 126, 484–490. [Google Scholar] [CrossRef]
- Long, X.; Li, Q.; Zhao, X. Free radical scavenging ability of soybean milk fermented by Lactobacillus plantarum YS4 isolated from yak yoghurt in vitro. IOP Conf. Ser. Earth Environ. Sci. 2021, 792, 012020. [Google Scholar] [CrossRef]
- Kumar, S.; Chouhan, V.S.; Sanghi, A.; Teotia, U.V.S. Antioxidative effect of yak milk caseinates hydrolyzed with three differenct proteases. Vet. World 2013, 6, 799–802. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Pei, J.; Jiang, H.; Li, X.; Jin, W.; Tao, Y. Antimicrobial peptides sourced from post-butter processing waste yak milk protein hydrolysates. AMB Express 2017, 7, 217. [Google Scholar] [CrossRef]
- Cheng, X.; Tang, X.; Wang, Q.; Mao, X.Y. Antibacterial effect and hydrophobicity of yak κ-casein hydrolysate and its fractions. Int. Dairy J. 2013, 31, 111–116. [Google Scholar] [CrossRef]
- Kaur, M.; Jangra, M.; Singh, H.; Tambat, R.; Singh, N.; Jachak, S.M.; Mishra, S.; Sharma, C.; Nandanwar, H.; Pinnaka, A.K. Pseudomonas koreensis Recovered From Raw Yak Milk Synthesizes a β-Carboline Derivative With Antimicrobial Properties. Front. Microbiol. 2019, 10, 1728. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Zhang, L.; Waqas, M.; Mehmood, K.; Iqbal, M.; Muyou, C.; Li, Z.; Lian, Y.; Sizhu, S.; et al. Probiotic potential of Lactobacillus on the intestinal microflora against Escherichia coli induced mice model through high-throughput sequencing. Microb. Pathog. 2019, 137, 103760. [Google Scholar] [CrossRef]
- Cao, F.; Liang, M.; Liu, J.; Liu, Y.; Renye, J.A.; Qi, P.X.; Ren, D. Characterization of an exopolysaccharide (EPS-3A) produced by Streptococcus thermophilus ZJUIDS-2-01 isolated from traditional yak yogurt. Int. J. Biol. Macromol. 2021, 192, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Song, J.; Zeng, W.; Wang, H.; Zhang, Y.; Xin, J.; Suo, H. A broad-spectrum novel bacteriocin produced by Lactobacillus plantarum SHY 21–2 from yak yogurt: Purification, antimicrobial characteristics and antibacterial mechanism. LWT 2021, 142, 110955. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Mohan, A. Mechanisms of food protein-derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Sánchez, D.; Kassan, M.; Contreras, M.d.M.; Carrón, R.; Recio, I.; Montero, M.-J.; Sevilla, M.-Á. Long-term intake of a milk casein hydrolysate attenuates the development of hypertension and involves cardiovascular benefits. Pharmacol. Res. 2011, 63, 398–404. [Google Scholar] [CrossRef]
- Lin, K.; Ma, Z.; Ramachandran, M.; De Souza, C.; Han, X.; Zhang, L.-w. ACE inhibitory peptide KYIPIQ derived from yak milk casein induces nitric oxide production in HUVECs and diffuses via a transcellular mechanism in Caco-2 monolayers. Process. Biochem. 2020, 99, 103–111. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, S.; Ren, F.; Luo, Z.; Zeng, S.S. Yak Milk Casein as a Functional Ingredient: Preparation and Identification of Angiotensin-I-Converting Enzyme Inhibitory Peptides. J. Dairy Res. 2007, 74, 18–25. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.-W.; Han, X.; Cheng, D.-Y. Novel angiotensin I-converting enzyme inhibitory peptides from protease hydrolysates of Qula casein: Quantitative structure-activity relationship modeling and molecular docking study. J. Funct. Foods 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Ni, J.-R.; Sun, W.-L.; Hao, P.-P.; Fan, L. Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides. Food Chem. 2007, 103, 1282–1287. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.; Han, X.; Meng, Z.; Zhang, J.; Wu, Y.; Cheng, D. Quantitative Structure–Activity Relationship Modeling Coupled with Molecular Docking Analysis in Screening of Angiotensin I-Converting Enzyme Inhibitory Peptides from Qula Casein Hydrolysates Obtained by Two-Enzyme Combination Hydrolysis. J. Agric. Food Chem. 2018, 66, 3221–3228. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, L.-w.; Han, X.; Xin, L.; Meng, Z.-x.; Gong, P.-m.; Cheng, D.-y. Yak milk casein as potential precursor of angiotensin I-converting enzyme inhibitory peptides based on in silico proteolysis. Food Chem. 2018, 254, 340–347. [Google Scholar] [CrossRef]
- Feng, R.; Zou, X.; Wang, K.; Liu, H.; Hong, H.; Luo, Y.; Tan, Y. Antifatigue and microbiome reshaping effects of yak bone collagen peptides on Balb/c mice. Food Biosci. 2023, 52, 102447. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Wu, S.; Li, H.; Li, Y.; Mi, F.; Wang, X.; Zhang, L. Anti-fatigue effect of yak milk powder in mouse model. Dairy Sci. Technol. 2015, 95, 245–255. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Zhang, L.; Chen, Q.; Tan, F.; Zhao, X. Effect of Lactobacillus fermentum HFY03 on the Antifatigue and Antioxidation Ability of Running Exhausted Mice. Oxid. Med. Cell. Longev. 2021, 2021, 8013681. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Suo, H.; Du, M.; Zhao, X.; Li, J.; Li, G.J.; Song, J.-L.; Liu, Z. Preventive effect of Lactobacillus fermentum Lee on activated carbon-induced constipation in mice. Exp. Med. 2015, 9, 272–278. [Google Scholar] [CrossRef]
- Zhao, X.; Qian, Y.; Li, G.; Yi, R.; Park, K.-Y.; Song, J.-L. Lactobacillus plantarum YS2 (yak yogurt Lactobacillus) exhibited an activity to attenuate activated carbon-induced constipation in male Kunming mice. J. Dairy Sci. 2019, 102, 26–36. [Google Scholar] [CrossRef]
- Zhao, X.; Yi, R.; Qian, Y.; Park, K.-Y. Lactobacillus plantarum YS-3 Prevents Activated Carbon-Induced Constipation in Mice. J. Med. Food 2018, 21, 575–584. [Google Scholar] [CrossRef]
- Ali, S.M.; Salem, F.E.; Aboulwafa, M.M.; Shawky, R.M. Hypolipidemic activity of lactic acid bacteria: Adjunct therapy for potential probiotics. PLoS ONE 2022, 17, e0269953. [Google Scholar] [CrossRef]
- Qian, Y.; Li, M.; Wang, W.; Wang, H.; Zhang, Y.; Hu, Q.; Zhao, X.; Suo, H. Effects of Lactobacillus Casei YBJ02 on Lipid Metabolism in Hyperlipidemic Mice. J. Food Sci. 2019, 84, 3793–3803. [Google Scholar] [CrossRef]
- Ding, W.; Shi, C.; Chen, M.; Zhou, J.; Long, R.; Guo, X. Screening for lactic acid bacteria in traditional fermented Tibetan yak milk and evaluating their probiotic and cholesterol-lowering potentials in rats fed a high-cholesterol diet. J. Funct. Foods 2017, 32, 324–332. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, S.; Cao, J.; Li, H.; Li, Y.; He, J.; Zhang, L. A preliminary study on anti-hypoxia activity of yak milk powder in vivo. Dairy Sci. Technol. 2014, 94, 633–639. [Google Scholar] [CrossRef]
- Gao, H.N.; Ren, F.Z.; Wen, P.C.; Xie, L.X.; Wang, R.; Yang, Z.N.; Li, Y.X. Yak milk–derived exosomal microRNAs regulate intestinal epithelial cells on proliferation in hypoxic environment. J. Dairy Sci. 2021, 104, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.N.; Hu, H.; Wen, P.C.; Lian, S.; Xie, X.L.; Song, H.L.; Yang, Z.N.; Ren, F.Z. Yak milk–derived exosomes alleviate lipopolysaccharide-induced intestinal inflammation by inhibiting PI3K/AKT/C3 pathway activation. J. Dairy Sci. 2021, 104, 8411–8424. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Kong, X.; Xerenbek, T.; Mamet, T. Yak (Bos grunniens) milk improves bone mass and microarchitecture in mice with osteoporosis. J. Dairy Sci. 2022, 105, 7878–7890. [Google Scholar] [CrossRef]
- Yi, R.; Tan, F.; Liao, W.; Wang, Q.; Mu, J.; Zhou, X.; Yang, Z.; Zhao, X. Isolation and Identification of Lactobacillus plantarum HFY05 from Natural Fermented Yak Yogurt and Its Effect on Alcoholic Liver Injury in Mice. Microorganisms 2019, 7, 530. [Google Scholar] [CrossRef]
- Jiang, Y.; Qi, Y.; Liu, X.; Fang, L.; Gao, Y.; Liu, C.; Wu, D.; Wang, X.; Zhao, F.; Wang, J.; et al. Neuroprotective effects of fermented yak milk-derived peptide LYLKPR on H2O2-injured HT-22 cells. Food Funct. 2022, 13, 12021–12038. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Hani, A.; Li, W.; Gao, L.e.; Ke, W.; Guo, X. Influence of a cholesterol-lowering strain Lactobacillus plantarum LP3 isolated from traditional fermented yak milk on gut bacterial microbiota and metabolome of rats fed with a high-fat diet. Food Funct. 2020, 11, 8342–8353. [Google Scholar] [CrossRef]
- Agyare, A.N.; Liang, Q. Nutrition of yak milk fat—Focusing on milk fat globule membrane and fatty acids. J. Funct. Foods 2021, 83, 104404. [Google Scholar] [CrossRef]
- Joshi, D.D.; Awasthi, B.D.; Sharma, M. An Assessment of the Yak Cheese Factories in Nepal; National Zoonoses and Food Research Center: Kathmandu, Nepal, 1999; p. 75. [Google Scholar]
- Singh, T.P.; Arora, S.; Borad, S.G.; Bam, J.; Paul, V.; Thomas, R.; Sarkar, M. Fatty acid and amino acid profiling, antioxidant activity and other quality characteristics of vacuum packed cheddar style-yak milk cheese during ripening. Food Biosci. 2023, 51, 102213. [Google Scholar] [CrossRef]
- Pei, J.; Li, X.; Han, H.; Tao, Y. Purification and characterization of plantaricin SLG1, a novel bacteriocin produced by Lb. plantarum isolated from yak cheese. Food Control 2018, 84, 111–117. [Google Scholar] [CrossRef]
- Kandeepan, G.; Sangma, S. Technologies for processing traditional yak milk products. Beverage Food World 2009, 36, 47–48. [Google Scholar]
- Kandeepan, G.; Sangma, S. Comparison of quality characteristics of full fat and low fat paneer developed from yak milk. Int. J. Dairy Technol. 2011, 64, 117–120. [Google Scholar] [CrossRef]
- Singh, T.P.; Raigar, R.K.; Bam, J.; Paul, V. Predictive modeling for physicochemical and microbial quality assessment of vacuum-packed yak milk paneer under various storage temperatures. J. Food Process. Preserv. 2022, 46, e16114. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, N.; Wang, Q.; Liu, Z.; Lee, Y.-K.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Microbial diversity and volatile profile of traditional fermented yak milk. J. Dairy Sci. 2020, 103, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Liu, W.; Yu, J.; Wang, W.; Qing, M.; Chen, X.; Wang, F.; Zhang, J.; Zhang, W.; Qiao, J. Isolation and identification of cultivable lactic acid bacteria in traditional yak milk products of Gansu Province in China. J. Gen. Appl. Microbiol. 2012, 58, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Yang, J.; Wang, Q.; Suo, H.; Hamdy, A.M.; Song, J. Antifungal Effect of Metabolites from a New Strain Lactiplantibacillus Plantarum LPP703 Isolated from Naturally Fermented Yak Yogurt. Foods 2023, 12, 181. [Google Scholar] [CrossRef]
- Wang, G.; Song, J.; Huang, Y.; Li, X.; Wang, H.; Zhang, Y.; Suo, H. Lactobacillus plantarum SHY130 isolated from yak yogurt attenuates hyperglycemia in C57BL/6J mice by regulating the enteroinsular axis. Food Funct. 2022, 13, 675–687. [Google Scholar] [CrossRef]
- Li, C.; Liu, H.; Yang, J.; Mu, J.; Wang, R.; Zhao, X. Effect of soybean milk fermented with Lactobacillus plantarum HFY01 isolated from yak yogurt on weight loss and lipid reduction in mice with obesity induced by a high-fat diet. RSC Adv. 2020, 10, 34276–34289. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Song, J.-L.; Wang, R.; Qian, Y.; Wang, Q. Gastric ulcer preventive activities of soybean milk fermented by Lactobacillus isolated from yak yogurt (647.9). FASEB J. 2014, 28, 647–649. [Google Scholar] [CrossRef]
- Phiri, B.S.J.; Sakumona, M.; Hang’ombe, B.M.; Fetsch, A.; Schaarschmidt, S. The traditional dairy value chain in Zambia and potential risk factors to microbiological food safety. Food Control 2021, 124, 107885. [Google Scholar] [CrossRef]
- Ao, X.; Zhang, X.; Zhang, X.; Shi, L.; Zhao, K.; Yu, J.; Dong, L.; Cao, Y.; Cai, Y. Identification of lactic acid bacteria in traditional fermented yak milk and evaluation of their application in fermented milk products. J. Dairy Sci. 2012, 95, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, W.; Yang, G.; Yu, J. Recent progress of yak milk products in China. Food Ferment. Ind. 2021, 47, 318–324. [Google Scholar] [CrossRef]
- Feng, D.; Wang, J.; Ji, X.-J.; Min, W.-X.; Yan, W.-J. Analysis of Volatile Organic Compounds by HS-GC-IMS in Powdered Yak Milk Processed under Different Sterilization Conditions. J. Food Qual. 2021, 2021, 5536645. [Google Scholar] [CrossRef]
| Yak Breeds (Country) | Milk Solids | Fat | Protein | Lactose | Ash |
|---|---|---|---|---|---|
| Tianzhu White yak (China) | 16.3–18.4 | 5.6–5.8 | 4.7–6.5 | 5.0–5.3 | 0.77–0.87 |
| Jiulong (China) | 17.3–17.8 | 6.9–7.2 | 4.9–4.9 | 4.7–4.8 | 0.79–0.83 |
| Maiwa (China) | 17.5 | 6.3 | 4.9 | 5.4 | 0.82 |
| Inner Mongolia (China) | 17.8 | 6.8 | 5.0 | 5.1 | 0.86 |
| Lulang (China) | 18.0 | 6.9 | 5.0 | 4.8 | 0.79 |
| Songduo (China) | 18.4 | 7.1 | 5.0 | 5.1 | 0.81 |
| Milashan (China) | 19.0 | 7.4 | 5.2 | 5.2 | 0.83 |
| Jiali (China) | 16.3 | 6.8 | 5.0 | 3.6 | 0. 95 |
| Pali (China) | 16.3 | 6.0 | 5.7 | 3.8 | / |
| Sibu (China) | 17.1 | 7.5 | 5.3 | 3.5 | / |
| Kirghizia | 17.4 | 6.6 | 6.3 | 4.6 | 0.87 |
| Nepal | 17.4 | 6.5 | 5.4 | 4.6 | 0.90 |
| India | 17.9 | 6.5 | 5.9 | 4.7 | 0.87 |
| Holsteinbovine | 11.8–13.7 | 2.8–4.0 | 2.8–4.0 | 4.6–4.9 | 0.60–0.80 |
| Yellow bovine (China) | 12.8 | 3.9 | 3.4 | 4.8 | 0.86 |
| Buffalo (China) | 18.4 | 7.6 | 4.9 | 4.7 | 0.85 |
| Zhongdian Yak | Gannan Yak | Maiwa Yak | Cow | Buffalo | Goat | |
|---|---|---|---|---|---|---|
| TN | 0.68 ± 0.02 | 0.84 ± 0.06 | 0.79 ± 0.04 | 3.25 ± 0.03 | 3.87 ± 0.02 | 2.95 ± 0.02 |
| NPN | 0.07 ± 0.11 | 0.03 ± 0.02 | 0.04 ± 0.01 | 0.33 ± 0.03 | 0.38 ± 0.02 | 0.39 ± 0.01 |
| NPN/TN | 10.29 | 3.58 | 5.06 | 10.15 | 9.82 | 13.22 |
| WPN | 0.13 ± 0.52 | 0.17 ± 0.04 | 0.15 ± 0.01 | 0.47 ± 0.01 | 0.68 ± 0.02 | 0.53 ± 0.02 |
| WPN/TN | 19.12 | 20.23 | 18.99 | 14.46 | 17.57 | 17.97 |
| CN | 0.48 ± 0.17 | 0.64 ± 0.06 | 0.60 ± 0.03 | 2.79 ± 0.02 | 3.20 ± 0.03 | 2.44 ± 0.03 |
| CN/TN | 70.59 | 76.19 | 75.95 | 85.85 | 82.69 | 82.71 |
| WPN/CN | 27.08 | 26.41 | 25 | 16.85 | 21.25 | 21.72 |
| Types of Proteins | Yak | Cow | Buffalo | Goat |
|---|---|---|---|---|
| Total casein (g/100 g) | 2.10–4.00 | 2.40–2.80 | 2.70–5.00 | 2.30–3.80 |
| αS1-casein (mg/100 g) | 416–1024 | 806–1508 | 1147–1924 | 135–1020 |
| αS2-casein (mg/100 g) | 288–576 | 182–390 | 222–629 | 270–750 |
| β-casein (mg/100 g) | 1184–1632 | 728–988 | 1295–1702 | 1020–1920 |
| κ-casein (mg/100 g) | 384–672 | 234–520 | 407–592 | 300–570 |
| Total whey proteins (g/100 g) | 1.10 | 0.50–0.70 | 0.60–1.00 | 0.30–1.20 |
| α-lactalbumin (mg/100 g) | 77–220 | 96–150 | 117–303 | 85–250 |
| β-lactoglobulin (mg/100 g) | 550–946 | 198–402 | 301–441 | 170–385 |
| Serum albumin (mg/100 g) | 77–165 | 36–45 | 2.1–35 | 25–110 |
| Lactoferrin (mg/kg) | 200–700 | 20–500 | 20–300 | 20–300 |
| Immunoglobulins (mg/kg) | 100–400 | 150–1000 | 500–1300 | 150–500 |
| Lactoperoxidase (units/mL) | 2.95 | 1.40 | 0.90 | 0.26–4.55 |
| Types of Amino Acids | Maiwa Yak | Gannan Yak | Human | Bovine | Goat |
|---|---|---|---|---|---|
| Thr | 0.18 | 0.21 | 0.05 | 0.15 | 0.16 |
| Val | 0.25 | 0.22 | 0.06 | 0.16 | 0.24 |
| Met | 0.11 | 0.13 | 0.02 | 0.06 | 0.08 |
| Ile | 0.23 | 0.20 | 0.06 | 0.14 | 0.21 |
| Leu | 0.42 | 0.46 | 0.10 | 0.29 | 0.31 |
| Phe | 0.21 | 0.23 | 0.05 | 0.16 | 0.16 |
| Lys | 0.37 | 0.39 | 0.07 | 0.27 | 0.29 |
| His | 0.11 | 0.11 | 0.02 | 0.10 | 0.09 |
| Trp | 0.06 | 0.06 | 0.02 | 0.05 | 0.04 |
| EAA | 1.94 | 2.00 | 0.45 | 1.33 | 1.58 |
| Cys | 0.03 | 0.03 | 0.02 | 0.02 | 0.05 |
| Arg | 0.15 | 0.15 | 0.04 | 0.11 | 0.12 |
| Pro | 0.45 | 0.48 | 0.08 | 0.32 | 0.37 |
| Asp | 0.33 | 0.36 | 0.08 | 0.26 | 0.21 |
| Ser | 0.23 | 0.27 | 0.04 | 0.16 | 0.18 |
| Glu | 1.03 | 1.13 | 0.17 | 0.77 | 0.63 |
| Gly | 0.09 | 0.10 | 0.03 | 0.06 | 0.05 |
| Ala | 0.14 | 0.16 | 0.04 | 0.10 | 0.12 |
| Tyr | 0.20 | 0.22 | 0.05 | 0.15 | 0.18 |
| NEAA | 2.66 | 2.92 | 0.55 | 1.95 | 1.91 |
| TAA | 4.60 | 4.91 | 1.00 | 3.33 | 3.49 |
| EAA/NEAA | 73% | 68% | 82% | 68% | 81% |
| EAA/TAA | 42% | 41% | 45% | 40% | 45% |
| Fatty Acid | Content | Fatty Acid | Content | Fatty Acid | Content |
|---|---|---|---|---|---|
| ECSFA | BCSFA | C22:1 c13 | 0.12 ± 0.06 | ||
| C4:0 | 2.12 ± 0.63 | C13:0 iso | 0.07 ± 0.01 | C24:1 c15 | 0.04 ± 0.02 |
| C6:0 | 1.49 ± 0.20 | C13:0 anteiso | 0.02 ± 0.00 | ∑ MUFA | 35.60 |
| C8:0 | 0.92 ± 0.11 | C14:0 iso | 0.22 ± 0.03 | PUFA | |
| C10:0 | 1.75 ± 0.23 | C15:0 iso | 0.46 ± 0.08 | C18:2 c9c12 | 1.13 ± 0.24 |
| C12:0 | 1.47 ± 0.26 | C15:0 anteiso | 0.79 ± 0.13 | C18:2 t9t12 | 0.18 ± 0.02 |
| C14:0 | 6.25 ± 0.94 | C16:0 iso | 0.35 ± 0.07 | ∑ Nonconjugated C18:2 others | 0.76 ± 0.14 |
| C16:0 | 22.06 ± 6.45 | C17:0 iso | 0.46 ± 0.08 | C18:3 c6c9c12 | 0.02 ± 0.01 |
| C18:0 | 15.03 ± 2.27 | C17:0 anteiso | 0.52 ± 0.11 | C18:3 c9c12c15 | 1.12 ± 0.24 |
| C20:0 | 0.69 ± 0.30 | C18:0 iso | 0.07 ± 0.03 | C18:2 c9t11 | 2.57 ± 1.17 |
| C22:0 | 0.35 ± 0.15 | ∑ BCSFA | 2.95 | C18:2 t10c12 | 0.03 ± 0.03 |
| C24:0 | 0.22 ± 0.07 | MUFA | C20:2 c11c14 | 0.02 ± 0.02 | |
| ∑ ECSFA | 52.33 | C10:1 c9 | 0.31 ± 0.08 | C20:3 c8c11c14 | 0.02 ± 0.02 |
| OCSFA | C12:1 c5 | 0.00 ± 0.01 | C20:4 c5c8c11c14 | 0.09 ± 0.02 | |
| C5:0 | 0.01 ± 0.01 | C12:1 c9 | 0.03 ± 0.01 | C20:3 c11c14c17 | 0.02 ± 0.01 |
| C7:0 | 0.02 ± 0.01 | C14:1 c9 | 0.29 ± 0.07 | C20:5 c5c8c11c14c17 | 0.06 ± 0.01 |
| C9:0 | 0.01 ± 0.01 | C16:1 c7 | 0.27 ± 0.09 | C22:2 c13c16 | 0.02 ± 0.02 |
| C11:0 | 0.02 ± 0.01 | C16:1 c9 | 1.75 ± 0.44 | C22:4 c7c10c13c16 | 0.03 ± 0.03 |
| C13:0 | 0.06 ± 0.01 | C17:1 c9 | 0.37 ± 0.13 | C22:5 c4c7c10c13c16 | 0.02 ± 0.02 |
| C15:0 | 1.31 ± 0.19 | ∑ C18:1 | 31.81 ± 7.61 | C22:5 c7c10c13c16c19 | 0.21 ± 0.04 |
| C17:0 | 0.84 ± 0.18 | C19:1 c10 | 0.26 ± 0.08 | C22:6 c4c7c10c13c16c19 | 0.03 ± 0.01 |
| C19:0 | 0.17 ± 0.06 | C19:1 t10 | 0.05 ± 0.04 | ∑ n-6 | 1.57 |
| C21:0 | 0.14 ± 0.06 | C20:1 c9 | 0.21 ± 0.08 | ∑ n-3 | 1.43 |
| C23:0 | 0.19 ± 0.06 | C20:1 c11 | 0.07 ± 0.02 | n-6/n-3 | 1.09 |
| ∑ OCSFA | 2.78 | C20:1 t11 | 0.01 ± 0.01 | ∑ PUFA | 6.33 |
| Maiwa Yak | Gannan Yak | Human | Bovine | Goat | |
|---|---|---|---|---|---|
| Cu | 0.45 ± 0.08 | 0.65 ± 0.02 | 0.6 | 0.1–0.6 | 0.5 |
| Mg | 154.10 ± 13.22 | 150.59 ± 13.98 | 40 | 90.00–140.00 | 160 |
| Zn | 7.31 ± 0.44 | 1.76 ± 0.33 | 3.8 | 2.00–6.00 | 5.6 |
| Fe | 0.57 ± 0.04 | 1.25 ± 0.05 | 2.0 | 0.012–0.035 | 0.7 |
| Mn | 0.06 ± 0.01 | 0.02 ± 0.02 | 0.7 | 0.16–0.35 | 0.32 |
| Ca | 1545.45 ± 145.61 | 1525.2 ± 177.0 | 330 | 1000.00–1300.00 | 1340 |
| P | 922.04 ± 70.13 | 1023.9 ± 81.2 | 430 | 900.00–1000.00 | 1210 |
| Maiwa Yak | Gannan Yak | Human | Bovine | Goat | |
|---|---|---|---|---|---|
| Vitamin B1 | 48.54 ± 11.54 | 23.56 ± 11.29 | 17.00 | 45.00 | 68.00 |
| Vitamin B2 | 79.49 ± 28.15 | 20.79 ± 5.74 | 20.00 | 175.00 | 376.00 |
| Vitamin B3 | 2.61 ± 3.21 | 1.58 ± 0.67 | 0.20 | 90.00 | 0.41 |
| Vitamin B6 | 40.75 ± 16.21 | 0.36 ± 0.18 | 0.01 | 0.23 | 0.08 |
| Vitamin A | 13.88 ± 4.52 | 89.79 ± 4.72 | 60.80 | 47.74 | 46.72 |
| Vitamin D | 0.15 ± 0.21 | 3.95 ± 0.30 | 0.04 | 0.06 | 0.18 |
| Vitamin E | 30.15 ± 7.30 | 91.85 ± 21.25 | / | 100.00 | / |
| Function | Active Ingredients | Cell Line or Model | Dose | Detailed Content | Research Evaluation | References |
|---|---|---|---|---|---|---|
| Antioxidant | Peptide (T10) KALNEINQF | H2O2-induced injury model of HUVECs | 25, 50 or 100 μg/mL | Involved in regulating the Nrf2 signaling pathway and cell apoptosis. | Provide a theoretical basis for the development of functional foods in the future. | [44] |
| Peptide (T8) Met-His-Gln-Pro-His-Gln-Pro-Leu-Pro-Pro-Thr-Val-Met-Phe | / | 6.25, 12.5, 25 and 50 μg/mL | Improve H2O2-induced oxidative stress in HUVEC cells by regulating the Nrf2 signaling pathway. | Promote the application of yak milk residue in functional foods. | [54] | |
| Casein hydrolysate peptide Glu-Leu-Glu-Glu-Leu | / | / | Scavenging activity against superoxide anion and hydroxyl radical (with IC50 values of 0.52 and 0.69 mg/mL). | Can serve as a source of natural antioxidant peptides. | [43] | |
| Casein hydrolysate | / | 2.5 mg/mL | Exhibit free radical scavenging activity against DPPH, superoxide, and hydrogen peroxide. | Yak casein hydrolysate exhibits antioxidant activity. | [55] | |
| Lactobacillus plantarum YS4 | / | / | Has the ability to scavenge DPPH, ABTS, and hydroxyl free radicals. | The effect was better than that of commercial Lactobacillus bulgaricus. | [56] | |
| Lactic acid bacteria in fermented yak milk | Ageing mice induced by D-galactose | 0.3 mL (1 × 1010 CFU/mL) | The activity of GSH-Px in livers and serum, as well as the activity of SOD in mouse serum and brains, significantly increased, while the level of MDA in mouse livers and serum significantly decreased. | It is a potential antioxidant strain for the production of functional foods. | [45] | |
| Casein hydrolysate | / | / | The antioxidant activity of trypsin hydrolysate is the highest. | Yak milk casein can serve as a resource for producing antioxidant peptides. | [57] | |
| Lactobacillus plantarum As21 | Caenorhabditis elegans | 0.3 mL (1 × 108 CFU/mL) | Reduced the production of ROS and MDA and promoted the production of SOD, CAT, and GSH. | It may be a potential probiotic strain for delaying aging and can be used in functional foods. | [39] | |
| Anti-cancer activity | Lan4 strain | Hela cells | / | Induced maximum apoptosis in Hela cells (87%) and was non-toxic to non-cancerous HEK293 cells. | This shows excellent probiotic properties and ideal health benefits. | [50] |
| Peptide TPVVVPPFL | MDA-MB-231 and MCF7 cells | 0, 62.5, 125, 250, 500, and 1000 µg/mL | Induces G2-M cell cycle arrest in MCF7 cells, S cell cycle arrest in MDA-MB-231 cells, and induces apoptosis in both cancer cell lines. | Yak milk is a good source of peptides with anti-breast cancer cell activity. | [49] | |
| Exopolysaccharides produced by Lactobacillus casei SB27 from yak milk | HT-29 colorectal cancer cells | / | It can significantly inhibit the proliferation of HT-29 colon cancer cells and upregulate the expression of the Bad, Bax, Caspase-3, and -8 genes. | It has the potential to be a functional food and can be a source of natural anti-tumor drugs. | [51] |
| Function | Active Ingredients | Cell Line or Model | Dose | Detailed Content | Research Evaluation | References |
|---|---|---|---|---|---|---|
| Anti-bacterial activity | Exopolysaccharide (EPS-3A) produced by Streptococcus thermophilus ZJUIDS-2-01 | / | 8 mg/mL | It has inhibitory effects against Staphylococcus aureus CMCC 26003 and Lactobacillus monocytogenes CMCC 54007. | It can be developed as a natural alternative to antibiotics. | [63] |
| Y5-P1 | / | / | Antibacterial compound Y5-P1, from this strain, has biologically active effects against both Gram-positive and Gram-negative bacteria. | It could be further developed as a candidate drug against highly resistant pathogens. | [61] | |
| Antimicrobial peptides Arg-Val-Met-Phe-Trp-Ala and Val-Ile-Ser-Met-Ile. | / | / | The peptide Arg-Val-Met-Phe-Lys-Trp-Ala has inhibitory effects against Bacillus subtilis, Staphylococcus aureus, Listeria innocua, Escherichia coli, Enterobacter cloacae, and Salmonella typhi. The peptide Lys-Val-Ile-Ser-Met-Ile also has inhibitory effects against fungi. | / | [59] | |
| κ-casein hydrolysate | / | / | Exhibits strong antibacterial activity against Escherichia coli. | It can serve as a potential inhibitor for Escherichia coli. | [60] | |
| Mouse diarrhea model | 1 × 109 CFU/day | Regulates intestinal microbiota in an E. coli-induced diarrhea mouse model, improving diarrhea. | Pre-supplementation of LR1 can alleviate clinical symptoms caused by E. coli and promote the health of the gut microbiota. | [62] | ||
| Lactobacillus Plantarum SHY21-2 | / | / | It has a broad spectrum of antibacterial activity against Gram-positive and Gram-negative bacteria as well as fungi. | It has the potential to be used as a biopreservative in food. | [64] | |
| Anti-hypertensive | Peptides in fermented foods with yak milk | / | / | It has strong ACE inhibitory activity. | Natural nutritional products rich in highly active ACE inhibitory peptides can be developed. | [24] |
| YQKFPQY LPQNIPPL SKVLPVPQK LPYPYY FLPYPYY | / | / | It has strong ACE inhibitory activity. | Yak cheese protein can be used as a resource for the production of anti-hypertensive peptides. | [68] | |
| Casein hydrolyzed peptide | / | / | Release of active and non-toxic ACE inhibitory peptides. | Yak cheese protein is a good precursor for the production of many bioactive peptides. | [72] | |
| Casein hydrolyzed peptide PPEIN, PLPLL | / | / | It has strong ACE inhibitory activity with an IC50 of 0.29 ± 0.01 mg/mL and 0.25 ± 0.01 mg/mL, respectively. | It can be used as a raw material for the production of functional foods. | [70] | |
| Casein hydrolyzed peptide KYIPIQ | / | / | It has strong ACE inhibitory activity with an IC50 of 7.28 μM. | May be a valuable source of ACE inhibitory peptides. | [69] | |
| KYIPIQ | Human vascular endothelial cells | / | Increased synthesis of NO and expression of eNOS in HUVECs. | It has the potential to be used as a therapeutic agent for the treatment of hypertension. | [67] | |
| Casein hydrolyzed peptide KFPQY | / | / | It has strong ACE inhibitory activity with an IC50 of 12.37 ± 0.43 μM. | / | [71] |
| Function | Active Ingredients | Cell Line or Model | Dose | Detailed Content | Research Evaluation | References |
|---|---|---|---|---|---|---|
| Anti-fatigue | Lactobacillus fermentum HFY03 | ICR mice | 1:0 × 108 and 1:0 × 109 CFU/kg | Reduces protein catabolism, improves hepatic glycogen storage capacity, reduces lactate accumulation, and increases fat consumption. | It can be used as an anti-fatigue, probiotic nutritional supplement. | [75] |
| Yak milk powder | Kunming mice | 2.6, 5.2, and 7.8 g·kg bw−1·day−1 | Dose-dependent increase in forced swimming time in mice; increased liver glycogen content; and decreased serum triglyceride levels. | It can improve physical endurance and relieve fatigue. | [74] | |
| Improve constipation | Lactobacillus fermentum Lee | ICR mice | 1 × 108 and 1 × 109 CFU/mL | Increased gastrointestinal transit ratio; increased MTL, Gas, ET, AChE, SP, and VIP levels. | Preventive effect on constipation in mice. | [77] |
| Lactobacillus plantarum YS2 | Kunming mice | 1.0 × 108 and 1.0 × 109 cfu/kg of BW | Reduces the time to first black stool defecation and promotes gastrointestinal motility in constipated mice; increases serum MTL, gastrin, AChE, SP, and VIP levels. | Ability to reduce activated charcoal-induced constipation in Kunming mice. | [78] | |
| Lactobacillus plantarum YS3 | Kunming mice | 1 × 108 and 1 × 109 CFU/kg | Reduced time required for first black stool evacuation; increased MTL, Gas, ET, AChE, SP, and VIP levels. | It can effectively suppress constipation. | [79] | |
| Hypolipidemic effect | Lactobacillus plantarum Lp3 | Sprague-Dawley rats | 109 CFU/mL | It significantly reduced serum and liver cholesterol and triglyceride levels and reduced lipid deposition in the cytoplasm of rat liver tissue. | May be a potential probiotic for the treatment of hyperlipidemia and may be used in functional foods. | [82] |
| Lactobacillus Casei YBJ02 | Kunming mice | 1 × 1010 and 1 × 109 CFU/kg | Regulates intestinal flora in mice and inhibits lipid accumulation by regulating obesity gene synthesis through the PPAR-alpha pathway. | It can be used as a probiotic due to its antilipidemic effect. | [81] | |
| Hypoxia resistant | Yak milk powder | BALB/c mice hypoxia model | 2.6, 5.2, 7.8 mg·g−1 bw·day−1 | Prolongs survival time under conditions of normoxia and sodium nitrite intoxication; increases red blood cell and hemoglobin levels. | It can be used as a novel anti-hypoxia functional food ingredient. | [83] |
| Yak milk exosome miRNA, bta-miR-34a | Intestinal epithelial cells | / | Protective effect on the survival of intestinal epithelial cells under hypoxic conditions. | Further study of the effects of lactogenic exosomal miRNAs on human intestinal health provides a scientific basis. | [84] | |
| Anti-inflammatory | Exosomal proteins | Intestinal epithelial cells 6 | 0, 50, 100 and 200 ng/μL | Activating the PI3K/AKT/C3 signaling pathway, thereby reducing the incidence and severity of intestinal inflammation. | May be a potential innovative treatment option for intestinal inflammation. | [85] |
| Casein hydrolysate | Mouse macrophages | / | Significantly reduced the production of NO and pro-inflammatory cytokines IL-1β, IL-6, and TNF-α in mouse macrophages. | May be used to prevent inflammation-related diseases. | [55] | |
| Improve osteoporosis | Yak Milk | C57BL/6J mice | 1000 and 2000 mg/kg/d | Can improve bone mass and microarchitecture by inhibiting bone resorption in osteoporotic mice. | / | [86] |
| Improve liver injury | Lactobacillus plantarum HFY05 | Mouse model of alcoholic liver injury | / | Reduced AST, ALT, ALP, TG, TC, BUN, NO, and MDA levels and up-regulated ALB, SOD, CAT, and GSH-Px levels in the serum of liver-injured mice; down-regulated IL-6, IL-12, TNF-α, and IFN-γ levels in the serum of liver-injured mice; and regulated intestinal flora. | Hepatoprotective effect on alcoholic liver injury in experimental mice. | [87] |
| Immune regulation | Casein hydrolysate | Mouse spleen cells | / | Promotes lymphoproliferative activity and IL-2 production in mouse splenocytes. | Casein protease hydrolysate has high immunomodulatory activity. | [25] |
| Neuroprotective effect | Fermented yak milk peptide LYLKPR | H2O2 damage in HT-22 cells | 25, 50, 100, 150, and 200 μM | LYLKPR ameliorates oxidative stress-mediated neuronal injury by inhibiting the NLRP3 inflammasome through regulation of the Nrf2/Keap-1/HO-1 pathway. | Helps with anti-aging. | [88] |
| Cholesterol regulation | Lactobacillus plantarum LP3 | Sprague-Dawley rats | 1 × 1010 CFU/mL | Reduces high cholesterol induced by a high-fat diet by modulating intestinal microbiota and metabolites. | Lactobacillus plantarum LP3 has good potential as a therapeutic probiotic. | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhou, Y.; Zheng, X.; Guo, J.; Duan, H.; Zhou, S.; Yan, W. Yak Milk: Nutritional Value, Functional Activity, and Current Applications. Foods 2023, 12, 2090. https://doi.org/10.3390/foods12112090
Wang D, Zhou Y, Zheng X, Guo J, Duan H, Zhou S, Yan W. Yak Milk: Nutritional Value, Functional Activity, and Current Applications. Foods. 2023; 12(11):2090. https://doi.org/10.3390/foods12112090
Chicago/Turabian StyleWang, Diandian, Yaxi Zhou, Xianping Zheng, Jinhong Guo, Hao Duan, Shiqi Zhou, and Wenjie Yan. 2023. "Yak Milk: Nutritional Value, Functional Activity, and Current Applications" Foods 12, no. 11: 2090. https://doi.org/10.3390/foods12112090
APA StyleWang, D., Zhou, Y., Zheng, X., Guo, J., Duan, H., Zhou, S., & Yan, W. (2023). Yak Milk: Nutritional Value, Functional Activity, and Current Applications. Foods, 12(11), 2090. https://doi.org/10.3390/foods12112090








