Co-Microencapsulation of Sacha Inchi (Plukenetia huayllabambana) Oil with Natural Antioxidants Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Dry Skin Antioxidant Extracts
Total Phenolic Content (TPC) of Antioxidant Extracts
2.3. Co-Microencapsulation of SIPHO with NAE
2.3.1. Moisture Determination
2.3.2. Particle Size Distribution and Morphology of Microcapsules
2.3.3. Total Phenolic Content (TPC) and Surface Phenolic Content (SPC)
2.3.4. Determination of Antioxidant Activity on DPPH Radical
2.3.5. Fatty Acid Composition
2.3.6. Sterol Composition
2.3.7. Oxidative Stability and Shelf-Life
2.4. Statistical Analysis
3. Results
3.1. Total Polyphenolic Content of Dried Skins
3.2. Moisture Determination and Particle Size Distribution
3.3. Morphology Analysis of Microcapsules
3.4. Total Phenolic Content (TPC) and Surface Phenolic Content (SPC) of Microcapsules
3.5. Antioxidant Activity of Microcapsules
3.6. Fatty Acid Composition of Microcapsules
3.7. Sterol Analysis of Microcapsules
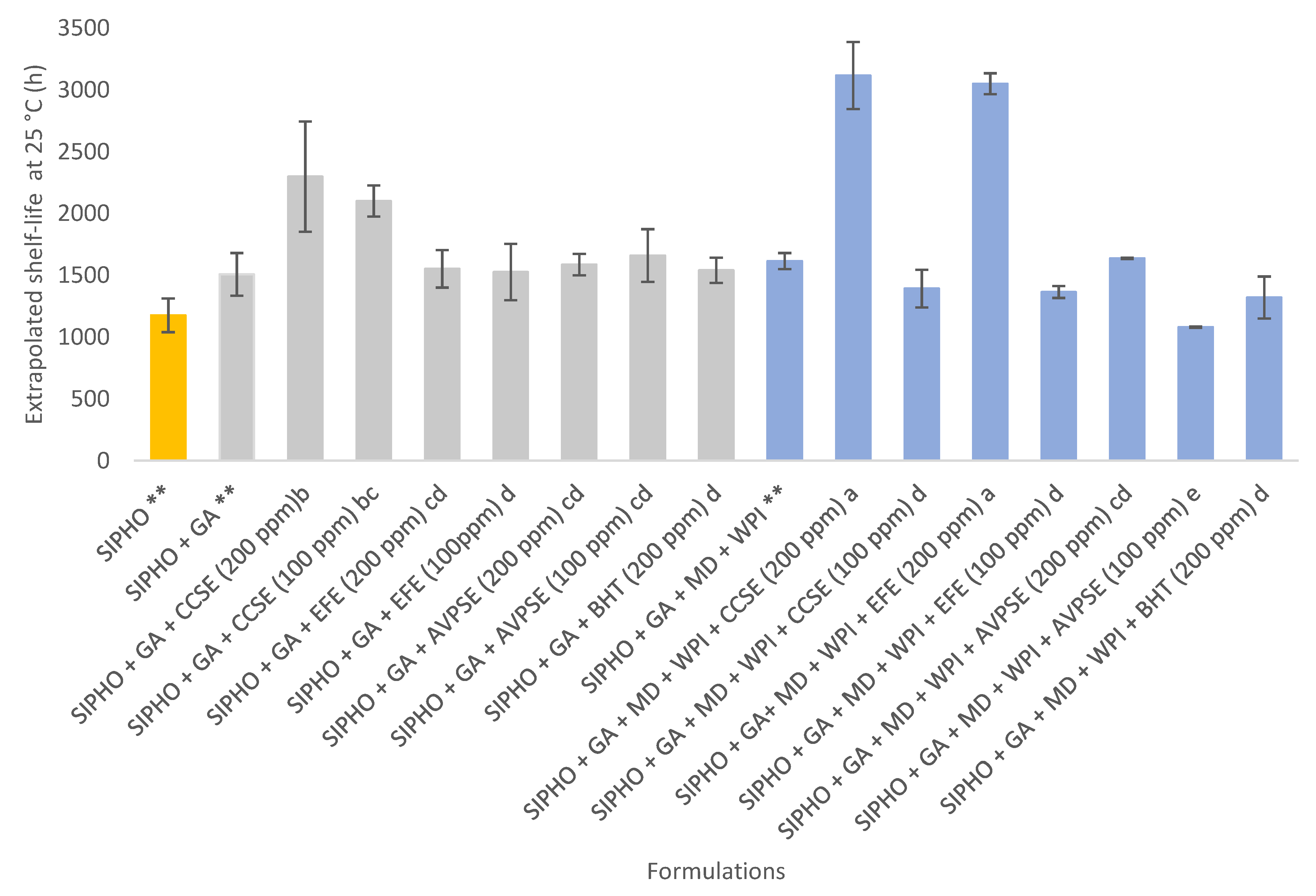
3.8. Oxidation Stability and Shelf-Life of Microcapsules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borsonelo, E.C.; Galduróz, J.C.F. The Role of Polyunsaturated Fatty Acids (PUFAs) in Development, Aging and Substance Abuse Disorders: Review and Propositions. Prostaglandins Leukot Essent Fat. Acids 2008, 78, 237–245. [Google Scholar] [CrossRef]
- Huerta-Yépez, S.; Tirado-Rodriguez, A.B.; Hankinson, O. Role of Diets Rich in Omega-3 and Omega-6 in the Development of Cancer. Bol. Med. Hosp. Infant. Mex. 2016, 73, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Singh, M. Essential Fatty Acids, DHA and Human Brain. Indian J. Pediatr. 2005, 72, 239–242. [Google Scholar] [CrossRef]
- Chasquibol, N.A.; Gómez-Coca, R.B.; Yácono, J.C.; Guinda, Á.; Moreda, W.; Del Aguila, C.; Pérez-Camino, M.C. Markers of Quality and Genuineness of Commercial Extra Virgin Sacha Inchi Oils. Grasas Y Aceites 2016, 67, e169. [Google Scholar] [CrossRef]
- Chasquibol, N.A.; Gallardo, G.; Gómez-Coca, R.B.; Trujillo, D.; Moreda, W.; Pérez-Camino, M.C. Glyceridic and Unsaponifiable Components of Microencapsulated Sacha Inchi (Plukenetia Huayllabambana L. and Plukenetia Volubilis L.) Edible Oils. Foods 2019, 8, 671. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Reinoso, Z.; Gutiérrez, L.F. Effects of the Emulsion Composition on the Physical Properties and Oxidative Stability of Sacha Inchi (Plukenetia Volubilis L.) Oil Microcapsules Produced by Spray Drying. Food Bioprocess Technol. 2017, 10, 1354–1366. [Google Scholar] [CrossRef]
- Landoni, L.; Alarcon, R.; Vilca, L.; Chasquibol, N.; Pérez-Camino, M.C.; Gallardo, G. Physicochemical Characterization and Oxidative Stability of Microencapsulated Edible Sacha Inchi Seed Oil by Spray Drying. Grasas Y Aceites 2020, 71, e387. [Google Scholar] [CrossRef]
- Aberkane, L.; Roudaut, G.; Saurel, R. Encapsulation and Oxidative Stability of PUFA-Rich Oil Microencapsulated by Spray Drying Using Pea Protein and Pectin. Food Bioprocess Technol. 2014, 7, 1505–1517. [Google Scholar] [CrossRef]
- Le Priol, L.; Gmur, J.; Dagmey, A.; Morandat, S.; El Kirat, K.; Saleh, K.; Nesterenko, A. Co-Encapsulation of Vegetable Oils with Phenolic Antioxidants and Evaluation of Their Oxidative Stability under Long-Term Storage Conditions. LWT 2021, 142, 111033. [Google Scholar] [CrossRef]
- Chang, C.; Nickerson, M.T. Encapsulation of Omega 3-6-9 Fatty Acids-Rich Oils Using Protein-Based Emulsions with Spray Drying. J. Food Sci. Technol. 2018, 55, 2850–2861. [Google Scholar] [CrossRef]
- Akram, S. Microencapsulation of Edible Oils Through Spray Drying. Ph.D. Thesis, National Institute of Food Science & Technology, Faisalabad, Pakistan, 2019. [Google Scholar]
- Fadini, A.L.; Alvim, I.D.; Ribeiro, I.P.; Ruzene, L.G.; da Silva, L.B.; Queiroz, M.B.; Miguel, A.M.R.d.O.; Chaves, F.C.M.; Rodrigues, R.A.F. Innovative Strategy Based on Combined Microencapsulation Technologies for Food Application and the Influence of Wall Material Composition. LWT 2018, 91, 345–352. [Google Scholar] [CrossRef]
- Pastuña-Pullutasig, A.; López-Hernández, O.; Debut, A.; Vaca, A.; Rodríguez-Leyes, E.; Vicente, R.; Gonzalez, V.; González-Sanabia, M.; Tapia-Hernández, F. Microencapsulación de Aceite de Sacha Inchi (Plukenetia Volubilis L.) Mediante Secado Por Aspersión. Rev. Colomb. De Cienc. Químico-Farm 2016, 45, 422–437. [Google Scholar] [CrossRef]
- Alarcón, R.; Pérez-Camino, M.C.; Chasquibol, N. Evalución de La Vida Útil de Los Aceites de Sacha Inchi (Plukenetia Huayllabambana y Plukenetia Volubilis) Microencapsulados. Rev. De La Soc. Química Del Perú 2019, 85, 327–337. [Google Scholar]
- Chawda, P.J.; Shi, J.; Xue, S.; Young Quek, S. Co-Encapsulation of Bioactives for Food Applications. Food Qual. Saf. 2017, 1, 302–309. [Google Scholar] [CrossRef]
- Sharif, H.R.; Goff, H.D.; Majeed, H.; Shamoon, M.; Liu, F.; Nsor-Atindana, J.; Haider, J.; Liang, R.; Zhong, F. Physicochemical Properties of β-Carotene and Eugenol Co-Encapsulated Flax Seed Oil Powders Using OSA Starches as Wall Material. Food Hydrocoll. 2017, 73, 274–283. [Google Scholar] [CrossRef]
- Yin, X.; Fu, X.; Cheng, H.; Wusigale; Liang, L. α-Tocopherol and Naringenin in Whey Protein Isolate Particles: Partition, Antioxidant Activity, Stability and Bioaccessibility. Food Hydrocoll. 2020, 106, 105895. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, Betalain Pattern, and Antioxidant Properties of Cactus Pear (Opuntia Spp.) Clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Gan, R.Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia Sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Chasquibol, N.; Alarcón, R.; Gonzales, B.F.; Sotelo, A.; Landoni, L.; Gallardo, G.; García, B.; Pérez-Camino, M.C. Design of Functional Powdered Beverages Containing Co-Microcapsules of Sacha Inchi P. Huayllabambana Oil and Antioxidant Extracts of Camu Camu and Mango Skins. Antioxidants 2022, 11, 1420. [Google Scholar] [CrossRef]
- Alarcón, R.; Gonzales, B.; Sotelo, A.; Gallardo, G.; Pérez-Camino, M.d.C.; Chasquibol, N. Microencapsulation of Sacha Inchi (Plukenetia Huayllabambana) Oil by Spray Drying with Camu Camu (Myrciaria Dubia (H.B.K.) Mc Vaugh) and Mango (Mangifera Indica) Skins. Proceedings 2020, 53, 11. [Google Scholar] [CrossRef]
- Yen, G.C.; Pin-Der, D. Scavenging Effect of Methanolic Extracts of Peanut Hulls on Free-Radical and Active-Oxygen Species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.; Yang, B.; Shen, G.; Rao, G. Identification of Bioactive Compounds in Phyllenthus Emblica L. Fruit and Their Free Radical Scavenging Activities. Food Chem. 2009, 114, 499–504. [Google Scholar] [CrossRef]
- García-González, A.; Velasco, J.; Velasco, L.; Ruiz-Méndez, M.V. An Analytical Simplification for Faster Determination of Fatty Acid Composition and Phytosterols in Seed Oils. Food Anal. Methods 2018, 11, 1234–1242. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, M.C.; Márquez-Ruiz, G. Application of the Accelerated Test Rancimat to Evaluate Oxidative Stability of Dried Microencapsulated Oils. Grasas Y Aceites 2000, 51, 261–267. [Google Scholar] [CrossRef]
- Montero, I.F.; Chagas, E.A.; Melo Filho, A.A.D.E.; Saravia, S.A.M.; Santos, R.C.; Chagas, P.C.; Ednalva, D.R.d.S.D. Evaluation of Total Phenolic Compounds and Antioxidant Activity in Amazon Fruit. Chem. Eng. Trans. 2018, 64, 649–654. [Google Scholar] [CrossRef]
- Conceição, N.; Albuquerque, B.R.; Pereira, C.; Corrêa, R.C.G.; Lopes, C.B.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. By-Products of Camu-Camu [Myrciaria Dubia (Kunth) McVaugh] as Promising Sources of Bioactive High Added-Value Food Ingredients: Functionalization of Yogurts. Molecules 2020, 25, 70. [Google Scholar] [CrossRef]
- Neves, L.C.; da Silva, V.X.; Pontis, J.A.; Flach, A.; Roberto, S.R. Bioactive Compounds and Antioxidant Activity in Pre-Harvest Camu-Camu [Myrciaria Dubia (H.B.K.) Mc Vaugh] Fruits. Sci. Hortic. 2015, 186, 223–229. [Google Scholar] [CrossRef]
- Bellumori, M.; Silva, N.A.C.; Vilca, L.; Andrenelli, L.; Cecchi, L.; Innocenti, M.; Balli, D.; Mulinacci, N. A Study on the Biodiversity of Pigmented Andean Potatoes: Nutritional Profile and Phenolic Composition. Molecules 2020, 25, 3169. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic Compound Contents and Antioxidant Activity in Plants with Nutritional and/or Medicinal Properties from the Peruvian Andean Region. Ind. Crops Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic Acid Derivatives, Ellagitannins, Proanthocyanidins and Other Phenolics, Vitamin C and Antioxidant Capacity of Two Powder Products from Camu-Camu Fruit (Myrciaria Dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Ocampo, E.; Torrejón-Valqui, L.; Muñóz-Astecker, L.D.; Medina-Mendoza, M.; Mori-Mestanza, D.; Castro-Alayo, E.M. Antioxidant Capacity, Total Phenolic Content and Phenolic Compounds of Pulp and Bagasse of Four Peruvian Berries. Heliyon 2021, 7, e07787. [Google Scholar] [CrossRef]
- Souza, A.L.R.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.C.; Tonon, R.V. Microencapsulation by Spray Drying of a Lycopene-Rich Tomato Concentrate: Characterization and Stability. LWT 2018, 91, 286–292. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro, L.F.; Thomazini, M.; Pallone, E.M.J.A.; do Amaral Sobral, P.J.; de Castro, I.A.; Favaro-Trindade, C.S. Echium Oil with Oxidative Stability Increased by Emulsion Preparation in the Presence of the Phenolic Compound Sinapic Acid Followed by Dehydration by Spray and Freeze Drying Processes. J. Food Sci. Technol. 2019, 56, 1155–1164. [Google Scholar] [CrossRef]
- Vishnu, K.V.; Chatterjee, N.S.; Ajeeshkumar, K.K.; Lekshmi, R.G.K.; Tejpal, C.S.; Mathew, S.; Ravishankar, C.N. Microencapsulation of Sardine Oil: Application of Vanillic Acid Grafted Chitosan as a Bio-Functional Wall Material. Carbohydr. Polym. 2017, 174, 540–548. [Google Scholar] [CrossRef]
- Binsi, P.K.; Nayak, N.; Sarkar, P.C.; Jeyakumari, A.; Muhamed Ashraf, P.; Ninan, G.; Ravishankar, C.N. Structural and Oxidative Stabilization of Spray Dried Fish Oil Microencapsulates with Gum Arabic and Sage Polyphenols: Characterization and Release Kinetics. Food Chem. 2017, 219, 158–168. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Mobli, H.; Rafiee, S.; Madadlou, A. Optimization of Emulsification Procedure for Mutual Maximizing the Encapsulation and Exergy Efficiencies of Fish Oil Microencapsulation. Powder Technol. 2012, 225, 107–117. [Google Scholar] [CrossRef]
- Yeşilsu, A.F.; Özyurt, G. Oxidative Stability of Microencapsulated Fish Oil with Rosemary, Thyme and Laurel Extracts: A Kinetic Assessment. J. Food Eng. 2019, 240, 171–182. [Google Scholar] [CrossRef]
- Sheu, T.-Y.; Rosenberg, M. Microencapsulation by Spray Drying Ethyl Caprylate in Whey Protein and Carbohydrate Wall Systems. J. Food Sci. 1995, 60, 98–103. [Google Scholar] [CrossRef]
- Akdeniz, B.; Sumnu, G.; Sahin, S. Microencapsulation of Phenolic Compounds Extracted from Onion (Allium Cepa) Skin. J. Food Process. Preserv. 2018, 42, e13648. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, Y.; Lin, Q.; Tian, Y.; Bao, Y. Study on the Antioxidant Activity of β-Sitosterol and Stigmasterol from Sacha Inchi Oil and Prinsepia Oil Added to Walnut Oil. Food Sci. Technol. 2022, 42, e69522. [Google Scholar] [CrossRef]
- Azevedo, L.; de Araujo Ribeiro, P.F.; de Carvalho Oliveira, J.A.; Correia, M.G.; Ramos, F.M.; de Oliveira, E.B.; Barros, F.; Stringheta, P.C. Camu-Camu (Myrciaria Dubia) from Commercial Cultivation Has Higher Levels of Bioactive Compounds than Native Cultivation (Amazon Forest) and Presents Antimutagenic Effects in Vivo. J. Sci. Food Agric. 2019, 99, 624–631. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Zynudheen, A.A.; Parvathy, U.; Binsi, P.K. Impact of Chitosan and Oregano Extract on the Physicochemical Properties of Microencapsulated Fish Oil Stored at Different Temperature. Int. J. Food Prop. 2018, 21, 942–955. [Google Scholar] [CrossRef]




| Formulations | Microencapsulated SIPHO with NAE Formulations |
|---|---|
| 1 | SIPHO + GA + CCSE (200 ppm) |
| 2 | SIPHO + GA + CCSE (100 ppm) |
| 3 | SIPHO + GA + EFE (200 ppm) |
| 4 | SIPHO + GA + EFE (100 ppm) |
| 5 | SIPHO + GA+ AVPSE (200 ppm) |
| 6 | SIPHO + GA+ AVPSE (100 ppm) |
| 7 | SIPHO + GA +BHT (200 ppm) |
| 8 | SIPHO + GA + MD + WPI + CCSE (200 ppm) |
| 9 | SIPHO + GA + MD + WPI + CCSE (100 ppm) |
| 10 | SIPHO + GA +MD +WPI+ EFE (200 ppm) |
| 11 | SIPHO + GA +MD +WPI+ EFE (100 ppm) |
| 12 | SIPHO + GA + MD + WPI + AVPSE (200 ppm) |
| 13 | SIPHO + GA+ MD + WPI + AVPSE (100 ppm) |
| 14 | SIPHO + GA + MD + WPI + BHT (200 ppm) |
| Microencapsulated SIPHO with NAE Formulations | Moisture Content (%) | D [4, 3] µm | Span | Volume Distribution µm | ||
|---|---|---|---|---|---|---|
| D (v, 0.1) | D (v, 0.5) | D (v, 0.9) | ||||
| SIPHO + GA ** | 5.87 ± 0.13 | 2.6 | 2.0 | 0.8 | 2.1 | 5.1 |
| 1 | 5.53 ± 0.04 d,e | 20.89 | 1.055 | 1.12 | 6.19 | 66.40 |
| 2 | 6.17 ± 0.04 c,d | - | - | - | - | - |
| 3 | 2.59 ± 0.73 h | 4.17 | 2.614 | 0.96 | 3.09 | 9.03 |
| 4 | 6.60 ± 0.13 c | - | - | - | - | - |
| 5 | 6.71 ± 0.01 b,c | 5.88 | 2.798 | 0.92 | 3.08 | 9.54 |
| 6 | 7.79 ± 0.04 a | - | - | - | - | - |
| 7 | 4.35 ± 0.21 f,g | - | - | - | - | - |
| SIPHO + GA + MD + WPI ** | 3.58 ± 0.09 | 6.1 | 2.9 | 0.9 | 3.2 | 10.1 |
| 8 | 4.00 ± 0.14 g | 6.42 | 2.146 | 1.18 | 4.69 | 11.24 |
| 9 | 6.28 ± 0.13 c,d | 7.47 | 2.360 | 1.27 | 5.68 | 14.66 |
| 10 | 4.18 ± 0.11 f,g | 10.19 | 2.071 | 1.76 | 8.90 | 20.19 |
| 11 | 3.53 ± 0.04 g | - | - | - | - | - |
| 12 | 7.50 ± 0.03 a,b | 7.08 | 2.262 | 1.32 | 5.88 | 14.62 |
| 13 | 7.59 ± 0.12 a | - | - | - | - | - |
| 14 | 4.94 ± 0.05 e,f | 12.41 | 2.089 | 2.08 | 9.60 | 22.14 |
| Microencapsulated SIPHO with NAEFormulations | TPC (µg GAE/g Powder) | SPC (µg GAE/g Powder) | PEE (%) |
|---|---|---|---|
| 1 | 3780.27 ± 14.38 b | 633.70 ± 18.60 c | 86.72 ± 0.60 b,c,d |
| 2 | 3245.00 ± 66.60 c | 775.20 ± 35.70 a | 79.69 ± 1.55 d,e |
| 3 | 757.70 ± 8.56 j | 107.50 ± 17.70 g | 92.81 ± 2.56 a,b |
| 4 | 589.70 ± 67.20 k | 114.80 ± 31.10 g | 85.61 ± 1.54 c,d,e |
| 5 | 976.72 ± 11.94 i | 196.46 ± 1.62 d | 88.97 ± 0.94 a,b,c |
| 6 | 522.27 ± 14.26 k | 79.48 ± 4.48 g | 61.93 ± 1.35 f |
| 7 | 501.48 ± 13.96 k | 213.90 ± 34.70 d | 56.34 ± 6.83 f |
| 8 | 4239.80 ± 26.40 a | 621.20 ± 22.00 c | 88.62 ± 0.64 a,b,c |
| 9 | 2823.80 ± 33.50 d | 710.10 ± 20.90 b | 79.06 ± 1.36 e |
| 10 | 2235.00 ± 35.90 f | 132.28 ± 8.91 e,f,g | 93.43 ± 0.29 a,b |
| 11 | 1970.00 ± 31.60 g | 182.23 ± 0.44 d,e,f | 93.49 ± 0.10 a,b |
| 12 | 1876.24 ± 14.17 g | 188.08 ± 7.95 d,e | 95.64 ± 2.16 a |
| 13 | 1478.60 ± 25.30 h | 225.95 ± 12.37 d | 82.77 ± 3.09 c,d,e |
| 14 | 2566.30 ± 99.50 e | 123.50 ± 15.67 f,g | 94.61 ± 0.42 a |
| Microencapsulated SIPHO with NAEFormulations | DPPH (µg trolox/g Powder) | Inhibition (%) |
|---|---|---|
| 1 | 12,716.00 ± 241.00 c | 78.97 ± 0.67 b |
| 2 | 11,270.00 ± 260.00 d | 69.82 ± 0.55 i |
| 3 | 10,353.00 ± 182.00 e,f | 64.41 ± 0.23 f,g |
| 4 | 10,137.90 ± 45.80 f | 63.81 ± 0.31 g,h |
| 5 | 12,769.90 ± 129.60 c | 73.70 ± 0.77 d,e,f |
| 6 | 11,458.50 ± 1.00 d | 69.01 ± 0.77 g,h |
| 7 | 10,608.00 ± 204.00 e | 70.93 ± 1.28 h |
| 8 | 12,454.00 ± 183.00 c | 77.36 ± 0.38 c,d,e |
| 9 | 11,451.40 ± 77.40 d | 71.72 ± 0.13 b,c |
| 10 | 10,691.12 ± 111.00 e | 65.71 ± 0.26 b,c,d |
| 11 | 9366.10 ± 31.10 g | 58.40 ± 0.09 e,f |
| 12 | 16,430.20 ± 104.20 a | 99.66 ± 0.29 a |
| 13 | 13,590.50 ± 29.00 b | 73.70 ± 0.77 d,e,f |
| 14 | 7408.00 ± 235.00 h | 51.28 ± 1.37 j |
| Microencapsulated SIPHO with NAEFormulations | C16:0 | C16:1 | C17:0 | C17:1 | C18:0 | C18:1 | C18:2 | C20:0 | C18:3 | C20:1 | trans |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SIPHO | 4.50 ± 0.24 bcd | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 1.75 ± 0.05 abcd | 7.95 ± 0.59 b | 26.10 ± 0.50 ab | 0.31 ± 0.15 | 58.17 ± 0.55 | 0.29 ± 0.15 ab | nd |
| 1 | 4.72 ± 0.28 abcd | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 1.82 ± 0.06 abcd | 8.77 ± 0.20 ab | 26.97 ± 0.88 ab | 0.29 ± 0.07 | 56.98 ± 0.57 | 0.19 ± 0.07 b | 0.07 ± 0.01 |
| 2 | 4.75 ± 0.14 abcd | 0.06 ± 0.11 | 0.06 ± 0.01 | 0.05 ± 0.01 | 1.85 ± 0.07 abcd | 8.79 ± 0.21 ab | 26.98 ± 0.81 ab | 0.29 ± 0.07 | 56.95 ± 0.85 | 0.13 ± 0.07 b | 0.07 ± 0.01 |
| 3 | 4.46 ± 0.27 bcd | nd | 0.05 ± 0.00 | nd | 1.67 ± 0.11 bcd | 8.54 ± 0.21 ab | 26.80 ± 0.72 ab | 0.26 ± 0.12 | 58.07 ± 0.82 | 0.16 ± 0.07 b | nd |
| 4 | 4.33 ± 0.14 cd | 0.04 ± 0.00 | 0.05 ± 0.00 | nd | 1.58 ± 0.07 d | 8.43 ± 0.28 ab | 26.45 ± 0.64 ab | 0.25 ± 0.11 | 58.36 ± 0.85 | 0.45 ± 0.06 a | nd |
| 5 | 4.46 ± 0.21 bcd | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 1.61 ± 0.10 cd | 8.13 ± 0.52 b | 28.72 ± 0.71 a | 0.26 ± 0.10 | 56.45 ± 0.78 | 0.18 ± 0.03 b | nd |
| 6 | 4.23 ± 0.10 d | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 1.63 ± 0.14 cd | 8.22 ± 0.68 b | 28.73 ± 0.85 a | 0.26 ± 0.06 | 56.57 ± 0.88 | 0.19 ± 0.04 b | nd |
| 7 | 4.50 ± 0.21 bcd | 0.04 ± 0.00 | 0.06 ± 0.00 | nd | 1.72 ± 0.10 abcd | 8.54 ± 0.37 ab | 26.67 ± 0.52 ab | 0.27 ± 0.10 | 57.82 ± 0.99 | 0.37 ± 0.08 ab | nd |
| 8 | 5.20 ± 0.24 ab | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 2.03 ± 0.08 ab | 9.25 ± 0.24 ab | 26.72 ± 0.71 ab | 0.29 ± 0.07 | 56.03 ± 0.66 | 0.16 ± 0.03 b | 0.07 ± 0.01 |
| 9 | 5.05 ± 0.17 abc | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 1.96 ± 0.08 abc | 9.00 ± 0.42 ab | 25.82 ± 0.57 b | 0.29 ± 0.11 | 56.41 ± 0.71 | 0.16 ± 0.03 b | 0.07 ± 0.01 |
| 10 | 5.40 ± 0.13 a | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 1.85 ± 0.10 abcd | 8.8 ± 0.23 ab | 27.00 ± 0.78 ab | 0.27 ± 0.11 | 57.10 ± 0.95 | 0.30 ± 0.08 ab | nd |
| 11 | 5.20 ± 0.16 ab | 0.06 ± 0.00 | 0.07 ± 0.00 | nd | 1.93 ± 0.08 abcd | 8.95 ± 0.29 ab | 26.36 ± 0.72 ab | 0.26 ± 0.10 | 56.81 ± 0.75 | 0.34 ± 0.03 ab | nd |
| 12 | 5.21 ± 0.21 ab | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 2.02 ± 0.07 ab | 9.14 ± 0.48 ab | 26.76 ± 0.64 ab | 0.29 ± 0.07 | 56.20 ± 0.85 | 0.16 ± 0.03 b | nd |
| 13 | 5.31 ± 0.18 a | 0.10 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 2.05 ± 0.08 a | 9.43 ± 0.41 ab | 26.65 ± 0.78 ab | 0.29 ± 0.08 | 55.89 ± 0.86 | 0.14 ± 0.01 b | nd |
| 14 | 5.42 ± 0.20 a | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 1.97 ± 0.13 abc | 9.91 ± 0.66 a | 26.32 ± 0.71 ab | 0.18 ± 0.01 | 55.83 ± 0.64 | 0.17 ± 0.01 b | nd |
| Microencapsulated SIPHO with NAE Formulations | Total Plant Sterols (mg/kg) | β-Sitosterol (Phytosterol Majority) (%) | Stigmasterol/Campesterol Ratio | Cholesterol (mg/kg) |
|---|---|---|---|---|
| SIPHO | 1856 ± 124 c | 56.7 ± 1.5 def | 5.96 ± 0.63 a | nd |
| 1 | 2411 ± 106 a | 60.1 ± 1.4 bcde | 4.62 ± 0.68 ab | nd |
| 2 | 2235 ± 113 abc | 59.8 ± 1.1 bcde | 5.57 ± 0.57 ab | nd |
| 3 | 1966 ± 115 bc | 61.8 ± 1.0 bc | 5.44 ± 0.59 ab | nd |
| 4 | 1987 ± 100 abc | 61.2 ± 1.1 bcde | 5.03 ± 0.58 ab | nd |
| 5 | 2187 ± 105 abc | 56.5 ± 1.0 ef | 5.76 ± 0.62 a | nd |
| 6 | 2173 ± 103 abc | 57.4 ± 1.4 cdef | 4.31 ± 0.55 ab | nd |
| 7 | 1941 ± 112 bc | 61.2 ± 1.3 bcde | 4.87 ± 0.54 ab | nd |
| 8 | 2068 ± 102 abc | 62.5 ± 0.8 ab | 5.08 ± 0.58 ab | 1051 ± 28 c |
| 9 | 2164 ± 108 abc | 62.5 ± 1.1 ab | 5.43 ± 0.57 ab | 907 ± 30 d |
| 10 | 2304 ± 110 ab | 53.8 ± 1.0 f | 3.33 ± 0.52 b | 1261 ± 38 b |
| 11 | 2082 ± 107 abc | 61.9 ± 1.4 abc | 4.96 ± 0.58 ab | 1458 ± 35 a |
| 12 | 2132 ± 100 abc | 61.1 ± 1.1 bcde | 5.42 ± 0.54 ab | 1258 ± 32 b |
| 13 | 1894 ± 101 bc | 66.6 ± 1.0 a | 6.33 ± 0.58 a | 1214 ± 31 b |
| 14 | 2007 ± 102 abc | 61.3 ± 1.3 bcd | 4.62 ± 0.54 ab | 1231 ± 29 b |
| Microencapsulated SIPHO with NAEFormulations | OOT (°C) | ||
|---|---|---|---|
| Average | SD | U | |
| SIPHO ** | 169 | 2 | 10 |
| SIPHO + GA + MD + WPI ** | 181 | 0.4 | 10 |
| 8 | 189 | <1 | ±11 |
| 9 | 187 | <1 | ±11 |
| 10 | 186 | 1 | ±11 |
| 11 | 179 | 1 | ±10 |
| 12 | 185 | <1 | ±11 |
| 13 | 185 | 1 | ±11 |
| 14 | 178 | 1 | ±10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chasquibol, N.; Gonzales, B.F.; Alarcón, R.; Sotelo, A.; Gallardo, G.; García, B.; Pérez-Camino, M.d.C. Co-Microencapsulation of Sacha Inchi (Plukenetia huayllabambana) Oil with Natural Antioxidants Extracts. Foods 2023, 12, 2126. https://doi.org/10.3390/foods12112126
Chasquibol N, Gonzales BF, Alarcón R, Sotelo A, Gallardo G, García B, Pérez-Camino MdC. Co-Microencapsulation of Sacha Inchi (Plukenetia huayllabambana) Oil with Natural Antioxidants Extracts. Foods. 2023; 12(11):2126. https://doi.org/10.3390/foods12112126
Chicago/Turabian StyleChasquibol, Nancy, Billy Francisco Gonzales, Rafael Alarcón, Axel Sotelo, Gabriela Gallardo, Belén García, and María del Carmen Pérez-Camino. 2023. "Co-Microencapsulation of Sacha Inchi (Plukenetia huayllabambana) Oil with Natural Antioxidants Extracts" Foods 12, no. 11: 2126. https://doi.org/10.3390/foods12112126
APA StyleChasquibol, N., Gonzales, B. F., Alarcón, R., Sotelo, A., Gallardo, G., García, B., & Pérez-Camino, M. d. C. (2023). Co-Microencapsulation of Sacha Inchi (Plukenetia huayllabambana) Oil with Natural Antioxidants Extracts. Foods, 12(11), 2126. https://doi.org/10.3390/foods12112126








