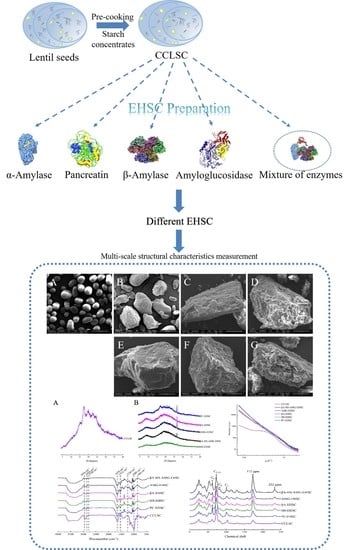
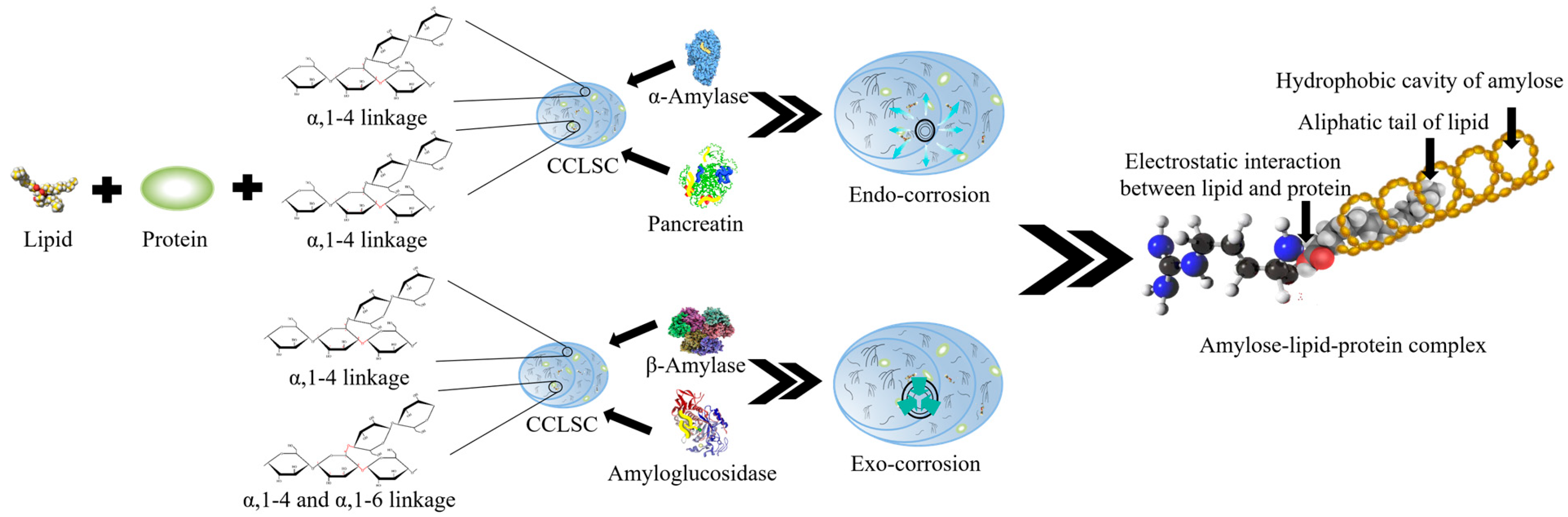
Multi-Scale Structural Insights into Enzymatically Hydrolyzed Lentil Starch Concentrates Prepared by In Vitro Method Using Different Types of Enzymes
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Pre-Cooking of Lentil Seeds and Enrichment of Starch
Protein and Lipid Content of CCLSC
2.3. Enzymatic Modification
2.3.1. Enzymatic Hydrolysis by β-Amylase
2.3.2. Enzymatic Hydrolysis by Thermostable α-Amylase
2.3.3. Enzymatic Hydrolysis by Pancreatin
2.3.4. Enzymatic Modification by Amyloglucosidase
2.3.5. Enzymatic Hydrolysis by the Action of Multi-Enzymes
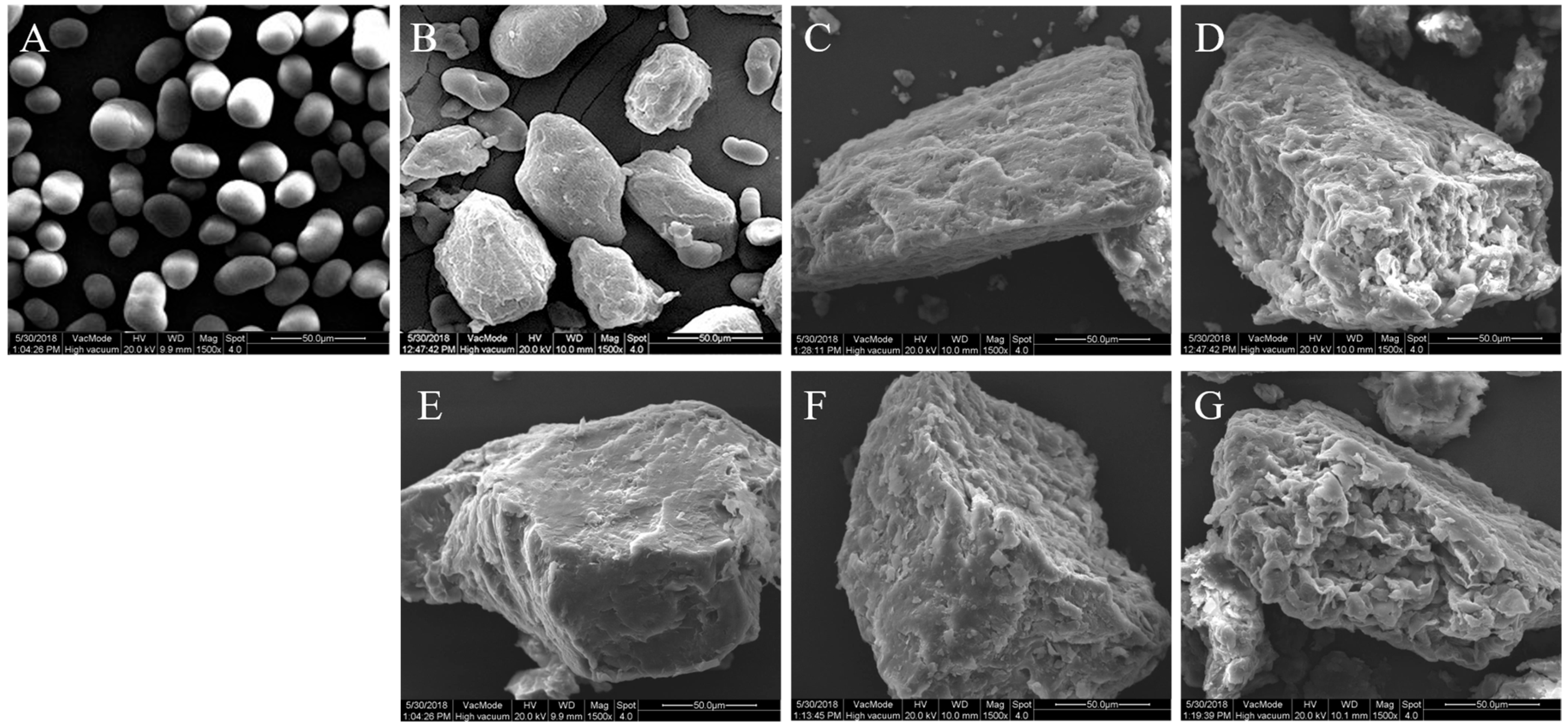
2.4. Scanning Electron Microscopy (SEM)
2.5. High-Performance Size-Exclusion Chromatography (HPSEC)
2.6. X-ray Diffraction (XRD)
2.7. Fourier-Transform Infrared (FTIR) Spectroscopy
2.8. Solid-State Nuclear Magnetic Resonance (13C NMR)
2.9. Small Angle X-ray Scattering (SAXS)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Morphological Features Obtained by Scanning Electron Microscope
3.2. Molecular Weight by HPSEC
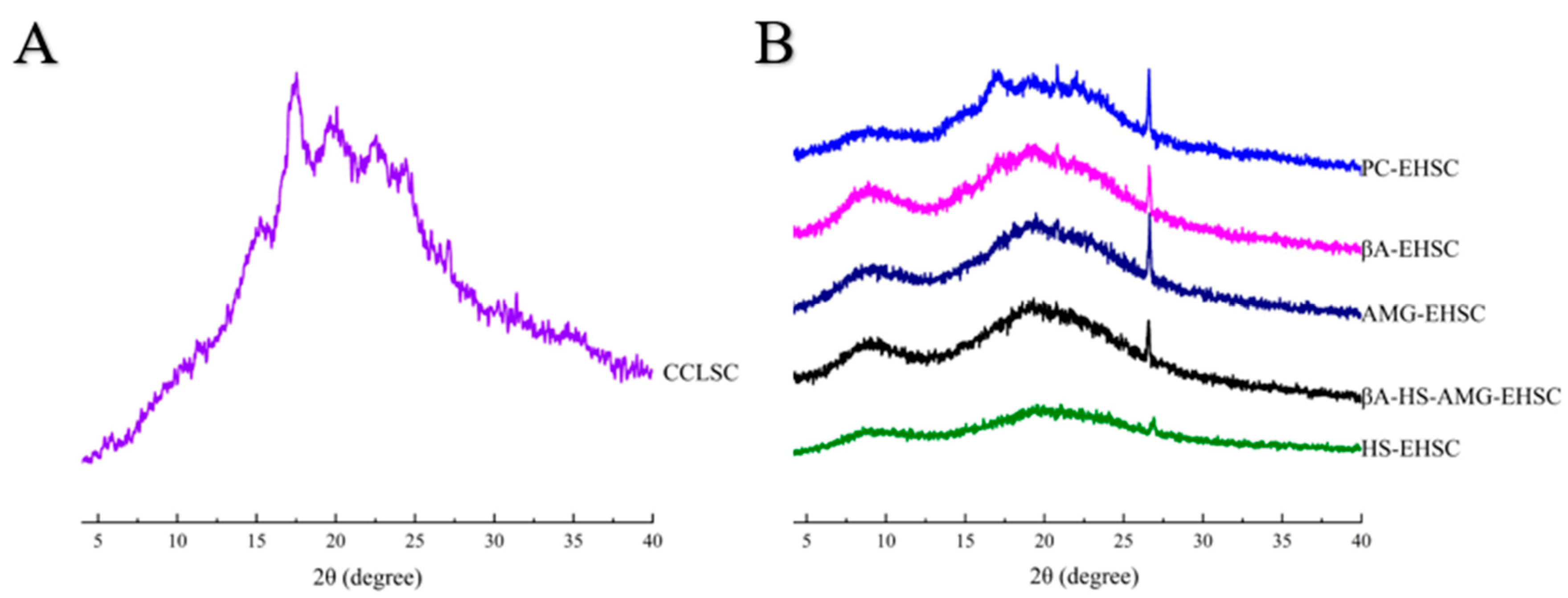
3.3. Crystalline Structure
3.3.1. XRD Analysis
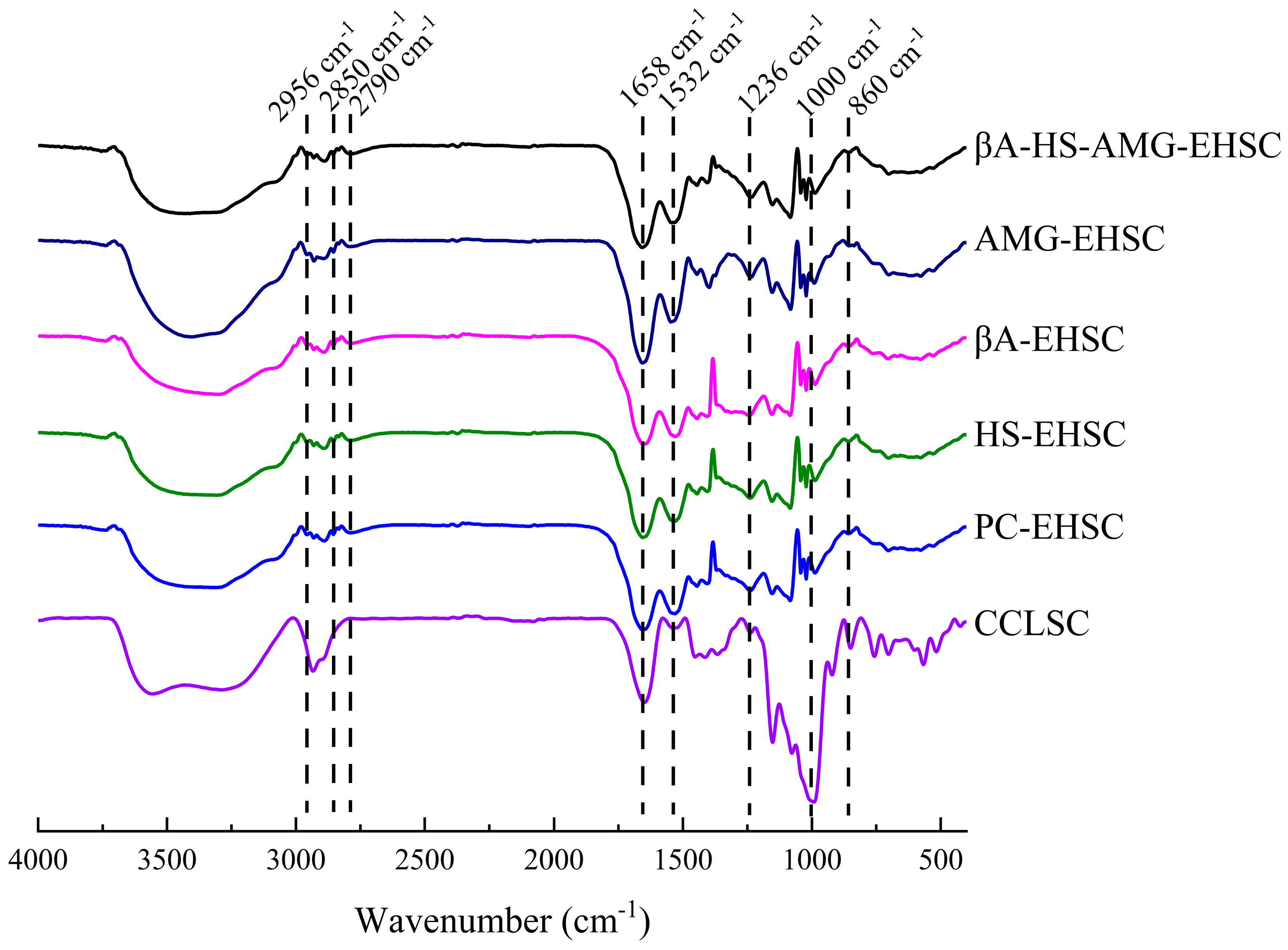
3.3.2. FTIR Analysis
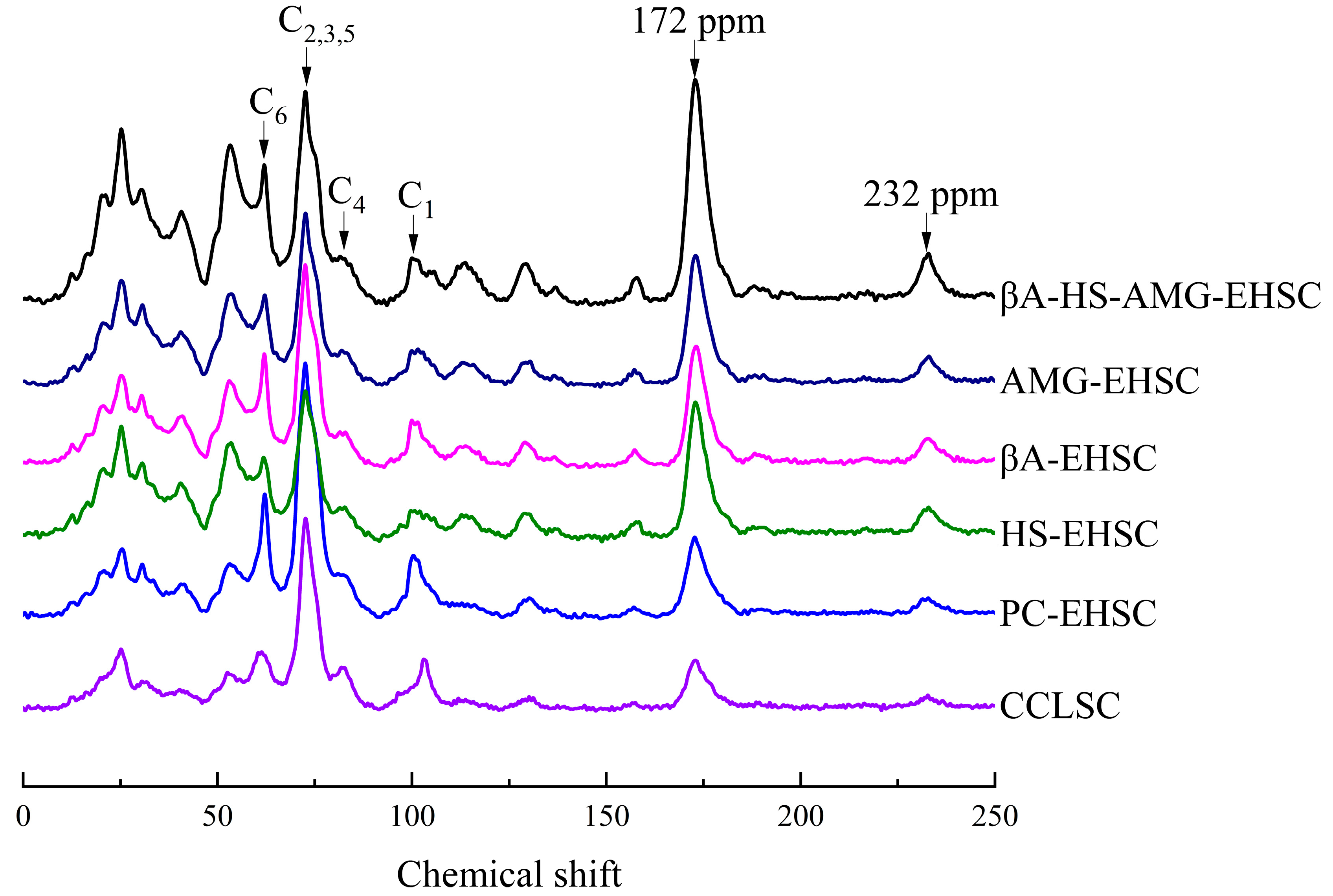
3.4. Structural Order by Solid-State 13C CP MAS NMR on the Molecular Level
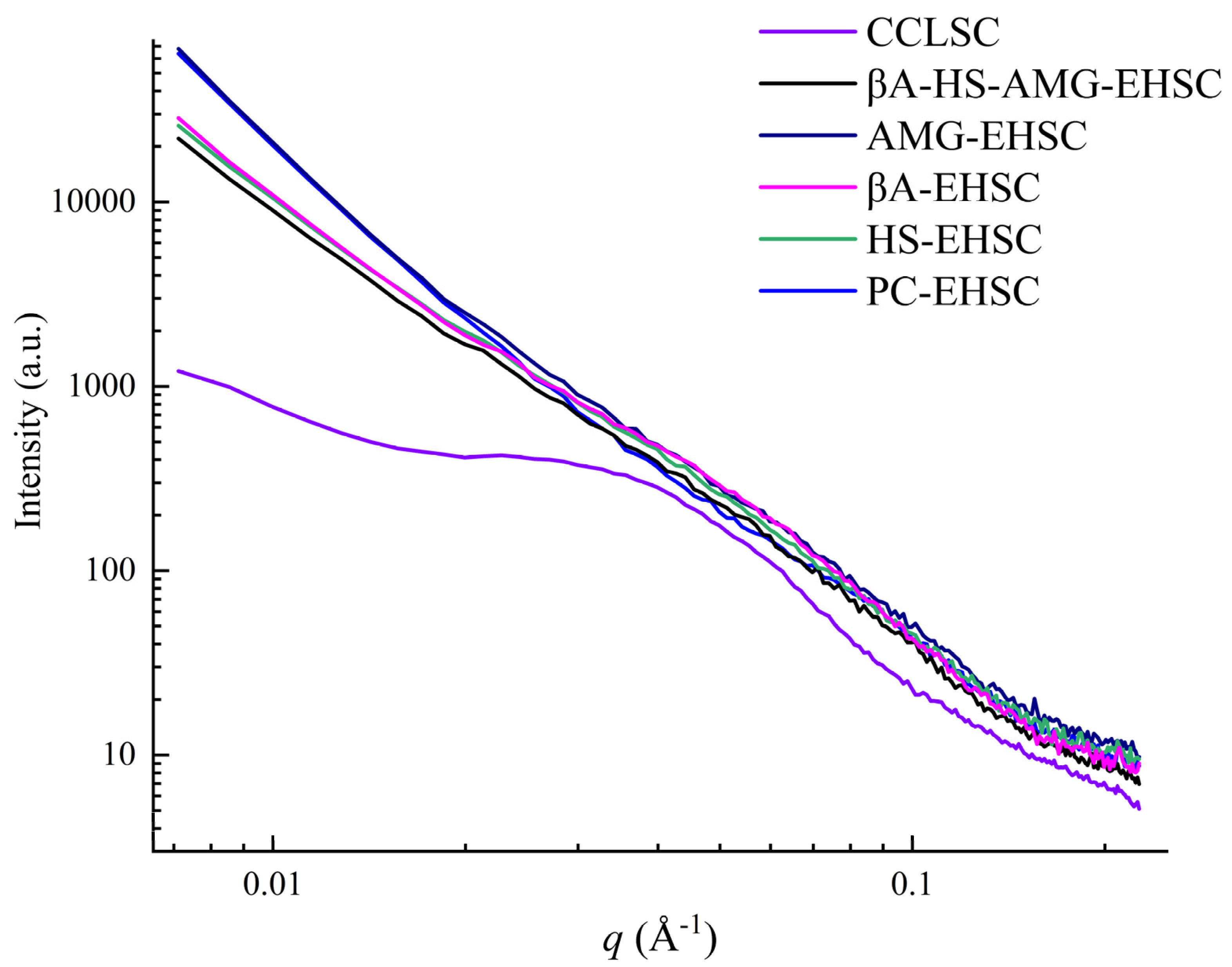
3.5. Lamellar Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Htoon, A.; Paterson, J. Alkaline extraction of starch from Australian lentil cultivars Matilda and Digger optimised for starch yield and starch and protein quality. Food Chem. 2007, 102, 551–559. [Google Scholar] [CrossRef]
- Ren, N.; Ma, Z.; Li, X.; Hu, X. Preparation of rutin-loaded microparticles by debranched lentil starch-based wall materials: Structure, morphology and in vitro release behavior. Int. J. Biol. Macromol. 2021, 173, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.; Meda, V.; Tyler, R.T. Resistant starch: A review of analytical protocols for determining resistant starch and of factors affecting the resistant starch content of foods. Food Res. Int. 2010, 43, 1959–1974. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Research advances on structural characterization of resistant starch and its structure-physiological function relationship: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1059–1083. [Google Scholar] [CrossRef]
- Lutfi, P.; Martha, P.W.; Yudi, P.; Lucia, D.W. Characterization of porous starch from edible canna (Canna edulis Kerr.) produced by enzymatic hydrolysis using thermostable α-amylase. Food Chem. Adv. 2023, 2, 100152. [Google Scholar]
- Tawil, G.; Vikso-Nielsen, A.; Rolland-Sabaté, A.; Colonna, P.; Buléon, A. Hydrolysis of concentrated raw starch: A new very efficient α-amylase from anoxybacillus flavothermus. Carbohydr. Polym. 2012, 87, 46–52. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Htoon, A.; Gilbert, E.P. Influence of Extrusion and Digestion on the Nanostructure of High-Amylose Maize Starch. Biomacromolecules 2007, 8, 1564–1572. [Google Scholar] [CrossRef]
- Zhang, B.; Dhital, S.; Gidley, M.J. Densely packed matrices as rate determining features in starch hydrolysis. Trends Food Sci. Technol. 2015, 43, 18–31. [Google Scholar] [CrossRef]
- Lee, B.-H.; Yan, L.; Phillips, R.J.; Reuhs, B.L.; Jones, K.; Rose, D.R.; Nichols, B.L.; Quezada-Calvillo, R.; Yoo, S.H.; Hamaker, B.R. Enzyme-Synthesized Highly Branched Maltodextrins Have Slow Glucose Generation at the Mucosal α-Glucosidase Level and Are Slowly Digestible in Vivo. PLoS ONE 2013, 8, e59745. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, W.; Qiu, X.; Sun, Y.; Xia, X.; Yang, L.; Fan, M.; Wang, L.; Qian, H. Effects of β-amylase hydrolysis on the structural, physicochemical and storage properties of wheat starch. J. Cereal Sci. 2023, 109, 103605. [Google Scholar] [CrossRef]
- Blazek, J.; Gilbert, E.P. Effect of Enzymatic Hydrolysis on Native Starch Granule Structure. Biomacromolecules 2010, 11, 3275–3289. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Luo, S.; McClements, D.J.; Liang, L.; Liu, C. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Res. Int. 2018, 106, 404–409. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, D.; Zhang, X.; Qu, L.; Zhang, Y.; Huang, Q. Effects of the Removal of Lipids and Surface Proteins on the Physicochemical and Structural Properties of Green Wheat Starches. Starch-Stärke 2020, 73, 2000046. [Google Scholar] [CrossRef]
- Miao, M.; Li, R.; Huang, C.; Jiang, B.; Zhang, T. Impact of β-amylase degradation on properties of sugary maize soluble starch particles. Food Chem. 2015, 177, 1–7. [Google Scholar] [CrossRef]
- Yin, X.; Ma, Z.; Hu, X.; Li, X.; Boye, J.I. Molecular rearrangement of Laird lentil (Lens culinaris Medikus) starch during different processing treatments of the seeds. Food Hydrocoll. 2018, 79, 399–408. [Google Scholar] [CrossRef]
- Wang, Y.; Chao, C.; Huang, H.; Wang, S.; Wang, S.; Wang, S.; Copeland, L. Revisiting Mechanisms Underlying Digestion of Starches. J. Agric. Food Chem. 2019, 67, 8212–8226. [Google Scholar] [CrossRef]
- Li, J.; Vasanthan, T.; Hoover, R.; Rossnagel, B. Starch from hull-less barley: V. In-vitro susceptibility of waxy, normal, and high-amylose starches towards hydrolysis by alpha-amylases and amyloglucosidase. Food Chem. 2004, 84, 621–632. [Google Scholar] [CrossRef]
- Ao, Z.; Simsek, S.; Zhang, G.; Venkatachalam, M.; Reuhs, B.L.; Hamaker, B.R. Starch with a Slow Digestion Property Produced by Altering Its Chain Length, Branch Density, and Crystalline Structure. J. Agric. Food Chem. 2007, 55, 4540–4547. [Google Scholar] [CrossRef]
- Jacobs, H.; Eerlingen, R.C.; Spaepen, H.; Grobet, P.J.; Delcour, J.A. Impact of annealing on the susceptibility of wheat, potato and pea starches to hydrolysis with pancreatin. Carbohydr. Res. 1997, 305, 193–207. [Google Scholar] [CrossRef]
- Obiro, W.C.; Sinha Ray, S.; Emmambux, M.N. V-amylose Structural Characteristics, Methods of Preparation, Significance, and Potential Applications. Food Rev. Int. 2012, 28, 412–438. [Google Scholar] [CrossRef]
- Jane, J.; Wong, K.; McPherson, A.E. Branch-structure difference in starches of A- and B-type X-ray patterns revealed by their Naegeli dextrins. Carbohydr. Res. 1997, 300, 219–227. [Google Scholar] [CrossRef]
- Dhital, S.; Warren, F.J.; Zhang, B.; Gidley, M.J. Amylase binding to starch granules under hydrolysing and non-hydrolysing conditions. Carbohydr. Polym. 2014, 113, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Munira, P.; Sarto; Hidayat, M. Inhibitor Effect (Lipid and Protein) in Starch Hydrolysis to Produce Glucose by using Amyloglucosidase. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 012022. [Google Scholar] [CrossRef]
- Liu, P.; Kang, X.; Cui, B.; Gao, W.; Wu, Z.; Yu, B. Effects of amylose content and enzymatic debranching on the properties of maize starch-glycerol monolaurate complexes. Carbohydr. Polym. 2019, 222, 115000. [Google Scholar] [CrossRef]
- Gelders, G.; Vanderstukken, T.; Goesaert, H.; Delcour, J. Amylose-lipid complexation: A new fractionation method. Carbohydr. Polym. 2004, 56, 447–458. [Google Scholar] [CrossRef]
- Mutungi, C.; Passauer, L.; Onyango, C.; Jaros, D.; Rohm, H. Debranched cassava starch crystallinity determination by Raman spectroscopy: Correlation of features in Raman spectra with X-ray diffraction and 13C CP/MAS NMR spectroscopy. Carbohydr. Polym. 2012, 87, 598–606. [Google Scholar] [CrossRef]
- Sneh, P.B.; Adeleke, O.A.; Arashdeep, S.V.C.; William, S.W. Enzymatic modification of starch: A green approach for starch applications. Carbohydr. Polym. 2022, 287, 119265. [Google Scholar]
- Lin, H.; Bean, S.R.; Tilley, M.; Peiris, K.H.S.; Brabec, D. Qualitative and Quantitative Analysis of Sorghum Grain Composition Including Protein and Tannins Using ATR-FTIR Spectroscopy. Food Anal. Methods 2020, 14, 268–279. [Google Scholar] [CrossRef]
- Fang, J. The preparation and characterisation of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Jafari, M.; Koocheki, A.; Milani, E. Effect of extrusion cooking on chemical structure, morphology, crystallinity and thermal properties of sorghum flour extrudates. J. Cereal Sci. 2017, 75, 324–331. [Google Scholar] [CrossRef]
- Cremer, D.R.; Kaletunç, G. Fourier transform infrared microspectroscopic study of the chemical microstructure of corn and oat flour-based extrudates. Carbohydr. Polym. 2013, 52, 53–65. [Google Scholar] [CrossRef]
- Zhang, G. Starch-free fatty acid complexation in the presence of whey protein. Carbohydr. Polym. 2004, 55, 419–424. [Google Scholar] [CrossRef]
- Olademehin, O.P.; Liu, C.; Rimal, B.; Adegboyega, N.F.; Chen, F.; Sim, C.; Kim, S.J. Dsi-RNA knockdown of genes regulated by Foxo reduces glycogen and lipid accumulations in diapausing Culex pipiens. Sci. Rep. 2010, 10, 17201. [Google Scholar] [CrossRef]
- Zhang, G.; Maladen, M.D.; Hamaker, B.R. Detection of a Novel Three Component Complex Consisting of Starch, Protein, and Free Fatty Acids. J. Agric. Food Chem. 2003, 51, 2801–2805. [Google Scholar] [CrossRef]
- Grewal, M.; Chandrapala, J.; Donkor, O.; Apostolopoulos, V.; Stojanovska, L.; Vasiljevic, T. Fourier transform infrared spectroscopy analysis of physicochemical changes in UHT milk during accelerated storage. Int. Dairy J. 2017, 66, 99–107. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Chi, C.; Ma, X. Effect of protein types on structure and digestibility of starch-protein-lipids complexes. LWT 2020, 134, 110175. [Google Scholar] [CrossRef]
- Lian, X.; Wang, C.; Zhang, K.; Li, L. The retrogradation properties of glutinous rice and buckwheat starches as observed with FT-IR, 13C NMR and DSC. Int. J. Biol. Macromol. 2014, 64, 288–293. [Google Scholar] [CrossRef]
- Govindaraju, I.; Zhuo, G.-Y.; Chakraborty, I.; Melanthota, S.K.; Mal, S.S.; Sarmah, B.; Baruah, V.J.; Mahato, K.K.; Mazumder, N. Investigation of structural and physico-chemical properties of rice starch with varied amylose content: A combined microscopy, spectroscopy, and thermal study. Food Hydrocoll. 2022, 122, 107093. [Google Scholar] [CrossRef]
- Zhang, X.; Do, M.D.; Hoobin, P.; Burgar, I. The phase composition and molecular motions of plasticized wheat gluten-based biodegradable polymer materials studied by solid-state NMR spectroscopy. Polymer 2016, 47, 5888–5896. [Google Scholar] [CrossRef]
- King, B.; Li, S.; Liu, C.; Joon Kim, S.; Sim, C. Suppression of glycogen synthase expression reduces glycogen and lipid storage during mosquito overwintering diapause. J. Insect Physiol. 2019, 120, 103971. [Google Scholar] [CrossRef] [PubMed]
- Jane, J.; Robyt, J.F.; Huang, D.-H. 13C-NMR study of the conformation of helical complexes of amylodextrin and of amylose in solution. Carbohydr. Res. 1985, 140, 21–35. [Google Scholar] [CrossRef] [PubMed]
- YashRoy, R.C. 13C-NMR studies of membrane lipid-protein interactions upon protein heat denaturation. J. Biochem. Biophys. Methods 1991, 23, 259–261. [Google Scholar] [CrossRef]
- Suzuki, T.; Chiba, A.; Yarno, T. Interpretation of small angle X-ray scattering from starch on the basis of fractals. Carbohydr. Polym. 1997, 34, 357–363. [Google Scholar] [CrossRef]
| Samples | Crystallinity by XRD (C1, %) | Crystallinity by 13CNMR (C2, %) | Double Helix Content by 13C NMR (%) | PPA by 13C NMR (%) | DO Value by FTIR | DD Value by FTIR | dc by SAXS | α by SAXS | Dm | Ds | Average Mw by SEC-MALLS (g/mol) | DPn by SEC-MALLS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCLSC | 1.06 ± 0.0169 d | 39.33 ± 0.8027 d | 31.62 ± 0.6207 a | 7.62 ± 0.0659 a | 1.0152 ± 0.0002 b | 0.9825 ± 0.0006 e | 4.68 | −2.10 | 2.10 | ND | 5.142 × 107 | 2.538 |
| PC-EHSC | 4.45 ± 0.3818 a | 77.92 ± 0.6977 a | 25.04 ± 1.0063 b | 8.19 ± 0.5233 a | 1.0196 ± 0.0005 a | 1.0452 ± 0.0012 e | 7.73 | −3.18 | ND | 2.82 | 3.309 × 104 | 1.037 |
| AMG-EHSC | 1.30 ± 0.0707 b | 23.26 ± 0.5650 e | 11.89 ± 1.2243 d | 6.95 ± 0.1199 b | 1.0167 ± 0.0002 b | 1.0440 ± 0.0000 b | 6.28 | −3.16 | ND | 2.84 | 2.395 × 106 | 1.132 |
| βA-EHSC | 0.81 ± 0.0707 c | 42.54 ± 0.6264 c | 14.63 ± 0. 5039 c | 4.60 ± 0.2407 c | 1.0147 ± 0.0011 d | 0.9745 ± 0.0002 d | 4.67 | −2.58 | 2.58 | ND | 5.690 × 104 | 1.038 |
| HS-EHSC | 0.61 ± 0.0354 c | 19.34 ± 0.5874 f | 9.87 ± 0.0056 d | 3.53 ± 0.0870 d | 1.0112 ± 0.0003 d | 0.9740 ± 0.0002 d | 4.38 | −2.46 | 2.46 | ND | 1.711 × 103 | 1.262 |
| βA-HS-AMG-EHSC | 1.30 ± 0.0778 b | 47.30 ± 0.5405 b | 15.98 ± 0.8533 c | 2.19 ± 0.2328 e | 1.0161 ± 0.0003 c | 0.9877 ± 0.0000 c | 4.87 | −2.45 | 2.45 | ND | 3.957 × 104 | 1.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, N.; Hu, X.; Ma, Z. Multi-Scale Structural Insights into Enzymatically Hydrolyzed Lentil Starch Concentrates Prepared by In Vitro Method Using Different Types of Enzymes. Foods 2023, 12, 2150. https://doi.org/10.3390/foods12112150
Ren N, Hu X, Ma Z. Multi-Scale Structural Insights into Enzymatically Hydrolyzed Lentil Starch Concentrates Prepared by In Vitro Method Using Different Types of Enzymes. Foods. 2023; 12(11):2150. https://doi.org/10.3390/foods12112150
Chicago/Turabian StyleRen, Namei, Xinzhong Hu, and Zhen Ma. 2023. "Multi-Scale Structural Insights into Enzymatically Hydrolyzed Lentil Starch Concentrates Prepared by In Vitro Method Using Different Types of Enzymes" Foods 12, no. 11: 2150. https://doi.org/10.3390/foods12112150
APA StyleRen, N., Hu, X., & Ma, Z. (2023). Multi-Scale Structural Insights into Enzymatically Hydrolyzed Lentil Starch Concentrates Prepared by In Vitro Method Using Different Types of Enzymes. Foods, 12(11), 2150. https://doi.org/10.3390/foods12112150