Proteins and Minerals in Whey Protein Supplements
Abstract
:1. Introduction
2. Materials and Methods
- Determination of total protein in protein supplements
- Digestion of samples (0.3–0.5 g) by addition of H2SO4 (98% concentration) (10 mL) and two catalyst tablets (Cu-Se) (Kjeldahl Catalyst Cu-Se, 1.5% CuSO4. 5H2O + 2%Se. Tablets. Panreac, AppliChem, Barcelona Spain) in a digester at a range of 350–380 °C for 1 h [25,26]. The organic nitrogen is converted to NH4+ under these conditions.
- Distillation of the digested solution using steam and a Kjeldahl distiller (FOSS KT 200 Kjeltec™ nitrogen distiller). Before starting the distillation, the flasks were prepared and ten drops of Mixed Indicator 5 (Methyl Red-Bromocresol Green, 283303, Panreac, AppliChem, Barcelona, Spain) and 30 mL of saturated H3BO3 were added, producing a fuchsia coloring with pH 4–5.5. The NH3+ was distilled and collected in a receiver vessel.
- Evaluation of the distillation results by addition of HCl (0.1000 mol/l) (181,023.1214, Panreac, AppliChem, Barcelona, Spain) with an electronic burette until the color changes from green to fuchsia with five drops of methyl red indicator to 0.1% (281,618.1208, Panreac, AppliChem, Barcelona, Spain). The green color is produced at pH 4.2–6.2, thus determining the protein nitrogen.
- 2.
- Element analysis in protein supplements
- 3.
- Statistical analysis
3. Results and Discussion
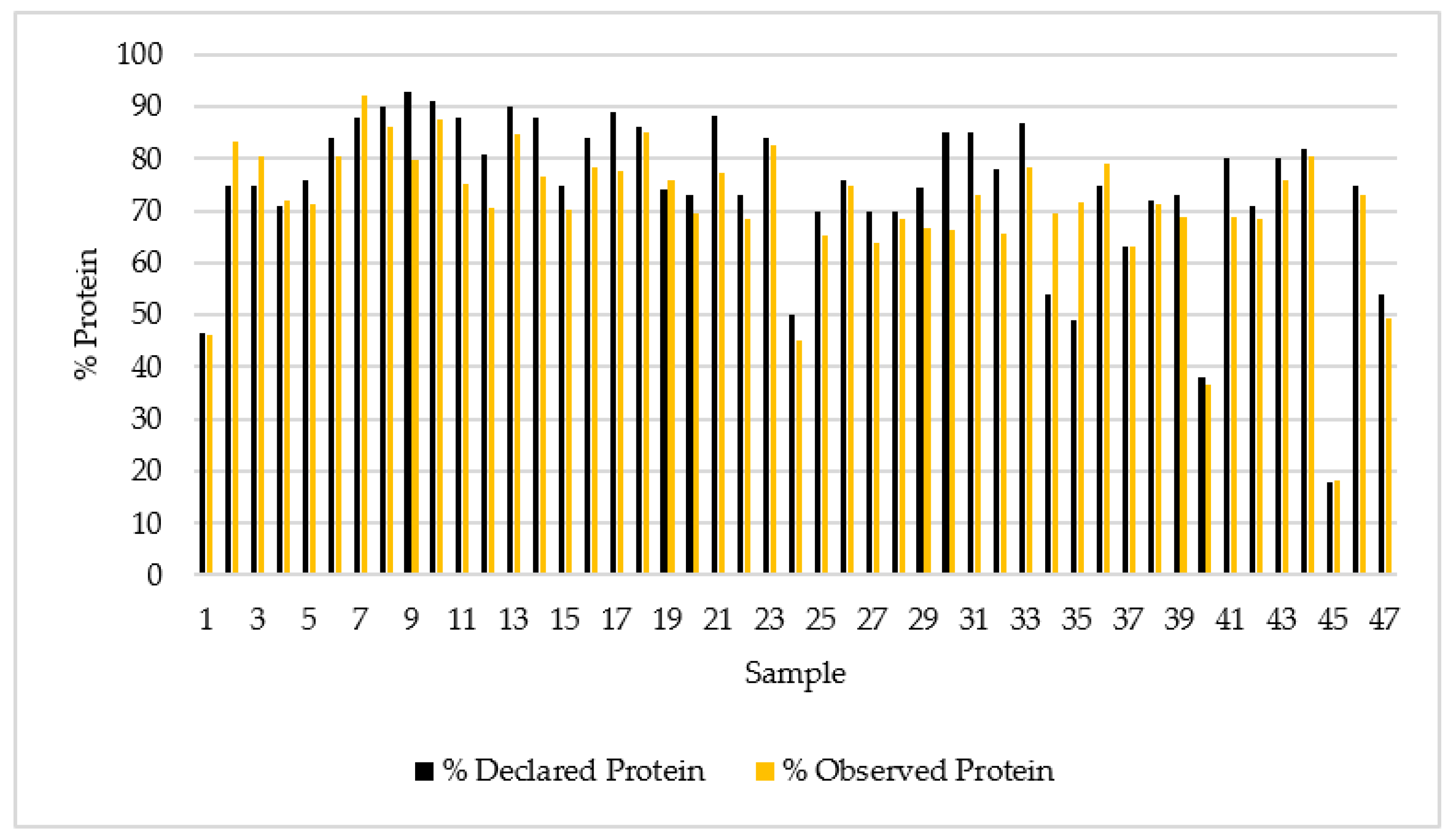
- Protein richness of whey protein supplements and discrepancies with labelling
- 2.
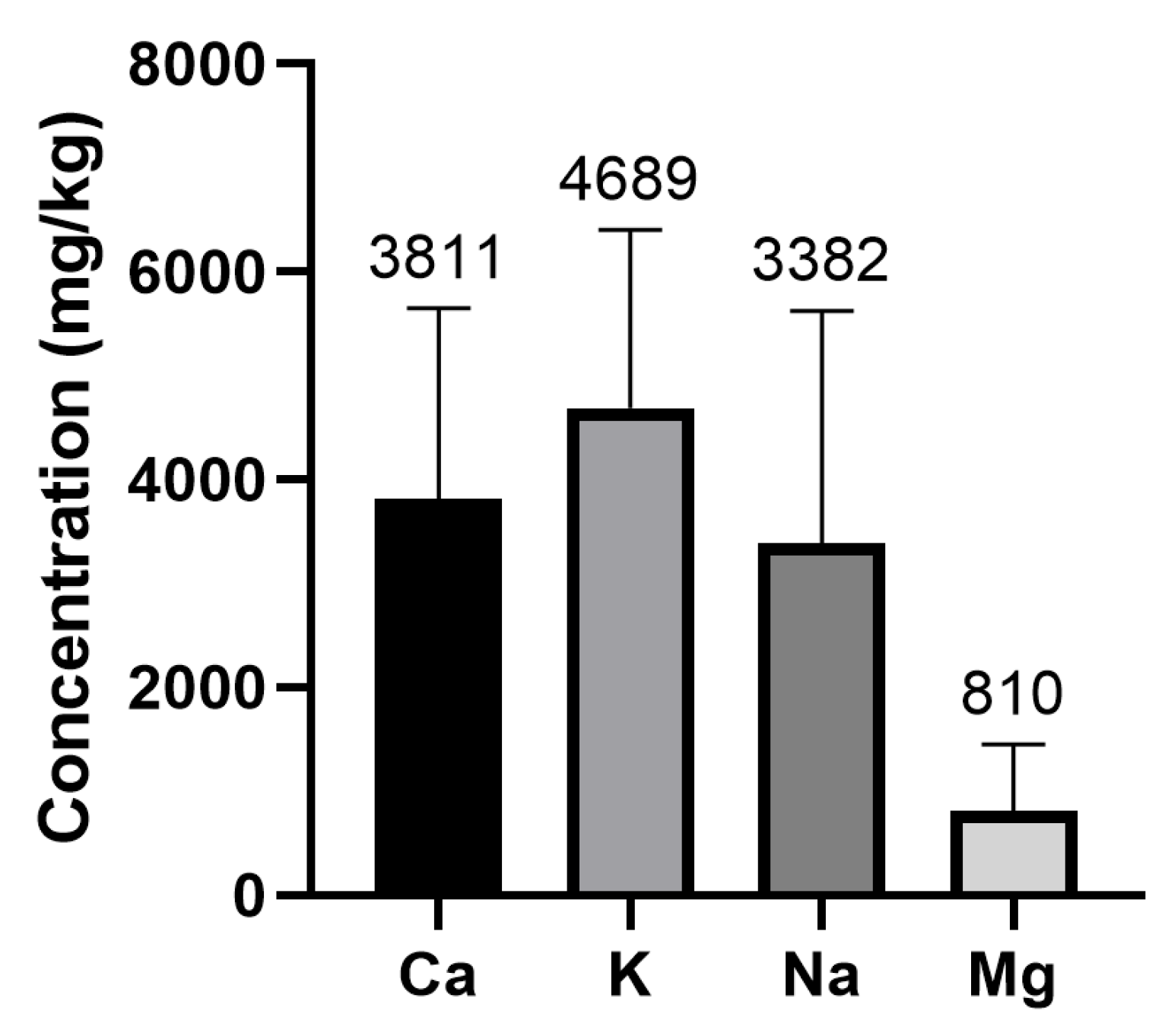
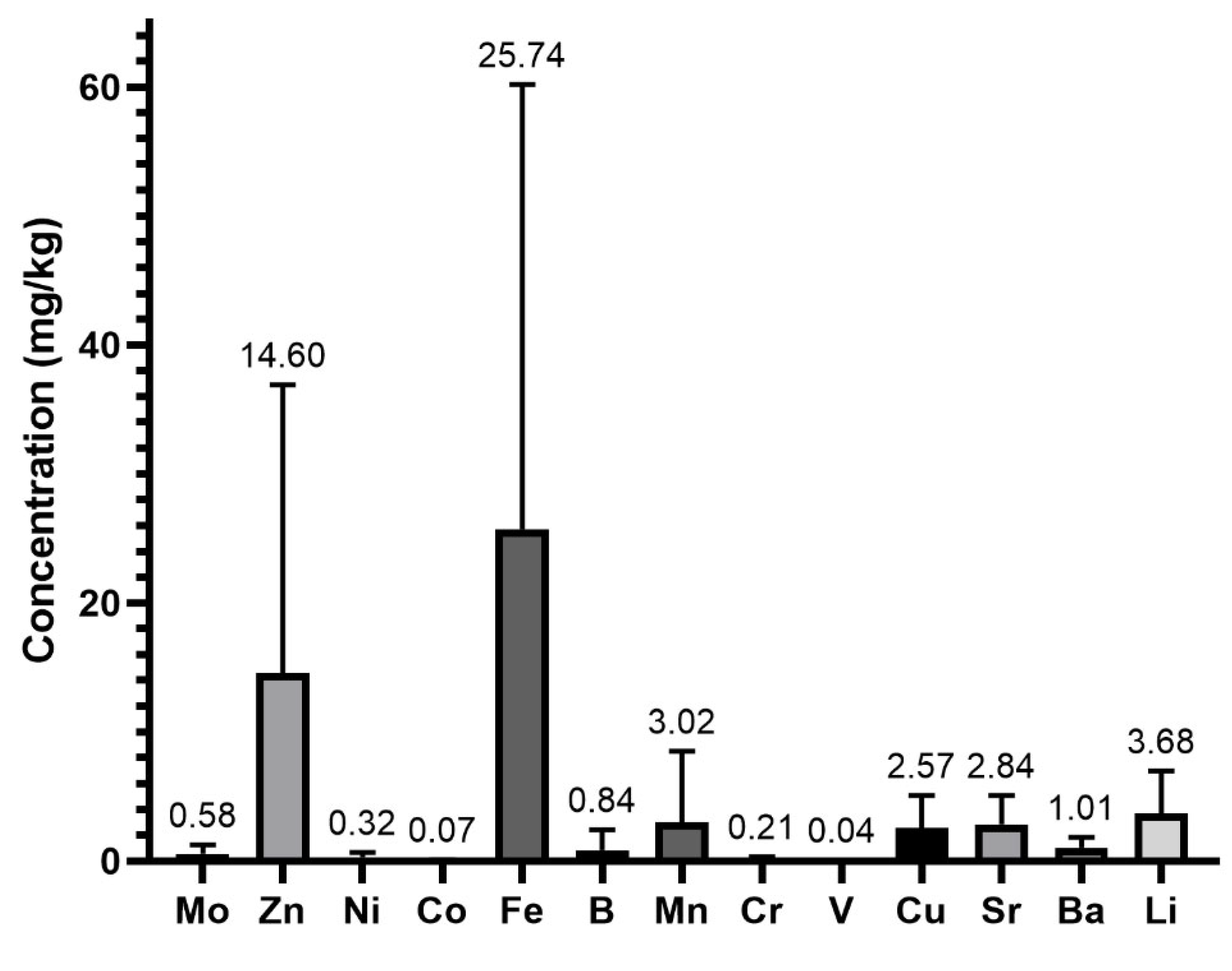
- Minerals in whey protein supplements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Mineral | Emission Wavelengths (nm) | Detection Limits (mg/L) | Quantification Limits (mg/L) |
|---|---|---|---|
| Al | 167.0 | 0.005 | 0.015 |
| B | 249.6 | 0.008 | 0.027 |
| Ba | 455.4 | 0.0006 | 0.002 |
| Ca | 315.8 | 1.629 | 5.432 |
| Co | 228.6 | 0.001 | 0.005 |
| Cr | 267.7 | 0.001 | 0.005 |
| Cu | 324.7 | 0.003 | 0.011 |
| Fe | 238.2 | 0.004 | 0.013 |
| K | 766.4 | 1.764 | 5.883 |
| Li | 670.7 | 0.013 | 0.031 |
| Mg | 383.8 | 1.580 | 5.268 |
| Mn | 257.6 | 0.0008 | 0.003 |
| Mo | 202.0 | 0.0016 | 0.005 |
| Na | 818.3 | 2.221 | 7.404 |
| Ni | 221.6 | 0.0009 | 0.003 |
| Sr | 407.7 | 0.003 | 0.011 |
| V | 292.4 | 0.0014 | 0.004 |
| Zn | 213.8 | 0.0027 | 0.009 |
| Lower 95% CI of the Mean | Upper 95% CI of the Mean | |
|---|---|---|
| Protein (%) | 17.43 | 33.79 |
| Mineral | ||
| Ca | 1875 | 2966 |
| Mg | 365.1 | 663.3 |
| K | 2364 | 3592 |
| Na | 1587 | 2709 |
| Al | 2.988 | 6.144 |
| B | 0.1957 | 0.7835 |
| Ba | 0.4511 | 0.8357 |
| Co | 0.01324 | 0.03487 |
| Cr | 0.09931 | 0.1704 |
| Cu | 1.086 | 2.175 |
| Fe | 9.383 | 23.31 |
| Mo | 0.2304 | 0.5115 |
| Mn | 0.8539 | 2.985 |
| Ni | 0.1296 | 0.2744 |
| Sr | 1.288 | 2.324 |
| V | 0.007289 | 0.01866 |
| Zn | 4.851 | 13.69 |
References
- Acosta Carrasco, M.R. La nutrición, suplementación e hidratación en el ámbito deportivo como base en el físico culturismo. Talentos 2020, 7, 31–47. Available online: https://talentos.ueb.edu.ec/index.php/talentos/article/view/200/282 (accessed on 5 December 2022). [CrossRef]
- AOAC Official Methods of the Analysis of the AOAC, 15th ed.; Association of the Official Analytical Chemist: Washington, DC, USA, 1990; pp. 237–273.
- Armendáriz-Anguiano, A.; Jimenez-Cruz, A.; Bacardi-Gascon, M.; Perez-Morales, E. Efectividad del uso de suplementos de proteína en entrenamientos de fuerza: Revisión sistemática. Arch. Latinoam. Nutr. 2010, 60, 113–118. Available online: https://www.alanrevista.org/ediciones/2010/2/art-1/ (accessed on 5 December 2022).
- Blasco Redondo, R. Las ayudas ergogénicas nutricionales en el ámbito deportivo. Primera parte. Aspectos generales. Nutr. Clínica Med. 2016, 10, 69–78. Available online: http://www.aulamedica.es/nutricionclinicamedicina/pdf/5038.pdf (accessed on 6 December 2022). [CrossRef]
- Elgammal, S.M.; Khorshed, M.A.; Ismail, E.H. Determination of heavy metal content in whey protein samples from markets in Giza, Egypt, using inductively coupled plasma optical emission spectrometry and graphite furnace atomic absorption spectrometry: A probabilistic risk assessment study. J. Food Compos. Anal. 2019, 84, 103300. [Google Scholar] [CrossRef]
- Guefai, F.Z.; Martínez-Rodríguez, A.; Grindlay, G.; Mora, J.; Gras, L. Elemental bioavailability in whey protein supplements. J. Food Compos. Anal. 2022, 112, 104696. Available online: https://www.sciencedirect.com/science/article/pii/S0889157522003143 (accessed on 5 December 2022). [CrossRef]
- Currie, L.A. Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- Arenas Jiménez, M.D. Cuando el deporte deja de ser salud: Dietas, suplementos y sustancias para aumentar el rendimiento y su relación con el riñón. Nefrología 2019, 39, 223–226. Available online: https://www.revistanefrologia.com/es-cuando-el-deporte-deja-ser-articulo-S0211699519300025 (accessed on 5 December 2022). [CrossRef]
- Jorquera Aguilera, C.; Rodríguez-Rodríguez, F.; Torrealba Vieira, M.I.; Campos Serrano, J.; Gracia Leiva, N. Consumo, características y perfil del consumidor de suplementos nutricionales en gimnasios de Santiago de Chile. Rev. Andal. Med. Del Deporte 2016, 9, 99–104. Available online: https://www.sciencedirect.com/science/article/pii/S1888754616300053 (accessed on 6 December 2022). [CrossRef] [Green Version]
- Knapik, J.J.; Steelman, R.A.; Hoedebecke, S.S.; Austin, K.G.; Farina, E.K.; Lieberman, H.R. Prevalence of Dietary Supplement Use by Athletes: Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Lofaso, M. Determination of Metals in Whey and Vegan Protein Supplements using Inductively Coupled Plasma Mass Spectrometry; University of Mississippi: Washington, DC, USA, 2021. [Google Scholar]
- Martínez-Sanz, J.M.; Sospedra, I.; Ortiz, C.M.; Baladía, E.; Gil-Izquierdo, A.; Ortiz-Moncada, R. Intended or Unintended Doping? A Review of the Presence of Doping Substances in Dietary Supplements Used in Sports. Nutrients 2017, 9, 1093. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sanz, J.M.; Mata, F.; Sala Ripoll, M.; Puya Braza, J.M.; Martínez Segura, A.; Sánchez-Oliver, A.J.; Cortell Tormo, J.M. Fraude en suplementos nutricionales para deportistas: Revisión narrativa. Nutr. Hosp. 2021, 38, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C.; et al. Effect of a Protein Supplement on the Gut Microbiota of Endurance Athletes: A Randomized, Controlled, Double-Blind Pilot Study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authorithy (EFSA). Dietary Reference Values [Internet] 2023. Available online: https://www.efsa.europa.eu/en/topics/topic/dietary-reference-values (accessed on 13 February 2023).
- Olivos, O.C.; Cuevas, M.A.; Álvarez, V.V.; Jorquera, A.C. Nutrición para el entrenamiento y la competición. Rev. Médica Clínica Las Condes 2012, 23. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/lil-733899 (accessed on 6 December 2022).
- Palacios, N.; Manonelles, P.; Redondo, R.; Contreras, C.; Franco, L.; Gaztañaga, T.; Manuz, B.; Galván, C.; del Valle, M.; García, A.; et al. Suplementos nutricionales para el deportista. AMD 2019, 36, 1–114. [Google Scholar]
- Pinto, E.; Ferreira Isabel, M.P.L.V.O.; Almeida, A. Essential and non-essential/toxic trace elements in whey protein supplements. J. Food Compos. Anal. 2020, 86, 103383. Available online: https://www.sciencedirect.com/science/article/pii/S0889157519309299 (accessed on 9 December 2022). [CrossRef]
- Pomerantz, A.; Blachman-Braun, R.; Vital-Flores, S.; Berebichez-Fridman, R.; Aguilar-Mendoza, J.P.; Lara-Villalón, D. Protein supplement consumption and its possible association with kidney damage in Mexican elite athletes. Rev. Med. Inst. Mex Seguro Soc. 2016, 54, 42–47. [Google Scholar] [PubMed]
- Pontes, T.d.C.; Fernandes Filho, G.M.C.; Trindade, A.d.S.P.; Sobral Filho, J.F. Incidence of acne vulgaris in young adult users of protein-calorie supplements in the city of João Pessoa--PB. An. Bras. Dermatol. 2013, 88, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Adeel, K.; Maqbool Hussain, A.; Muhammad Kashi, U.R.; Qamar uz, Z.; Raza, U. Official Methods of Analysis. In Association of Official Analytical Chemists, 14th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1984. [Google Scholar]
- UNE UNE-EN ISO 8968-4:2016. 2016. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?Tipo=N&c=N0057028 (accessed on 9 December 2022).
- Bonnet Lemus, R.; Álvarez, C.; Ramirez Mendoza, A.A.; Mendoza Magaña, M.L.; Ramírez-Herrera, M.; Pérez González, J. Análisis del Contenido Proteico de los Suplementos Alimenticios; UMA: Clearwater, FL, USA, 2020; pp. 196–201. [Google Scholar]
- Goulding, D.A.; Fox, P.F.; O’Mahony, J.A. Chapter 2—Milk proteins: An overview. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 21–98. [Google Scholar]
- Análisis de Nitrógen el Método de Johan Kjeldahl. Available online: https://www.gerhardt.de/fileadmin/Redaktion/downloads/Stickstoffanalyse_-_Die_Methode_von_Johan_Kjeldahl_gekuerzt_f_Homepage-spa-ES.pdf (accessed on 10 December 2022).
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; García Asuero, A.; Wybraniec, S. An Overview of the Kjeldahl Method of Nitrogen Determination. Part I. Early History, Chemistry of the Procedure, and Titrimetric Finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Determinación de Nitrógeno por el Método Kjeldahl. Available online: https://www.itwreagents.com/uploads/20180122/A173_ES.pdf (accessed on 10 December 2022).
- Kamizake, N.K.K.; Gonçalves, M.M.; Zaia Cássia, T.B.V.; Zaia, D.A.M. Determination of total proteins in cow milk powder samples: A comparative study between the Kjeldahl method and spectrophotometric methods. J. Food Compos. Anal. 2003, 16, 507–516. Available online: https://www.sciencedirect.com/science/article/pii/S0889157503000048 (accessed on 9 December 2022). [CrossRef]
- R Core Team R: A Language and environment for statistical computing. (Version 4.0). 2021, 4. Available online: https://www.scirp.org/(S(czeh2tfqw2orz553k1w0r45))/reference/referencespapers.aspx?referenceid=3131254 (accessed on 29 December 2022).
- Reilly, D. Robert Boyle and his background. J. Chem. Educ. 1951, 28, 178. [Google Scholar] [CrossRef]
- Rubio-Arméndariz, C.; Paz, S.; Gutiérrez, A.J.; González-Weller, D.; Revert, C.; Hardisson, A. Human Exposure to Toxic Metals (Al, Cd, Cr, Ni, Pb, Sr) from the Consuption of cereals in Canary Islands. Food 2021, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Paz, S.; Gutiérrez, A.J.; Furtado, V.G.; González-Weller, D.; Revert, C.; Hardisson, A. Toxic Metals in Cereals in Cape Verde: Risk Assessment Evaluation. Int. J. Environ. Res. Public Health 2021, 18, 3833. [Google Scholar] [CrossRef]
- Rodríguez, F.; Crovetto, M.; Andrea González, A.; Nikol Morant, C.; Francisco Santibáñez, T. Consumo de Suplementos Nutricionales en Gimnasios, Perfil del Consumidor Y Características de su uso. Rev. Chil. Nutr. 2011, 38, 157–166. [Google Scholar] [CrossRef] [Green Version]
- The Jamovi Project. Jamovi. (Version 2.2) [Computer Software]. 2021. Available online: https://www.jamovi.org (accessed on 10 December 2022).
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.W. Mann-Whitney U test and t-test. J. Vis. Impair. Blind. 2023, 1, 99–100. [Google Scholar] [CrossRef]
- Vierra, A.; Razzaq, A.; Andreadis, A. Continuous variable analyses: T-test, Mann–Whitney U, Wilcoxon sign rank. In Translational Surgery; Academic Press: Cambridge, MA, USA, 2023; pp. 165–170. [Google Scholar] [CrossRef]
- Hespanhol, L.; Vallio, C.S.; Costa, L.M.; Saragiotto, B.T. Understanding and interpreting confidence and credible intervals around effect estimates. Braz. J. Phys. Ther. 2019, 23, 290–301. [Google Scholar] [CrossRef]
- Schönfeldt, H.C.; Hall, N.; Pretorius, B. 12th IFDC 2017 Special Issue—High protein sports supplements: Protein quality and label compliance. J. Food Compos. Anal. 2019, 83, 103293. Available online: https://www.sciencedirect.com/science/article/pii/S0889157519311901 (accessed on 10 December 2022). [CrossRef]
- Garrido, B.C.; Souza, G.H.; Lourenço, D.C.; Fasciotti, M. Proteomics in quality control: Whey protein-based supplements. J. Proteom. 2016, 147, 48–55. [Google Scholar] [CrossRef]
- Pellegrino, L.; Hogenboom, J.A.; Rosi, V.; Sindaco, M.; Gerna, S.; D’Incecco, P. Focus on the Protein Fraction of Sports Nutrition Supplements. Molecules 2022, 27, 3487. [Google Scholar] [CrossRef]
- Sánchez Oliver, A.J. Suplementación Nutricional en la Actividad Físico-Deportiva: Análisis de la Calidad del Suplemento Proteico Consumido; Universidad de Granada: Granada, Spain, 2013. [Google Scholar]
| Declared | Observed | |
|---|---|---|
| Samples (N) | 47 | 47 |
| Mean | 74.3% | 70.9% |
| Std. error mean | 2.22 | 1.96 |
| Median | 75.0% | 71.9% |
| Mode | 75.0% | 71.3% |
| Sum | 3493 | 3334 |
| Standard deviation | 15.2 | 13.5 |
| Minimum | 18% | 18.2% |
| Maximum | 93.0% | 92.3% |
| Shapiro–Wilk W | 0.849 | 0.842 |
| Shapiro–Wilk p | <0.001 | <0.001 |
| 25th percentile | 71.0% | 71.9% |
| 50th percentile | 75.0% | 71.9% |
| 75th percentile | 85.0% | 78.8% |
| Minerals | N | N (>LQ) | Mean Concentration ± SD (g/Kg) | Maximum Value (g/Kg) | Minimum Value (g/Kg) |
|---|---|---|---|---|---|
| Ca | 47 | 47 | 3.81 ± 1.84 | 11.00 | 0.48 |
| Mg | 47 | 47 | 0.81 ± 0.64 | 3.25 | 0.06 |
| K | 47 | 47 | 4.70 ± 1.71 | 9.69 | 0.24 |
| Na | 47 | 47 | 3.38 ± 2.24 | 11.42 | 0.24 |
| Minerals | N | N (>LQ) | Mean Concentration ± SD (mg/Kg) | Maximum Value (mg/Kg) | Minimum Value (mg/Kg) |
| Ba | 47 | 47 | 1.01 ± 0.84 | 5.05 | 0.23 |
| B * | 47 | 43 | 0.84 ± 1.58 | 10.56 | <LQ |
| Co | 47 | 28 | 0.07 ± 0.06 | 0.31 | <LQ |
| Cu * | 47 | 47 | 2.57 ± 2.51 | 10.42 | 0.38 |
| Cr | 47 | 47 | 0.21 ± 0.14 | 0.60 | 0.03 |
| Fe | 47 | 47 | 25.74 ± 34.45 | 175.64 | 2.19 |
| Li | 47 | 46 | 3.68 ± 3.31 | 14.11 | <LQ |
| Mn | 47 | 47 | 3.02 ± 5.49 | 26.87 | 0.09 |
| Mo * | 47 | 47 | 0.58 ± 0.68 | 4.26 | 0.05 |
| Ni | 47 | 47 | 0.32 ± 0.34 | 1.40 | 0.01 |
| Sr | 47 | 47 | 2.84 ± 2.22 | 10.36 | 0.37 |
| V * | 47 | 22 | 0.04 ± 0.03 | 0.14 | <LQ |
| Zn * | 47 | 47 | 14.60 ± 22.23 | 101.51 | 1.35 |
| Al | 47 | 47 | 7.19 ± 7.37 | 35.22 | 0.40 |
| Whey Protein Supplement Concentrate | Whey Protein Supplement Isolate | ||||||
|---|---|---|---|---|---|---|---|
| Mineral | N | N (>LQ) | Mean Concentration ± SD (g/kg) (Cmin–Cmax) | Mineral | N | N (>LQ) | Mean Concentration ± SD (g/kg) (Cmin–Cmax) |
| Ca | 22 | 22 | 3.17 ± 1.14 (0.48–4.73) | Ca | 21 | 21 | 4.59 ± 2.18 (2.31–11.43) |
| Mg | 22 | 22 | 0.70 ± 0.54 (0.56–2.69) | Mg | 21 | 21 | 0.96 ± 0.77 (0.36–3.25) |
| K | 22 | 22 | 4.48 ± 1.57 (0.24–7.13) | K | 21 | 21 | 5.11 ± 1.91 (1.62–9.70) |
| Na | 22 | 22 | 3.22 ± 1.89 (0.24–7.19) | Na | 21 | 21 | 3.53 ± 2.66 (0.69–11.43) |
| Mineral | N | N (>LQ) | Mean Concentration ± SD (mg/kg) (Cmin–Cmax) | Mineral | N | N (>LQ) | Mean Concentration ± SD (mg/kg) (Cmin–Cmax) |
| Ba | 22 | 22 | 1.06 ± 1.11 (0.23–5.05) | Ba | 21 | 21 | 0.92 ± 0.42 (0.40–2.05) |
| B * | 22 | 19 | 1.18 ± 2.33 (0.17–10.56) | B | 21 | 21 | 0.54 ± 0.39 (0.15–1.65) |
| Co | 22 | 11 | 0.08 ± 0.08 (0.03–0.31) | Co | 21 | 14 | 0.05 ± 0.02 (0.02–0.10) |
| Cu * | 22 | 22 | 2.65 ± 2.66 (0.38–10.42) | Cu | 21 | 21 | 2.40 ± 2.42 (0.49–9.50) |
| Cr | 22 | 22 | 0.19 ± 0.14 (0.03–0.59) | Cr | 21 | 21 | 0.21 ± 0.12 (0.05–0.46) |
| Fe | 22 | 22 | 20.18 ± 36.75 (2.19–175.64) | Fe | 21 | 21 | 26.94 ± 24.27 (3.96–74.71) |
| Li | 22 | 21 | 3.61 ± 3.70 (0.35–13.84) | Li | 21 | 21 | 3.75 ± 3.29 (0.51–14.11) |
| Mn | 22 | 22 | 2.41 ± 4.87 (0.09–19.74) | Mn | 21 | 21 | 3.70 ± 6.45 (0.23–26.87) |
| Mo * | 22 | 22 | 0.82 ± 0.86 (0.05–4.26) | Mo | 21 | 21 | 0.28 ± 0.27 (0.06–1.04) |
| Ni | 22 | 22 | 0.31 ± 0.38 (0.02–1.40) | Ni | 21 | 21 | 0.32 ± 0.31 (0.03–1.13) |
| Sr | 22 | 22 | 2.44 ± 2.13 (0.37–10.36) | Sr | 21 | 21 | 3.02 ± 1.97 (1.37–10.01) |
| V * | 22 | 8 | 0.05 ± 0.04 (0.02–0.14) | V | 21 | 11 | 0.04 ± 0.02 (0.02–0.08) |
| Zn * | 22 | 22 | 10.00 ± 13.94 (1.35–55.0) | Zn | 21 | 21 | 18.63 ± 28.67 (1.36–101.51) |
| Al | 22 | 22 | 7.70 ± 9.30 (0.42–35.22) | Al | 21 | 21 | 6.05 ± 3.91 (1.82–13.46) |
| Elgammal et al. [5] | Guefai et al. [6] | Lofaso et al. [11] | Pinto et al. [18] | This Study | |
|---|---|---|---|---|---|
| Ca | 4423 | 1064 | - | - | 3811 |
| Mg | 962 | 724 | - | - | 809 |
| K | - | 4978 | - | - | 4689 |
| Na | 2830 | 3575 | - | - | 3382 |
| Ba | - | 0.46 | - | 1.38 | 1.01 |
| B | - | - | - | - | 0.84 |
| Co | N.D.–< 0.5 | 0.35 | 0.01 | 0.04 | 0.07 |
| Cu | N.D.–16.78 | 4.00 | 1.91 | 1.90 | 2.57 |
| Cr | N.D.–0.69 | 1.40 | 0.05 | 0.46 | 0.21 |
| Fe | 17.51 | 22.00 | 11.5 | 13.70 | 25.74 |
| Li | - | 1.19 | - | 0.02 | 3.68 |
| Mn | 3.56 | 8.70 | 0.20 | 1.60 | 3.02 |
| Mo | - | - | - | 0.92 | 0.58 |
| Ni | N.D.–0.93 | 0.52 | - | 0.35 | 0.32 |
| Sr | - | 3.10 | - | 3.20 | 2.84 |
| V | - | 0.44 | 0.002 | 0.025 | 0.04 |
| Zn | 17.66 | 29.10 | - | 6.70 | 14.60 |
| Al | <5–16.26 | 8.00 | - | 3.00 | 7.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Weller, D.; Paz-Montelongo, S.; Bethencourt-Barbuzano, E.; Niebla-Canelo, D.; Alejandro-Vega, S.; Gutiérrez, Á.J.; Hardisson, A.; Carrascosa, C.; Rubio, C. Proteins and Minerals in Whey Protein Supplements. Foods 2023, 12, 2238. https://doi.org/10.3390/foods12112238
González-Weller D, Paz-Montelongo S, Bethencourt-Barbuzano E, Niebla-Canelo D, Alejandro-Vega S, Gutiérrez ÁJ, Hardisson A, Carrascosa C, Rubio C. Proteins and Minerals in Whey Protein Supplements. Foods. 2023; 12(11):2238. https://doi.org/10.3390/foods12112238
Chicago/Turabian StyleGonzález-Weller, Dailos, Soraya Paz-Montelongo, Elena Bethencourt-Barbuzano, Daniel Niebla-Canelo, Samuel Alejandro-Vega, Ángel J. Gutiérrez, Arturo Hardisson, Conrado Carrascosa, and Carmen Rubio. 2023. "Proteins and Minerals in Whey Protein Supplements" Foods 12, no. 11: 2238. https://doi.org/10.3390/foods12112238








