The Effects of Synbiotics on Dextran-Sodium-Sulfate-Induced Acute Colitis: The Impact of Chitosan Oligosaccharides on Endogenous/Exogenous Lactiplantibacillus plantarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Chitosan Oligosaccharides
2.2. Strain Cultures and Synbiotic Preparation
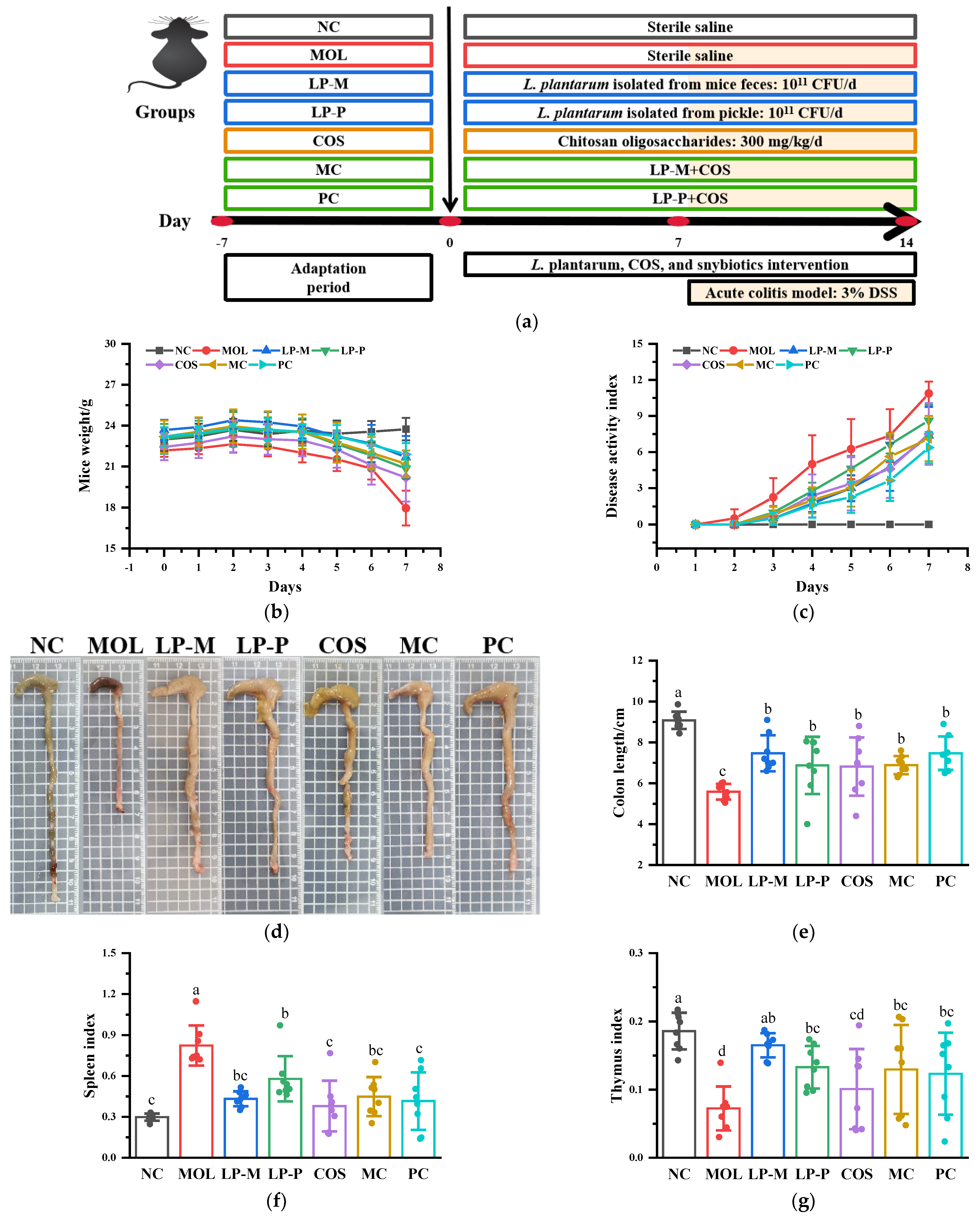
2.3. Animal Experimental Design and Tissue Collection
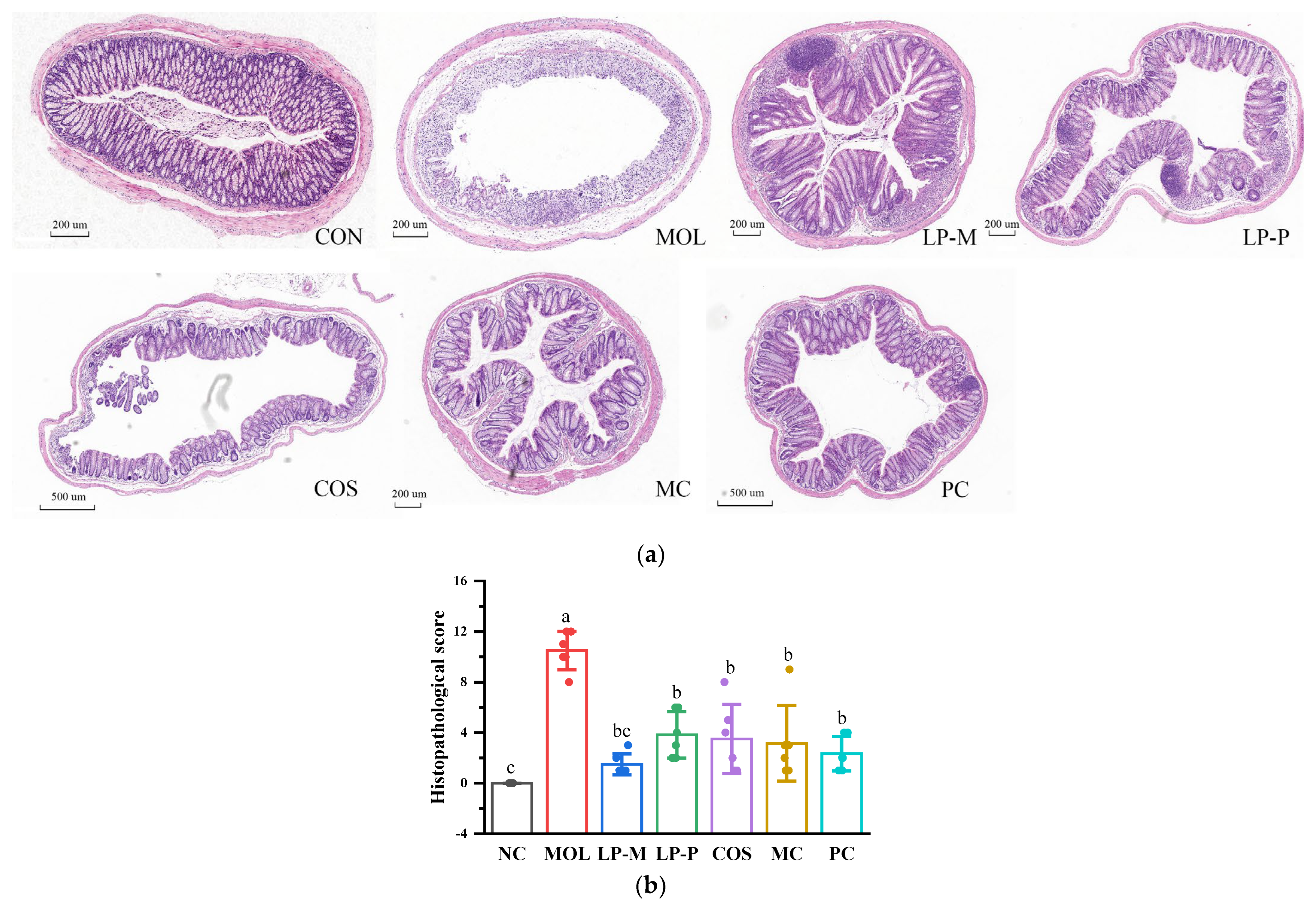
2.4. Histopathological Evaluation
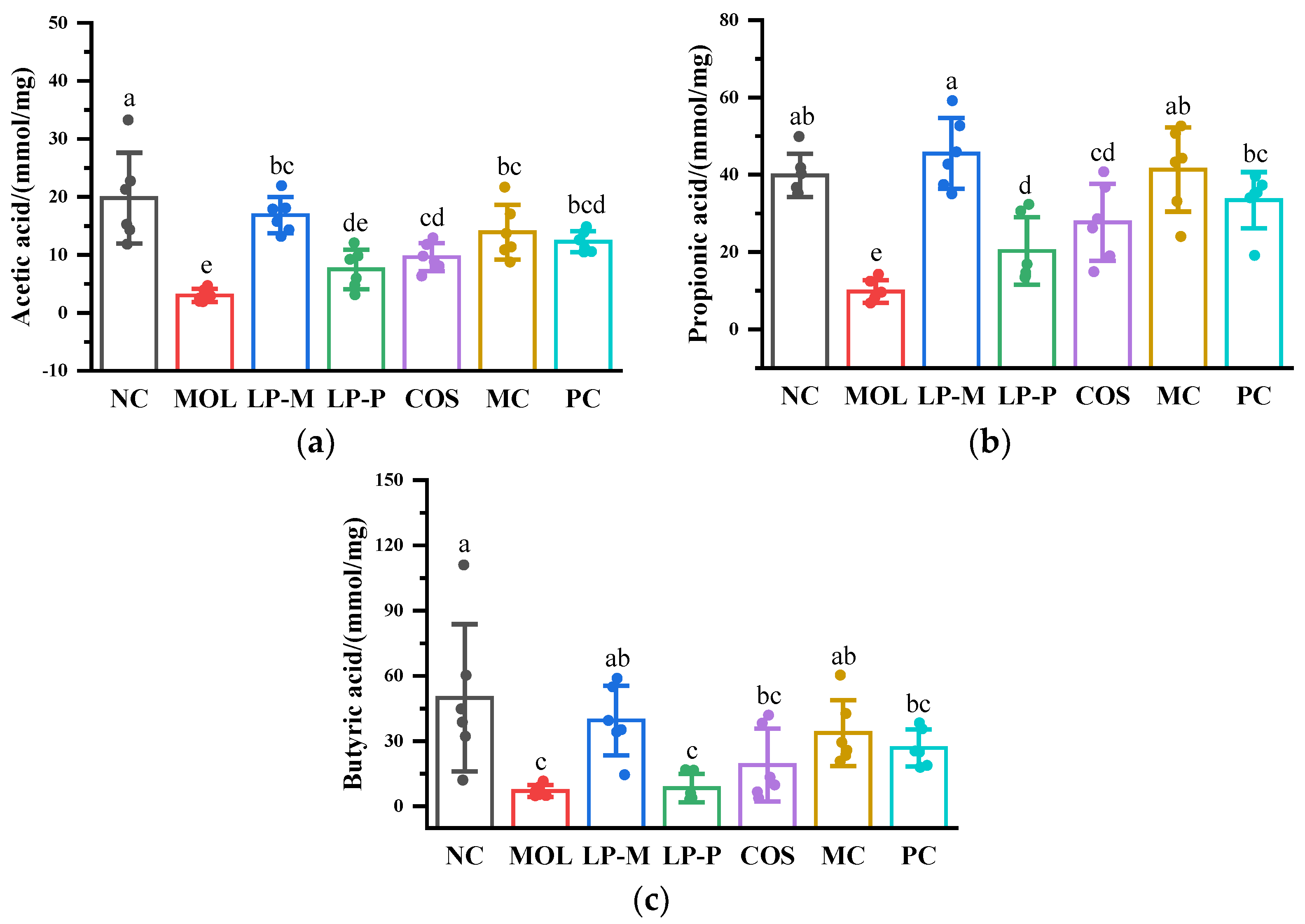
2.5. Measurement of Short-Chain Fatty Acids in Cecum Contents
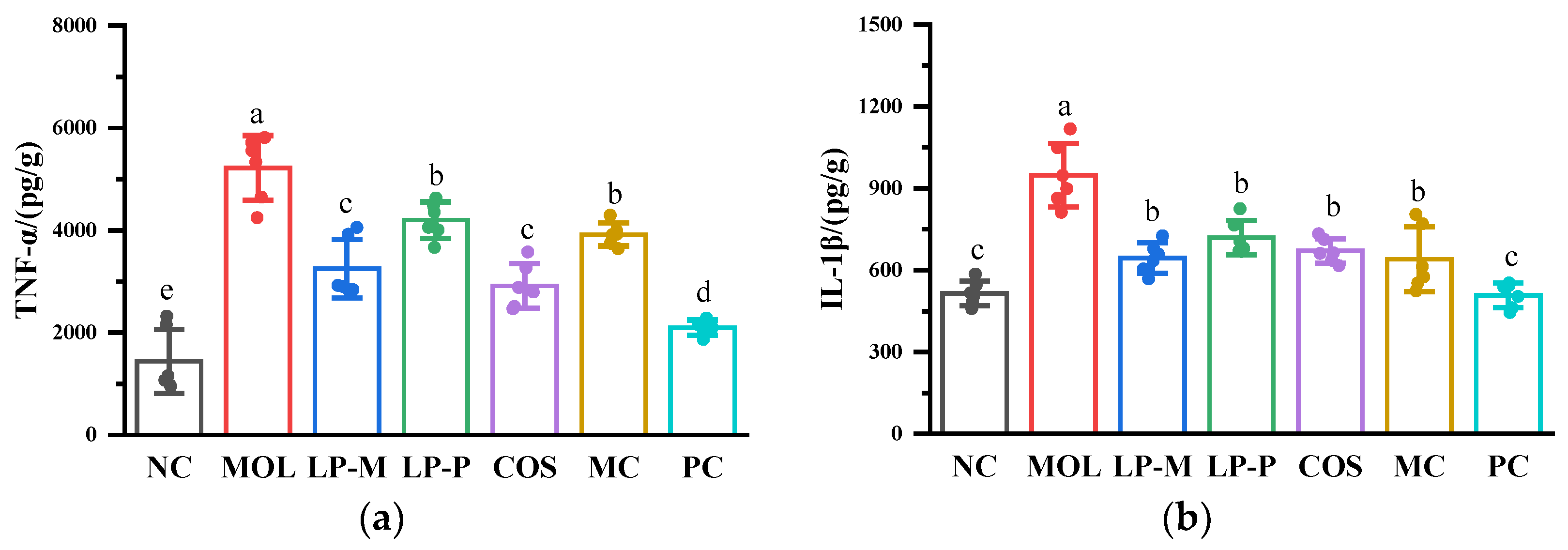
2.6. Measurement of Cytokine Levels
2.7. Measurement of Myeloperoxidase Activity
2.8. Analysis of Gut Microbiota
2.9. Statistical Analysis
3. Results
3.1. L. plantarum, COS, and Synbiotics Alleviated the DSS-Induced Colitis Symptoms
3.2. L. plantarum, COS, and Synbiotics Alleviated the Colon Damage in DSS-Induced Colitis Mice
3.3. L. plantarum, COS, and Synbiotics Improved the Short-Chain Fatty Acids in Cecum Contents of DSS-Induced Colitis Mice
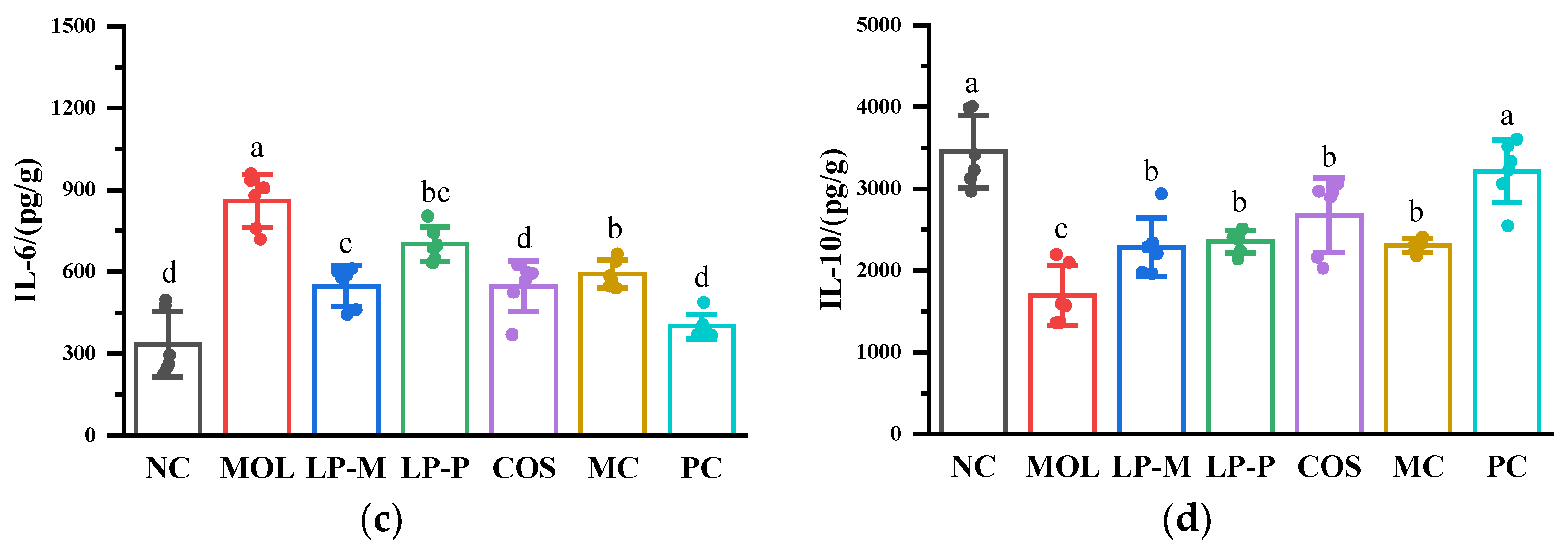
3.4. L. plantarum, COS, and Synbiotics Influenced Cytokine Levels Positively in the Colon of DSS-Induced Colitis Mice
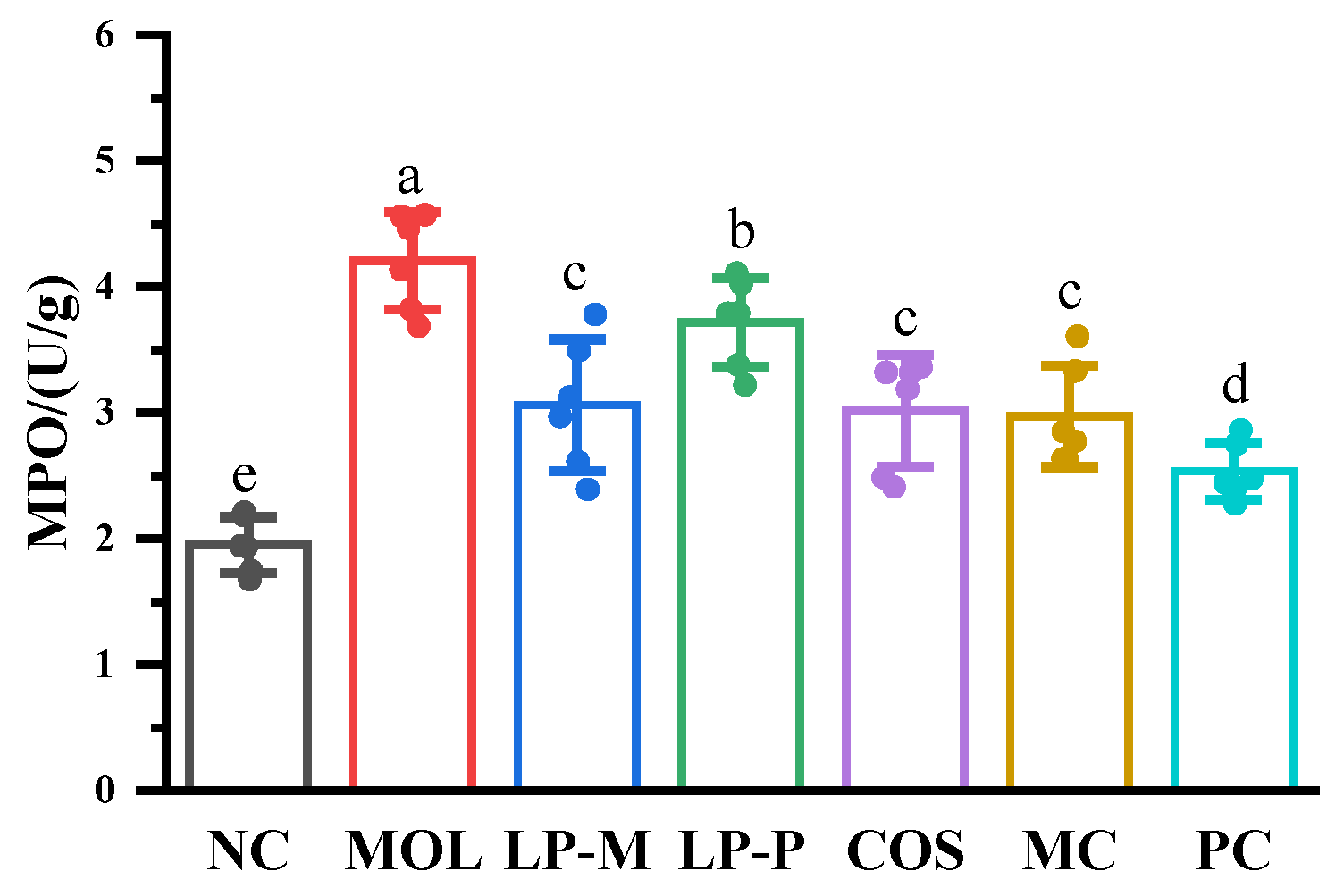
3.5. L. plantarum, COS, and Synbiotics Inhibited the MPO Activity in the Colon of DSS-Induced Colitis Mice
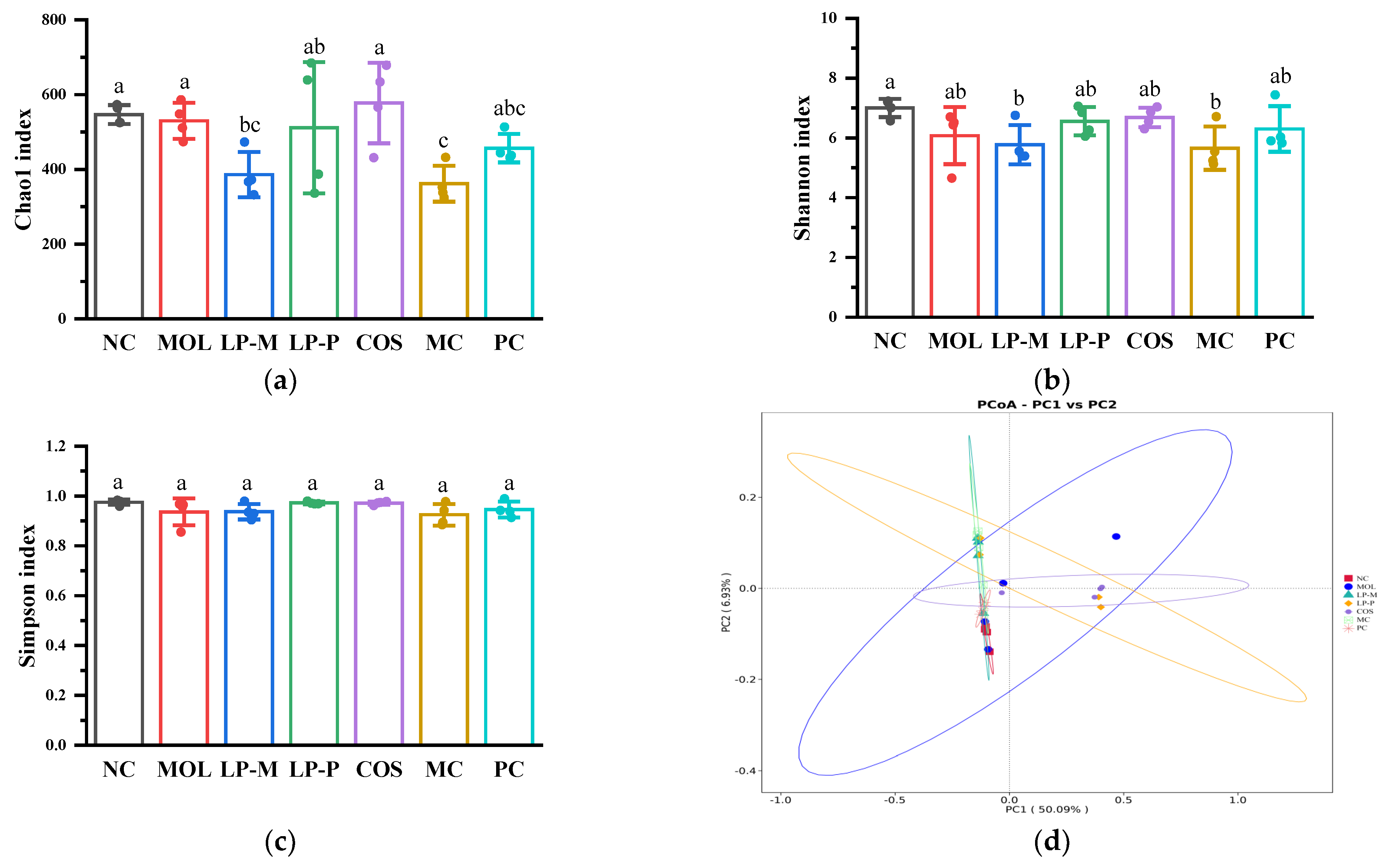
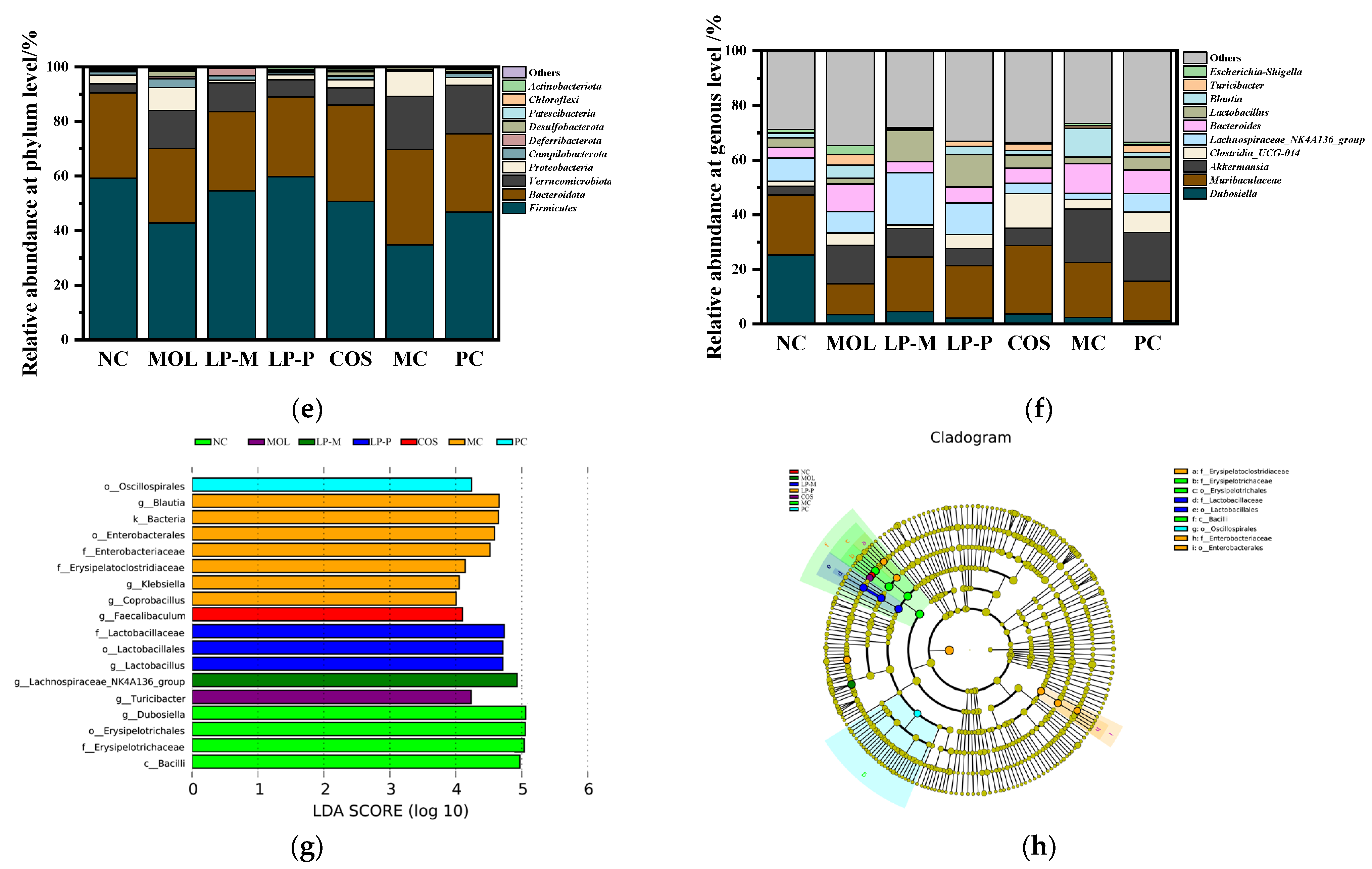
3.6. L. plantarum, COS, and Synbiotics Restored the Gut Microbiota in Colon Contents of DSS-Induced Colitis Mice
3.7. The Differential Analysis of the Effects of COS on Gut Microbiota Regulation in DSS-Induced Colitis Mice of Endogenous and Exogenous L. plantarum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, M.; Burisch, J. Impact of genes and the environment on the pathogenesis and disease course of inflammatory bowel disease. Dig. Dis. Sci. 2019, 64, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Chen, X.Q.; Xu, R.L.; Dong, H.M.; Yang, F.Y.; Wang, Y.; Zhang, Z.H.; Ju, J.M. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: A review. Carbohydr. Polym. 2021, 254, 117189. [Google Scholar] [CrossRef] [PubMed]
- Sireswar, S.; Ghosh, I.; Dey, G. First and second generation probiotic therapeutics for inflammatory bowel disease. PharmaNutrition 2019, 9, 100159. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific ssociation for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.d.; Ibrahim, S.A. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Wang, L.; He, Z.; Tian, P.; Wang, G. Lactic acid bacteria and host immunity. In Lactic Acid Bacteria: Omics and Functional Evaluation; Chen, W., Ed.; Springer Nature: Singapore, 2019; pp. 261–296. [Google Scholar]
- Al-Sahlany, S.T.G.; Niamah, A.K. Bacterial viability, antioxidant stability, antimutagenicity and sensory properties of onion types fermentation by using probiotic starter during storage. Nutr. Food Sci. 2022, 52, 901–916. [Google Scholar] [CrossRef]
- Jana, U.K.; Suryawanshi, R.K.; Prajapati, B.P.; Kango, N. Prebiotic mannooligosaccharides: Synthesis, characterization and bioactive properties. Food Chem. 2021, 342, 128328. [Google Scholar] [CrossRef]
- Jiang, H.R.; Cai, M.M.; Shen, B.Y.; Wang, Q.; Zhang, T.C.; Zhou, X. Synbiotics and gut microbiota: New perspectives in the treatment of type 2 diabetes mellitus. Foods 2022, 11, 2438. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Kesika, P.; Khongtan, S.; Khampithum, N.; Thangaleela, S.; Peerajan, S.; Bumrungpert, A.; Chaiyasut, K.; Sirilun, S.; et al. Synbiotic supplementation improves obesity index and metabolic biomarkers in thai obese adults: A randomized clinical trial. Foods 2021, 10, 1580. [Google Scholar] [CrossRef]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current status of probiotic and related health benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Liu, N.; Wang, H.; Yang, Z.; Zhao, K.; Li, S.; He, N. The role of functional oligosaccharides as prebiotics in ulcerative colitis. Food Funct. 2022, 13, 6875–6893. [Google Scholar] [CrossRef]
- Zhang, N.; Jin, M.; Wang, K.; Zhang, Z.; Shah, N.P.; Wei, H. Functional oligosaccharide fermentation in the gut: Improving intestinal health and its determinant factors-a review. Carbohydr. Polym. 2022, 284, 119043. [Google Scholar] [CrossRef]
- Liao, M.J.; Zhang, Y.F.; Qiu, Y.L.; Wu, Z.C.; Zhong, Z.H.; Zeng, X.Q.; Zeng, Y.L.; Xiong, L.; Wen, Y.; Liu, R.S. Fructooligosaccharide supplementation alleviated the pathological immune response and prevented the impairment of intestinal barrier in dss-induced acute colitis mice. Food Funct. 2021, 12, 9844–9854. [Google Scholar] [CrossRef]
- Xue, Z.H.; Li, R.L.; Liu, J.Y.; Zhou, J.N.; Zhang, X.Y.; Zhang, T.T.; Zhang, M.; Yang, Y.; Chen, H.X. Preventive and synbiotic effects of the soluble dietary fiber obtained from lentinula edodes byproducts and lactobacillus plantarum lp90 against dextran sulfate sodium-induced colitis in mice. J. Sci. Food Agric. 2023, 103, 616–626. [Google Scholar] [CrossRef]
- Simeoli, R.; Raso, G.M.; Lama, A.; Pirozzi, C.; Santoro, A.; Di Guida, F.; Sanges, M.; Aksoy, E.; Calignano, A.; D’Arienzo, A.; et al. Preventive and therapeutic effects of lactobacillus paracasei b21060-based synbiotic treatment on gut inflammation and barrier integrity in colitic mice. J. Nutr. 2015, 145, 1202–1210. [Google Scholar] [CrossRef]
- Akman, P.K.; Ozulku, G.; Tornuk, F.; Yetim, H. Potential probiotic lactic acid bacteria isolated from fermented gilaburu and shalgam beverages. LWT-Food Sci. Technol. 2021, 149, 111705. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Siddiq, M.; Zhao, H.; Zhu, J.; Yan, L.; Shao, D.; Xu, X.; Shi, J. Identification, characterization, and probiotic potential of lactobacillus rhamnosus isolated from human milk. LWT-Food Sci. Technol. 2017, 84, 271–280. [Google Scholar] [CrossRef]
- Yang, B.; Li, M.; Wang, S.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus ruminis alleviates dss-induced colitis by inflammatory cytokines and gut microbiota modulation. Foods 2021, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.; Costa, V.M.; Franchi Vasconcelos Gomes, M.I.; Golim, M.A.; Modolo, J.R.; Langoni, H. Effects of synbiotic-based bifidobacterium animalis in female rats experimentally infected with toxoplasma gondii. Comp. Immunol. Microb. 2011, 34, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.Y.; Lin, Q.L.; Li, X.H.; Nie, Y.; Wang, L.; Shi, L.M.; Xu, W.; Hu, T.; Guo, T.; Luo, F.J. Octacosanol attenuates inflammation in both raw264.7 macrophages and a mouse model of colitis. J. Agric. Food Chem. 2017, 65, 3647–3658. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Campos-Vega, R.; de Mejia, E.G.; Loarca-Pina, G. Consumption of a baked corn and bean snack reduced chronic colitis inflammation in cd-1 mice via downregulation of il-1 receptor, tlr, and tnf-alpha associated pathways. Food Res. Int. 2020, 132, 109097. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yeom, J.; Lim, Y.H. Dairy propionibacterium freudenreichii ameliorates acute colitis by stimulating muc2 expression in intestinal goblet cell in a dss-induced colitis rat model. Sci. Rep. 2020, 10, 5523. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Xiang, Q.R.; Mao, B.Y.; Tang, X.; Cui, S.M.; Li, X.F.; Zhao, J.X.; Zhang, H.; Chen, W. Protective effects of microbiome-derived inosine on lipopolysaccharide-induced acute liver damage and inflammation in mice via mediating the tlr4/nf-kappa b pathway. J. Agric. Food Chem. 2021, 69, 7619–7628. [Google Scholar] [CrossRef]
- Li, Q.; Chen, H.X.; Zhang, M.; Wu, T.; Liu, R. Altered short chain fatty acid profiles induced by dietary fiber intervention regulate ampk levels and intestinal homeostasis. Food Funct. 2019, 10, 7174–7187. [Google Scholar] [CrossRef]
- Subramanya, S.B.; Chandran, S.; Almarzooqi, S.; Raj, V.; Al Zahmi, A.S.; Al Katheeri, R.A.; Al Zadjali, S.A.; Collin, P.D.; Adrian, T.E. Frondanol, a nutraceutical extract from cucumaria frondosa, attenuates colonic inflammation in a dss-induced colitis model in mice. Mar. Drugs 2018, 16, 148. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Liu, R.; Zhang, M.; Wang, R. Soluble dietary fiber reduces trimethylamine metabolism via gut microbiota and co-regulates host ampk pathways. Mol. Nutr. Food Res. 2017, 61, 1700473. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Merikas, E.; Georgopoulos, F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des. Dev. Ther. 2011, 5, 185–210. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. Nlrp6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Giromini, C.; Baldi, A.; Rebucci, R.; Lanzoni, D.; Policardi, M.; Sundaram, T.S.; Purup, S. Role of short chain fatty acids to counteract inflammatory stress and mucus production in human intestinal ht29-mtx-e12 cells. Foods 2022, 11, 1983. [Google Scholar] [CrossRef]
- van der Beek, C.M.; Bloemen, J.G.; van den Broek, M.A.; Lenaerts, K.; Venema, K.; Buurman, W.A.; Dejong, C.H. Hepatic uptake of rectally administered butyrate prevents an increase in systemic butyrate concentrations in humans. J. Nutr. 2015, 145, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Singer, J.; Kwan, T.K.; Loh, Y.W.; Wang, C.; Tan, J.; Li, Y.J.; Lai, S.W.C.; Macia, L.; Alexander, S.I.; et al. Gut microbial netabolites induce donor-specific tolerance of kidney allografts through induction of t regulatory cells by short-chain fatty acids. J. Am. Soc. Nephrol. 2020, 31, 1445–1461. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.X.; Li, S.Q.; Zhao, Z.; An, L.L. Sodium butyrate as an effective feed additive to improve growth performance and gastrointestinal development in broilers. Vet. Med. Sci. 2020, 6, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (dss)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, ibd, and colitis-associated cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef]
- Moon, H.J.; Cha, Y.S.; Kim, K.A. Blackcurrant alleviates dextran sulfate sodium (dss)-induced colitis in mice. Foods 2023, 12, 1073. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an active disease biomarker: Recent biochemical and pathological perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Conte, M.P.; Schippa, S.; Zamboni, I.; Penta, M.; Chiarini, F.; Seganti, L.; Osborn, J.; Falconieri, P.; Borrelli, O.; Cucchiara, S. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 2006, 55, 1760–1767. [Google Scholar] [CrossRef]
- Zhai, Z.Y.; Zhang, F.; Cao, R.H.; Ni, X.J.; Xin, Z.Q.; Deng, J.P.; Wu, G.Y.; Ren, W.K.; Yin, Y.L.; Deng, B.C. Cecropin a alleviates inflammation through modulating the gut microbiota of c57bl/6 mice with dss-induced ibd. Front. Microbiol. 2019, 10, 1595. [Google Scholar] [CrossRef]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, J.; Ding, Y.; Zhou, J.; Gao, G.; Han, H.; Zhou, J.; Ke, L.; Rao, P.; Chen, T.; et al. Nanoparticles isolated from porcine bone soup ameliorated dextran sulfate sodium-induced colitis and regulated gut microbiota in mice. Front. Nutr. 2022, 9, 821404. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.G.; Chen, Q.; Liu, J.N.; Chen, X.Q.; Luo, H.Q.; Ye, Z.W.; Liu, J.C. Microbiome analysis reveals gut microbiota alteration in mice with the effect of matrine. Microb. Pathog. 2021, 156, 104926. [Google Scholar] [CrossRef]
- Li, X.; Qiao, G.; Chu, L.; Lin, L.; Zheng, G. Smilax china l. Polysaccharide alleviates dextran sulphate sodium-induced colitis and modulates the gut microbiota in mice. Foods 2023, 12, 1632. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Xia, X.X.; Lin, H.; Luo, F.J.; Wu, X.X.; Zhu, L.F.; Chen, S.L.; Luo, H.; Ye, F.; Peng, X.; Zhang, Y.; et al. Oryzanol ameliorates dss-stimulated gut barrier damage via targeting the gut microbiota accompanied by the tlr4/nf-κb/ nlrp3 cascade response in vivo. J. Agric. Food Chem. 2022, 70, 15747–15762. [Google Scholar] [CrossRef]
- Jin, S.; Zhao, D.; Cai, C.W.; Song, D.J.; Shen, J.; Xu, A.T.; Qiao, Y.Q.; Ran, Z.H.; Zheng, Q. Low-dose penicillin exposure in early life decreases th17 and the susceptibility to dss colitis in mice through gut microbiota modification. Sci. Rep. 2017, 7, 43662. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of ph in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef]
- Xu, J.; Chen, N.; Wu, Z.; Song, Y.; Zhang, Y.F.; Wu, N.; Zhang, F.; Ren, X.H.; Liu, Y. 5-aminosalicylic acid alters the gut bacterial microbiota in patients with ulcerative colitis. Front. Microbiol. 2018, 9, 1274. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Rabbi, M.F.; Khafipour, E.; Ghia, J.E. Acute dextran sulfate sodium (dss)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 2016, 56, 986–998. [Google Scholar] [CrossRef]
- Chung, Y.; Ryu, Y.; An, B.C.; Yoon, Y.S.; Choi, O.; Kim, T.Y.; Yoon, J.; Ahn, J.Y.; Park, H.J.; Kwon, S.K.; et al. A synthetic probiotic engineered for colorectal cancer therapy modulates gut microbiota. Microbiome 2021, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.A.; Ma, Y.; Xiong, K.; Wang, Y.R.; Liu, Y.J.; Sun, Y.Y.; Yang, Y.X.; Ma, A.G. Ameliorating effects of vitamin k2 on dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Mol. Sci. 2023, 24, 2986. [Google Scholar] [CrossRef]
- Yang, Z.D.; Ye, S.M.; Xu, Z.M.; Su, H.H.; Tian, X.; Han, B.; Shen, B.C.; Liao, Q.F.; Xie, Z.Y.; Hong, Y.J. Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.P.; Culpepper, T.; Saldivar, B.; Anton, S.; Stoll, S.; Handberg, E.M.; Xu, K.; Pepine, C.; Triplett, E.W.; Aggarwal, M. A six-day, lifestyle-based immersion program mitigates cardiovascular risk factors and induces shifts in gut microbiota, specifically lachnospiraceae, ruminococcaceae, faecalibacterium prausnitzii: A pilot study. Nutrients 2021, 13, 3459. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.R.; Bucci, V.; Toussaint, N.C.; Buffie, C.G.; Ratsch, G.; Pamer, E.G.; Sander, C.; Xavier, J.B. Ecological modeling from time-series inference: Insight into dynamics and stability of intestinal microbiota. PLoS Comput. Biol. 2013, 9, e1003388. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.W.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives t(h)1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, Y.Z.; Niu, Q.Y.; Wang, J.; Wang, J.; Zhu, W.Y. The changes of colonic bacterial composition and bacterial metabolism induced by an early food introduction in a neonatal porcine model. Curr. Microbiol. 2018, 75, 745–751. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef]
- Li, R.N.; Li, L.Q.; Hong, P.; Lang, W.Y.; Hui, J.N.; Yang, Y.; Zheng, X. Beta-carotene prevents weaning-induced intestinal inflammation by modulating gut microbiota in piglets. Anim. Biosci. 2021, 34, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.R.; Liu, J.; Zhang, X.; Wu, X.N.; Tang, S.X.; Sun, R.; Qian, C.L.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in dss-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, S.Y.; Zhang, Y.; Huang, K.; Liang, T.; Chen, Y.; Guan, Y.J.; Shang, R.Z.; Guan, T.; Wu, J.; et al. Amino acid-balanced diets improved dss-induced colitis by alleviating inflammation and regulating gut microbiota. Eur. J. Nutr. 2022, 61, 3531–3543. [Google Scholar] [CrossRef] [PubMed]

| Score | Weight Loss | Stool Consistency | Bleeding |
|---|---|---|---|
| 0 | <1% | Normal | Normal |
| 1 | 1–5% | Slightly soft | Occult blood and weak positive |
| 2 | 5–10% | Soft | Occult blood and positive |
| 3 | 10–20% | Loose | Visible blood |
| 4 | >20% | Diarrhea | Gross bleeding |
| Score | Epithelial Cell Destruction | Crypt Loss | Inflammatory Cell Infiltration |
|---|---|---|---|
| 0 | Normal | Normal | Normal |
| 1 | Localized and mild | Localized and mild | Localized and mild |
| 2 | Localized and moderate | Localized and moderate | Localized and moderate |
| 3 | Extensive and moderate | Extensive and moderate | Extensive and moderate |
| 4 | Extensive and severe | Extensive and severe | Extensive and severe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xue, L.; Li, S.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. The Effects of Synbiotics on Dextran-Sodium-Sulfate-Induced Acute Colitis: The Impact of Chitosan Oligosaccharides on Endogenous/Exogenous Lactiplantibacillus plantarum. Foods 2023, 12, 2251. https://doi.org/10.3390/foods12112251
Zhao Y, Xue L, Li S, Wu T, Liu R, Sui W, Zhang M. The Effects of Synbiotics on Dextran-Sodium-Sulfate-Induced Acute Colitis: The Impact of Chitosan Oligosaccharides on Endogenous/Exogenous Lactiplantibacillus plantarum. Foods. 2023; 12(11):2251. https://doi.org/10.3390/foods12112251
Chicago/Turabian StyleZhao, Yunjiao, Liangyu Xue, Shunqin Li, Tao Wu, Rui Liu, Wenjie Sui, and Min Zhang. 2023. "The Effects of Synbiotics on Dextran-Sodium-Sulfate-Induced Acute Colitis: The Impact of Chitosan Oligosaccharides on Endogenous/Exogenous Lactiplantibacillus plantarum" Foods 12, no. 11: 2251. https://doi.org/10.3390/foods12112251
APA StyleZhao, Y., Xue, L., Li, S., Wu, T., Liu, R., Sui, W., & Zhang, M. (2023). The Effects of Synbiotics on Dextran-Sodium-Sulfate-Induced Acute Colitis: The Impact of Chitosan Oligosaccharides on Endogenous/Exogenous Lactiplantibacillus plantarum. Foods, 12(11), 2251. https://doi.org/10.3390/foods12112251







