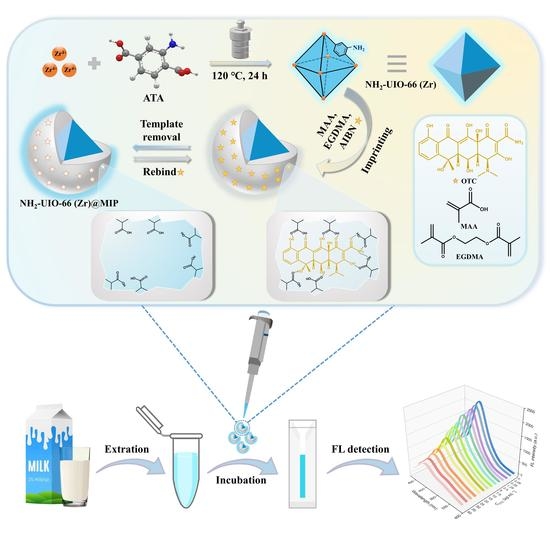
A Turn-Off Fluorescent Biomimetic Sensor Based on a Molecularly Imprinted Polymer-Coated Amino-Functionalized Zirconium (IV) Metal–Organic Framework for the Ultrasensitive and Selective Detection of Trace Oxytetracycline in Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatuses
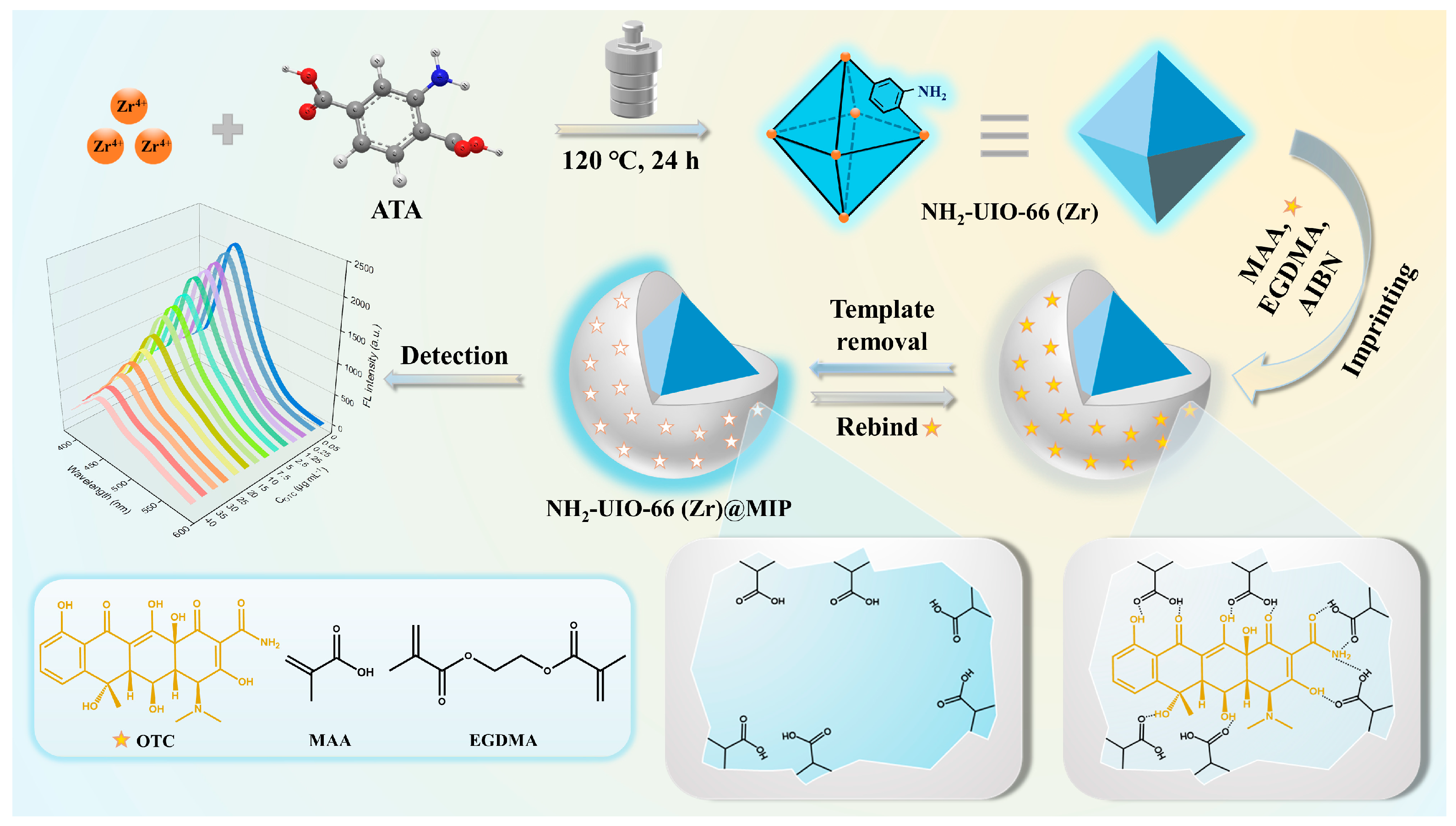
2.2. The Synthesis of NH2-UIO-66 (Zr)
2.3. The Fabrication of NH2-UIO-66 (Zr) Imprinted Polymers
2.4. FL Sensing Procedure
2.5. Real Samples Analysis
2.6. HPLC Analysis
3. Results and Discussion
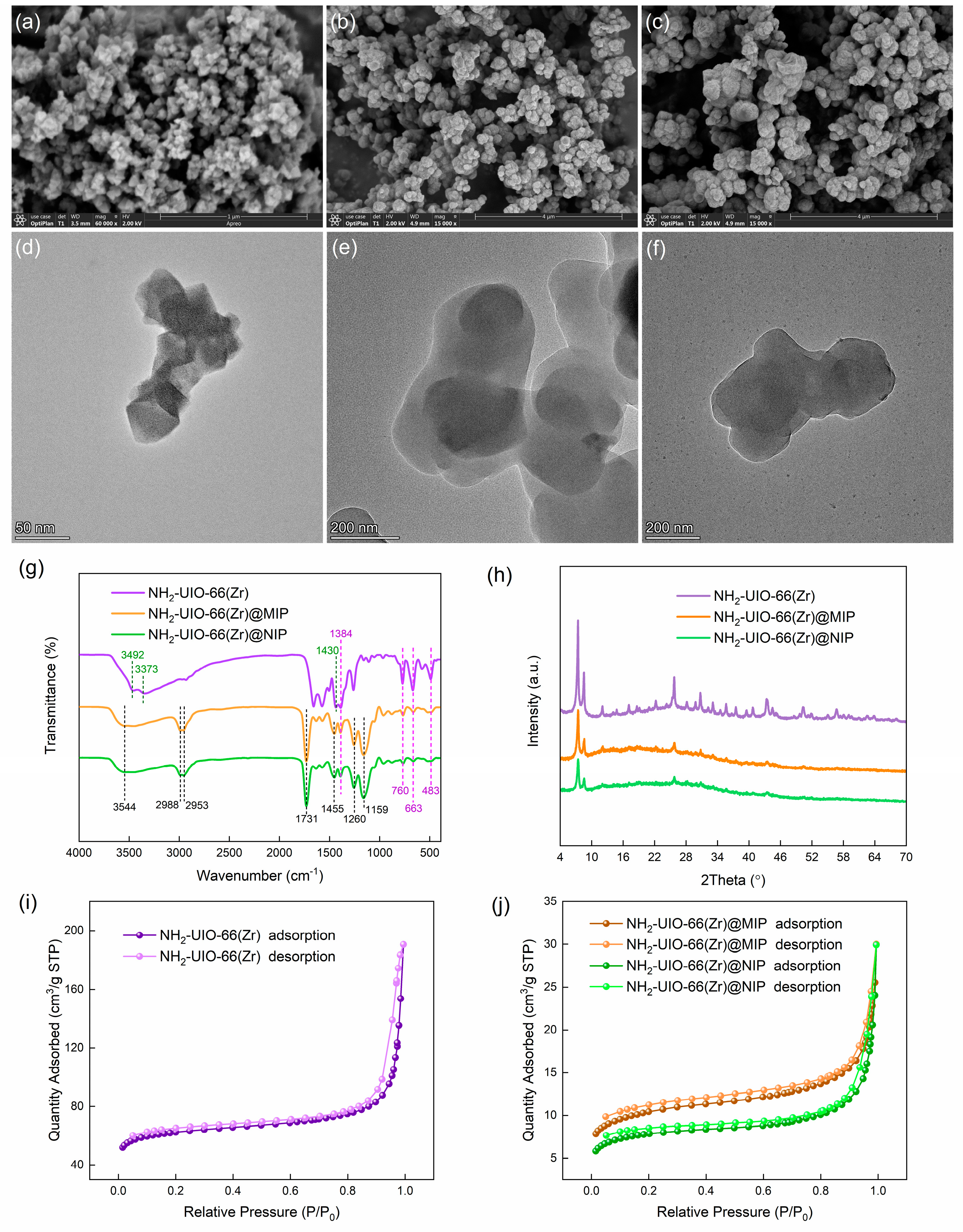
3.1. Synthesis of NH2-UIO-66 (Zr) and NH2-UIO-66 (Zr)@MIP
3.2. Characterization
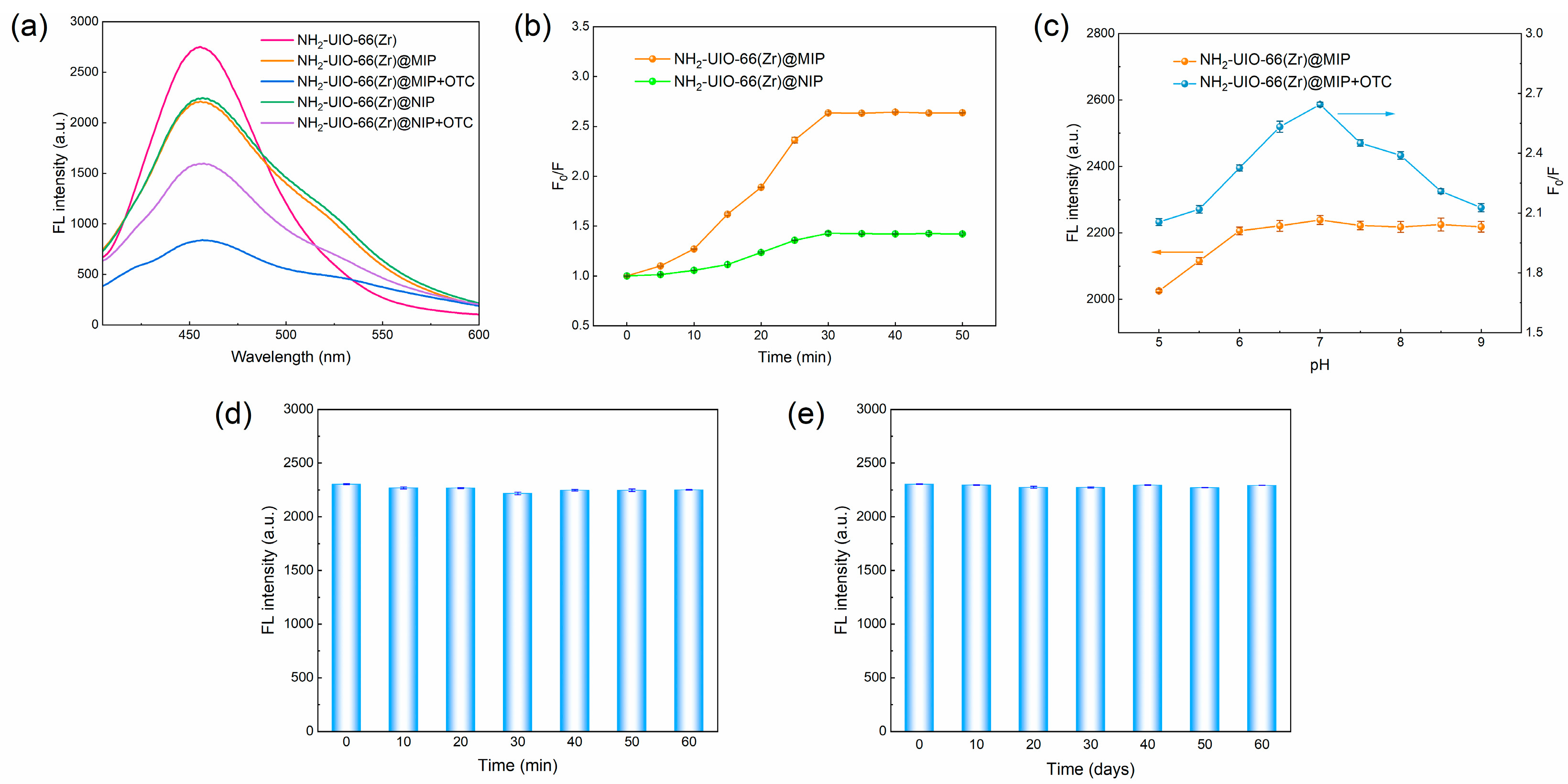
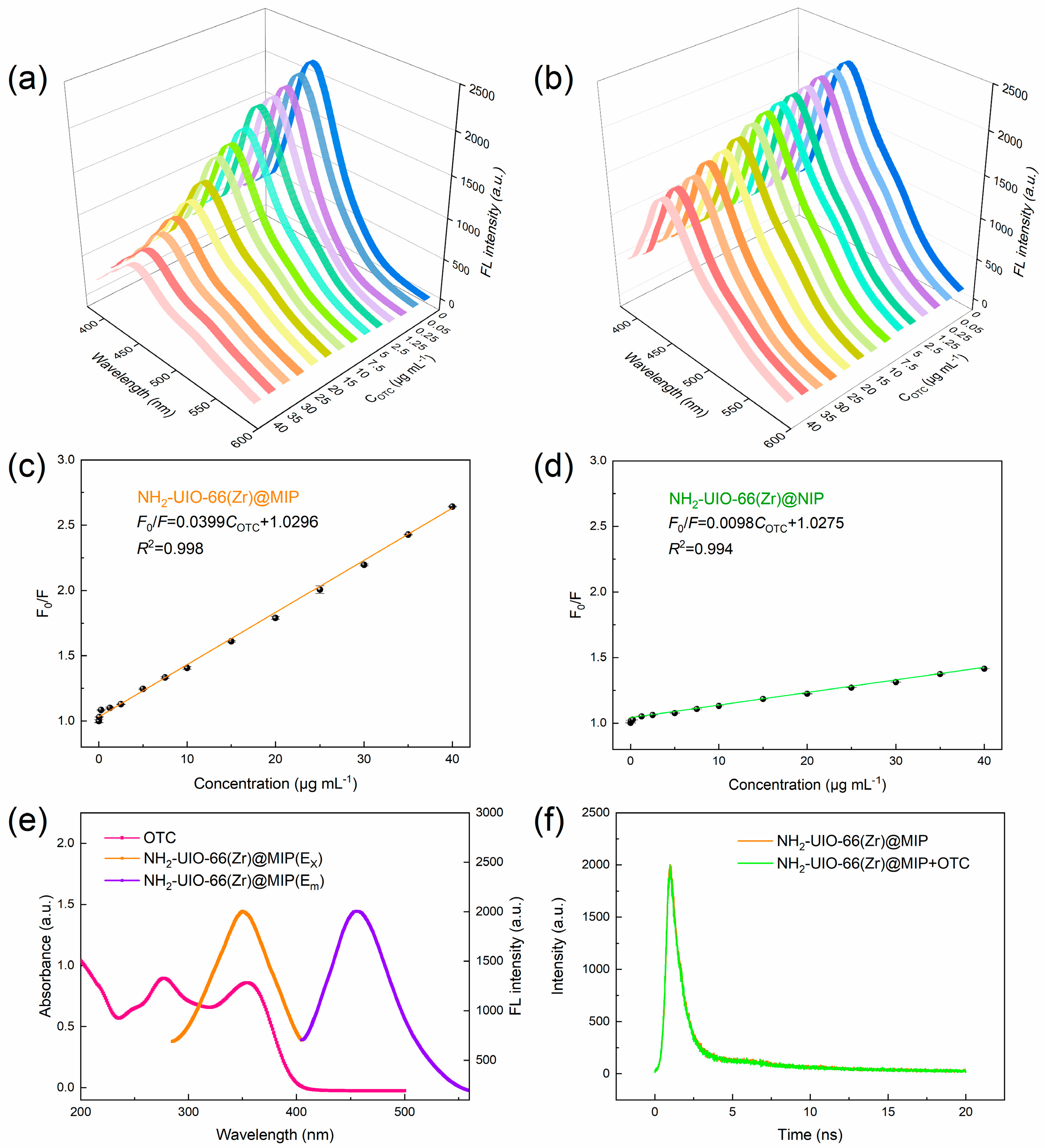
3.3. Optical and Adsorption Properties of NH2-UIO-66 (Zr)@MIP
3.4. Construction of FL Detection System for OTC
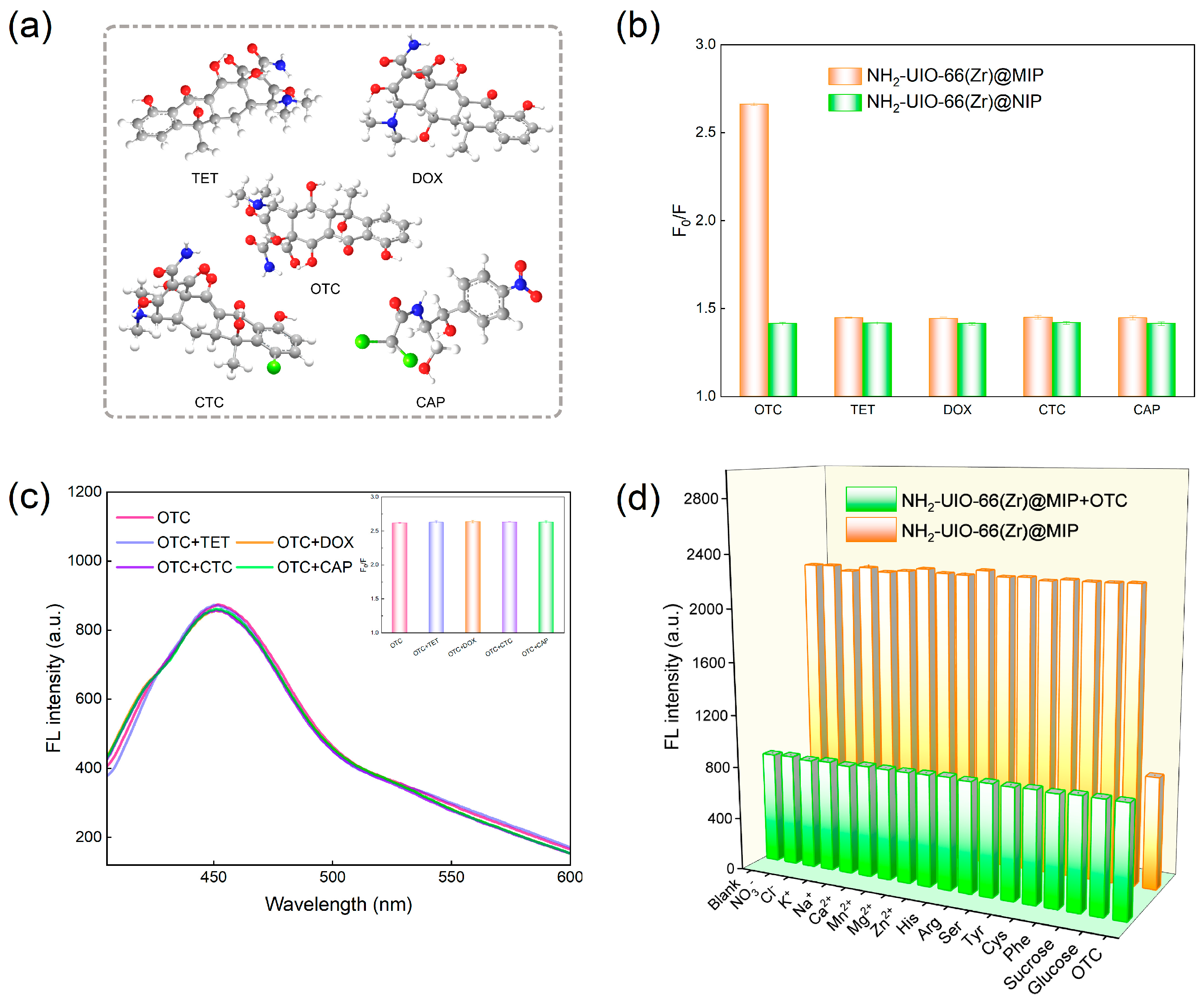
3.5. Selectivity and Specificity Analysis
3.6. Real Sample Analysis
3.7. Accuracy Assessment of the Established Fluorescence Method
3.8. Approach Performance Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.J.; Yue, Y.F.; Qian, G.D.; Chen, B.L. Luminescent Functional Metal-Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dou, Y.B.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Wang, N.; Xie, M.G.; Wang, M.K.; Li, Z.X.; Su, X.G. UiO-66-NH2 MOF-based ratiometric fluorescent probe for the detection of dopamine and reduced glutathione. Talanta 2020, 220, 7. [Google Scholar] [CrossRef]
- Zhu, H.L.; Huang, J.P.; Zhou, Q.Y.; Lv, Z.W.; Li, C.Y.; Hu, G. Enhanced luminescence of NH2-UiO-66 for selectively sensing fluoride anion in water medium. J. Lumin. 2019, 208, 67–74. [Google Scholar] [CrossRef]
- Yang, J.; Dai, Y.; Zhu, X.Y.; Wang, Z.; Li, Y.S.; Zhuang, Q.X.; Shi, J.L.; Gu, J.L. Metal-organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J. Mater. Chem. A 2015, 3, 7445–7452. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, X.F. UiO-66-NH2 based fluorescent sensing for detection of tetracyclines in milk. RSC Adv. 2022, 12, 23427–23436. [Google Scholar] [CrossRef]
- Zhang, H.F.; Kang, Z.W.; Zhu, H.; Lin, H.T.; Yang, D.P. ZnO/C nanocomposite grafted molecularly imprinted polymers as photoelectrochemical sensing interface for ultrasensitive and selective detection of chloramphenicol. Sci. Total Environ. 2023, 859, 160284. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Hashemzadeh, A.; Darroudi, M. A novel molecularly imprinted polymer decorated by CQDs@HBNNS nanocomposite and UiO-66-NH2 for ultra-selective electrochemical sensing of Oxaliplatin in biological samples. Sens. Actuators B-Chem. 2020, 307, 127614. [Google Scholar] [CrossRef]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies-Synthesis and Applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [Green Version]
- Cowen, T.; Cheffena, M. Template Imprinting Versus Porogen Imprinting of Small Molecules: A Review of Molecularly Imprinted Polymers in Gas Sensing. Int. J. Mol. Sci. 2022, 23, 9642. [Google Scholar] [CrossRef]
- Afshar, E.A.; Taher, M.A.; Karimi, F.; Karaman, C.; Moradi, O. Ultrasensitive and highly selective “turn-on” fluorescent sensor for the detection and measurement of melatonin in juice samples. Chemosphere 2022, 295, 133869. [Google Scholar] [CrossRef] [PubMed]
- Amiripour, F.; Ghasemi, S.; Azizi, S.N. Fo euro rster resonance energy transfer-based molecularly imprinted polymer/amine-functionalized metal-organic framework nanocomposite for trace level detection of 4-nitrophenol. Anal. Chim. Acta 2022, 1202, 339638. [Google Scholar] [CrossRef]
- Amiripour, F.; Ghasemi, S.; Azizi, S.N. Design of turn-on luminescent sensor based on nanostructured molecularly imprinted polymer-coated zirconium metal-organic framework for selective detection of chloramphenicol residues in milk and honey. Food Chem. 2021, 347, 129034. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, V. Advances in biosensor development for the screening of antibiotic residues in food products of animal origin—A comprehensive review. Biosens. Bioelectron. 2017, 90, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [Green Version]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232. [Google Scholar] [CrossRef] [Green Version]
- Singer, R.S.; Finch, R.; Wegener, H.C.; Bywater, R.; Walters, J.; Lipsitch, M. Antibiotic resistance—The interplay between antibiotic use in animals and human beings. Lancet Infect. Dis. 2003, 3, 47–51. [Google Scholar] [CrossRef]
- Wang, J.X.; Zou, L.H.; Xu, J.J.; Zhang, R.; Zhang, H.B. Molecularly imprinted fluoroprobes doped with Ag nanoparticles for highly selective detection of oxytetracycline in real samples. Anal. Chim. Acta 2021, 1161, 338326. [Google Scholar] [CrossRef]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.L. Occurrence of antibiotics in the aquatic environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- Li, C.H.; Zhu, L.; Yang, W.X.; He, X.; Zhao, S.L.; Zhang, X.S.; Tang, W.Z.; Wang, J.L.; Yue, T.L.; Li, Z.H. Amino-Functionalized Al-MOF for Fluorescent Detection of Tetracyclines in Milk. J. Agric. Food Chem. 2019, 67, 1277–1283. [Google Scholar] [CrossRef]
- Bai, X.Y.; Zhang, Y.; Gao, W.K.; Zhao, D.Y.; Yang, D.P.; Jia, N.Q. Hollow ZnS-CdS nanocage based photoelectrochemical sensor combined with molecularly imprinting technology for sensitive detection of oxytetracycline. Biosens. Bioelectron. 2020, 168, 112522. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.T.; Lian, Z.R. Fluorescence assay of oxytetracycline in seawater after selective capture using magnetic molecularly imprinted nanoparticles. Mar. Pollut. Bull. 2021, 163, 111962. [Google Scholar] [CrossRef]
- Mu, G.F.; Liu, H.T.; Xu, L.N.; Tian, L.H.; Luan, F. Matrix Solid-Phase Dispersion Extraction and Capillary Electrophoresis Determination of Tetracycline Residues in Milk. Food Anal. Meth. 2012, 5, 148–153. [Google Scholar] [CrossRef]
- Gajda, A.; Posyniak, A. Liquid chromatography—Tandem mass spectrometry method for the determination of ten tetracycline residues in muscle samples. Bull. Vet. Inst. Pulawy 2015, 59, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.N.; Kong, D.Z.; Liu, L.Q.; Song, S.S.; Kuang, H.; Xu, C.L. Development of an ELISA and Immunochromatographic Assay for Tetracycline, Oxytetracycline, and Chlortetracycline Residues in Milk and Honey Based on the Class-Specific Monoclonal Antibody. Food Anal. Meth. 2016, 9, 905–914. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.L.; Peng, J.D.; He, Y.T. High-performance liquid chromatography with resonance Rayleigh scattering detection for determining four tetracycline antibiotics. Anal. Methods 2014, 6, 9361–9366. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Huang, L.; Zhao, S.J.; Xing, X.J.; Lan, M.H.; Song, X.Z. A carbon dot-based fluorometric probe for oxytetracycline detection utilizing a Forster resonance energy transfer mechanism. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2021, 246, 6. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.J.; Huang, L.; Zhao, S.J.; Xiao, J.F.; Lan, M.H. S,N-Doped carbon dots for tetracyclines sensing with a fluorometric spectral response. Microchem. J. 2020, 157, 105065. [Google Scholar] [CrossRef]
- Satana Kara, H.E.; Demirhan, B.; Er Demirhan, B. Highly luminescent water-dispersed silicon quantum dots for fluorometric determination of oxytetracycline in milk samples. Turk. J. Chem. 2020, 44, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Xu, Y.Q.; Liu, X.Q.; Peng, L.; Huang, T.; Yan, Y.S.; Li, C.X. Efficient fabrication of ratiometric fluorescence imprinting sensors based on organic-inorganic composite materials and highly sensitive detection of oxytetracycline in milk. Microchem. J. 2020, 157, 105053. [Google Scholar] [CrossRef]
- Hu, X.L.; Guo, Y.; Wang, T.; Liu, C.; Yang, Y.K.; Fang, G.Z. A selectivity-enhanced ratiometric fluorescence imprinted sensor based on synergistic effect of covalent and non-covalent recognition units for ultrasensitive detection of ribavirin. J. Hazard. Mater. 2022, 421, 126748. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, H.; Amirzehni, M.; Asadollahzadeh, H.; Hassanzadeh, J.; Eslami, P.A. MIP-capped terbium MOF-76 for the selective fluorometric detection of cefixime after its preconcentration with magnetic graphene oxide. Sens. Actuators B-Chem. 2018, 275, 145–154. [Google Scholar] [CrossRef]
- Hu, X.L.; Guo, Y.; Zhang, J.N.; Wang, X.H.; Fang, G.Z.; Wang, S. A signal-amplified ratiometric fluorescence biomimetic sensor based on the synergistic effect of IFE and AE for the visual smart monitoring of oxytetracycline. Chem. Eng. J. 2022, 433, 134499. [Google Scholar] [CrossRef]
- Qian, S.H.; Qiao, L.N.; Xu, W.X.; Jiang, K.; Wang, Y.H.; Lin, H.W. An inner filter effect-based near-infrared probe for the ultrasensitive detection of tetracyclines and quinolones. Talanta 2019, 194, 598–603. [Google Scholar] [CrossRef]
- Hu, X.L.; Cao, Y.C.; Cai, L.; Wang, H.Y.; Fang, G.Z.; Wang, S. A smartphone-assisted optosensing platform based on chromium-based metal-organic framework signal amplification for ultrasensitive and real-time determination of oxytetracycline. J. Hazard. Mater. 2023, 444, 130395. [Google Scholar] [CrossRef]
- Chen, Q.; He, Q.Q.; Lv, M.M.; Xu, Y.L.; Yang, H.B.; Liu, X.T.; Wei, F.Y. Selective adsorption of cationic dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Fang, X.; Wu, S.B.; Wu, Y.H.; Yang, W.; Li, Y.L.; He, J.Y.; Hong, P.D.; Nie, M.X.; Xie, C.; Wu, Z.J.; et al. High-efficiency adsorption of norfloxacin using octahedral UIO-66-NH2 nanomaterials: Dynamics, thermodynamics, and mechanisms. Appl. Surf. Sci. 2020, 518, 146226. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Liu, Y.T.; Chen, S.Y. Adsorption of fluoride to UiO-66-NH2 in water: Stability, kinetic, isotherm and thermodynamic studies. J. Colloid Interface Sci. 2016, 461, 79–87. [Google Scholar] [CrossRef]
- Li, C.M.; Huang, J.P.; Zhu, H.L.; Liu, L.L.; Feng, Y.M.; Hu, G.; Yu, X.B. Dual-emitting fluorescence of Eu/Zr-MOF for ratiometric sensing formaldehyde. Sens. Actuators B-Chem. 2017, 253, 275–282. [Google Scholar] [CrossRef]
- Hu, X.L.; Zhao, Y.Q.; Dong, J.Y.; Liu, C.; Qi, Y.; Fang, G.Z.; Wang, S. A strong blue fluorescent nanoprobe based on Mg/N co-doped carbon dots coupled with molecularly imprinted polymer for ultrasensitive and highly selective detection of tetracycline in animal-derived foods. Sens. Actuators B-Chem. 2021, 338, 129809. [Google Scholar] [CrossRef]
- Han, S.; Yao, A.X.; Ding, Y.X.; Leng, Q.X.; Teng, F.; Zhao, L.; Sun, R.N.; Bu, H.Z. A dual-template imprinted polymer based on amino-functionalized zirconium-based metal-organic framework for delivery of doxorubicin and phycocyanin with synergistic anticancer effect. Eur. Polym. J. 2022, 170, 111161. [Google Scholar] [CrossRef]
- Liang, Y.T.; He, J.; Huang, Z.P.; Li, H.Y.; Zhang, Y.X.; Wang, H.G.; Rui, C.F.; Li, Y.Y.; You, L.Q.; Li, K.; et al. An amino-functionalized zirconium-based metal-organic framework of type UiO-66-NH2 covered with a molecularly imprinted polymer as a sorbent for the extraction of aflatoxins AFB1, AFB2, AFG1 and AFG2 from grain. Microchim. Acta 2020, 187, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Feng, W.B.; Liang, K.S.; Chen, C.Y.; Cai, C.Q. A novel fluorescence molecularly imprinted sensor for Japanese encephalitis virus detection based on metal organic frameworks and passivation-enhanced selectivity. Talanta 2020, 212, 120744. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.H.; Pan, M.F.; Fang, G.Z.; Wang, S. Carbon dots embedded metal-organic framework@molecularly imprinted nanoparticles for highly sensitive and selective detection of quercetin. Sens. Actuators B-Chem. 2019, 286, 321–327. [Google Scholar] [CrossRef]
- Hu, X.L.; Cao, Y.C.; Tian, Y.Y.; Qi, Y.; Fang, G.Z.; Wang, S. A molecularly imprinted fluorescence nanosensor based on upconversion metal-organic frameworks for alpha-cypermethrin specific recognition. Microchim. Acta 2020, 187, 632. [Google Scholar] [CrossRef]
- Zeng, L.S.; Zhang, X.; Wang, X.; Cheng, D.Q.; Li, R.F.; Han, B.; Wu, M.M.; Zhuang, Z.J.; Ren, A.N.; Zhou, Y.K.; et al. Simultaneous fluorescence determination of bisphenol A and its halogenated analogs based on a molecularly imprinted paper-based analytical device and a segment detection strategy. Biosens. Bioelectron. 2021, 180, 113106. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Zhang, D.N.; Gan, N.; Li, Q.P.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B-Chem. 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wu, X.H.; Mao, S.; Tao, W.Q.; Li, Z. Highly luminescent sensing for nitrofurans and tetracyclines in water based on zeolitic imidazolate framework-8 incorporated with dyes. Talanta 2019, 204, 344–352. [Google Scholar] [CrossRef]
- Tan, H.L.; Wu, X.Y.; Weng, Y.H.; Lu, Y.J.; Huang, Z.Z. Self-Assembled FRET Nanoprobe with Metal-Organic Framework As a Scaffold for Ratiometric Detection of Hypochlorous Acid. Anal. Chem. 2020, 92, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Yuan, Y.Q.; Yin, J.H.; Wang, X.; Meng, L. One-pot hydrothermal synthesis of luminescent silicon-based nanoparticles for highly specific detection of oxytetracycline via ratiometric fluorescent strategy. RSC Adv. 2017, 7, 48429–48436. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.Q.; Huang, T.; Wang, S.O.; Yan, Y.S. Mesoporous silica-based molecularly imprinted fluorescence sensor for the ultrafast and sensitive recognition of oxytetracycline. J. Food Compos. Anal. 2022, 108, 9. [Google Scholar] [CrossRef]
- Cai, Z.F.; Li, H.Y.; Wang, X.S.; Min, C.; Wen, J.Q.; Fu, R.X.; Dai, Z.Y.; Chen, J.; Guo, M.Z.; Yang, H.J.; et al. Highly luminescent copper nanoclusters as temperature sensors and "turn off" detection of oxytetracycline. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 647, 10. [Google Scholar] [CrossRef]
- Wu, H.F.; Xu, M.Q.; Chen, Y.B.; Zhang, H.L.; Shen, Y.J.; Tang, Y.F. A Highly Sensitive and Selective Nano-Fluorescent Probe for Ratiometric and Visual Detection of Oxytetracycline Benefiting from Dual Roles of Nitrogen-Doped Carbon Dots. Nanomaterials 2022, 12, 4306. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Du, P.Y.; Zhang, L.B.; Liu, Y.; Lu, X.Q. Fabrication of carbon dots@hierarchical mesoporous ZIF-8 for simultaneous ratiometric fluorescence detection and removal of tetracycline antibiotics. Sens. Actuator B-Chem. 2022, 358, 8. [Google Scholar] [CrossRef]
| Sample | Spiked (μg mL−1) | NH2-UIO-66 (Zr)@MIP | HPLC | ||||
|---|---|---|---|---|---|---|---|
| Found (μg mL−1) | Recovery (%) | RSD (%, n = 3) | Found (μg mL−1) | Recovery (%) | RSD (%, n = 3) | ||
| Milk 1 | 0.00 | 0.00 | - | - | 0.00 | - | - |
| 1.00 | 0.95 ± 0.02 | 95.38 ± 2.47 | 2.59 | 0.96 ± 0.02 | 96.16 ± 2.50 | 2.60 | |
| 15.00 | 14.50 ± 0.29 | 96.65 ± 1.96 | 2.03 | 14.73 ± 0.10 | 98.23 ± 0.66 | 0.67 | |
| 25.00 | 24.45 ± 0.16 | 97.79 ± 0.65 | 0.67 | 24.54 ± 0.13 | 98.14 ± 0.51 | 0.52 | |
| Milk 2 | 0.00 | 0.00 | - | - | 0.00 | - | - |
| 1.00 | 0.94 ± 0.02 | 93.56 ± 1.86 | 1.98 | 0.97 ± 0.02 | 96.68 ± 1.88 | 1.94 | |
| 15.00 | 14.67 ± 0.14 | 97.78 ± 0.91 | 0.93 | 14.78 ± 0.04 | 98.51 ± 0.26 | 0.27 | |
| 25.00 | 24.33 ± 0.11 | 97.32 ± 0.43 | 0.44 | 24.52 ± 0.12 | 98.10 ± 0.47 | 0.48 | |
| Milk 3 | 0.00 | 0.00 | - | - | 0.00 | - | - |
| 1.00 | 0.94 ± 0.01 | 93.67 ± 1.00 | 1.07 | 0.96 ± 0.01 | 95.59 ± 1.10 | 1.15 | |
| 15.00 | 14.73 ± 0.17 | 98.21 ± 1.10 | 1.12 | 14.64 ± 0.01 | 97.62 ± 0.04 | 0.04 | |
| 25.00 | 24.41 ± 0.16 | 97.65 ± 0.64 | 0.65 | 24.61 ± 0.09 | 98.45 ± 0.35 | 0.35 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, C.; Cao, Y.; Cai, L.; Wang, H.; Fang, G. A Turn-Off Fluorescent Biomimetic Sensor Based on a Molecularly Imprinted Polymer-Coated Amino-Functionalized Zirconium (IV) Metal–Organic Framework for the Ultrasensitive and Selective Detection of Trace Oxytetracycline in Milk. Foods 2023, 12, 2255. https://doi.org/10.3390/foods12112255
Wang X, Liu C, Cao Y, Cai L, Wang H, Fang G. A Turn-Off Fluorescent Biomimetic Sensor Based on a Molecularly Imprinted Polymer-Coated Amino-Functionalized Zirconium (IV) Metal–Organic Framework for the Ultrasensitive and Selective Detection of Trace Oxytetracycline in Milk. Foods. 2023; 12(11):2255. https://doi.org/10.3390/foods12112255
Chicago/Turabian StyleWang, Xiaohui, Chang Liu, Yichuan Cao, Lin Cai, Haiyang Wang, and Guozhen Fang. 2023. "A Turn-Off Fluorescent Biomimetic Sensor Based on a Molecularly Imprinted Polymer-Coated Amino-Functionalized Zirconium (IV) Metal–Organic Framework for the Ultrasensitive and Selective Detection of Trace Oxytetracycline in Milk" Foods 12, no. 11: 2255. https://doi.org/10.3390/foods12112255