Determination of Freshness of Mackerel (Scomber japonicus) Using Shortwave Infrared Hyperspectral Imaging
Abstract
:1. Introduction
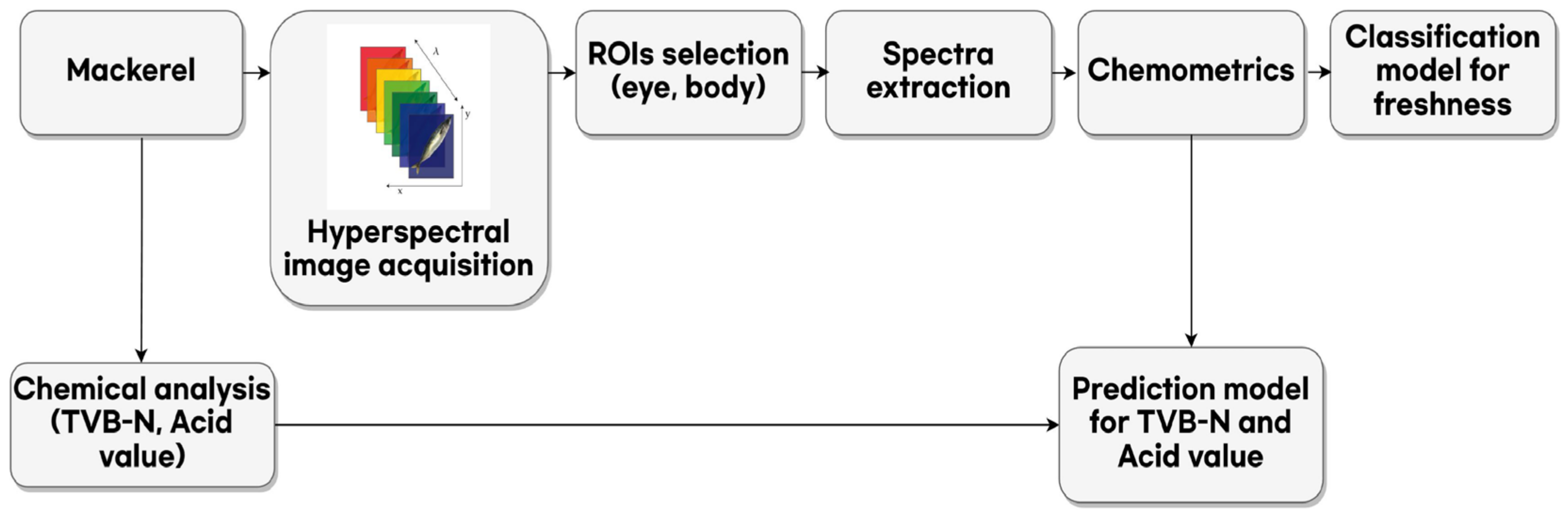
2. Materials and Methods
2.1. Sample Preparation
2.2. Hyperspectral Image Acquisition
2.3. Chemical Compounds Analysis
2.3.1. TVB-N Measurement
2.3.2. Acid Value Measurement
2.4. Data Analysis
2.4.1. Partial Least Squared (PLS) Analysis
2.4.2. Spectrum Preprocessing
2.4.3. Statistical Analysis
3. Results and Discussion
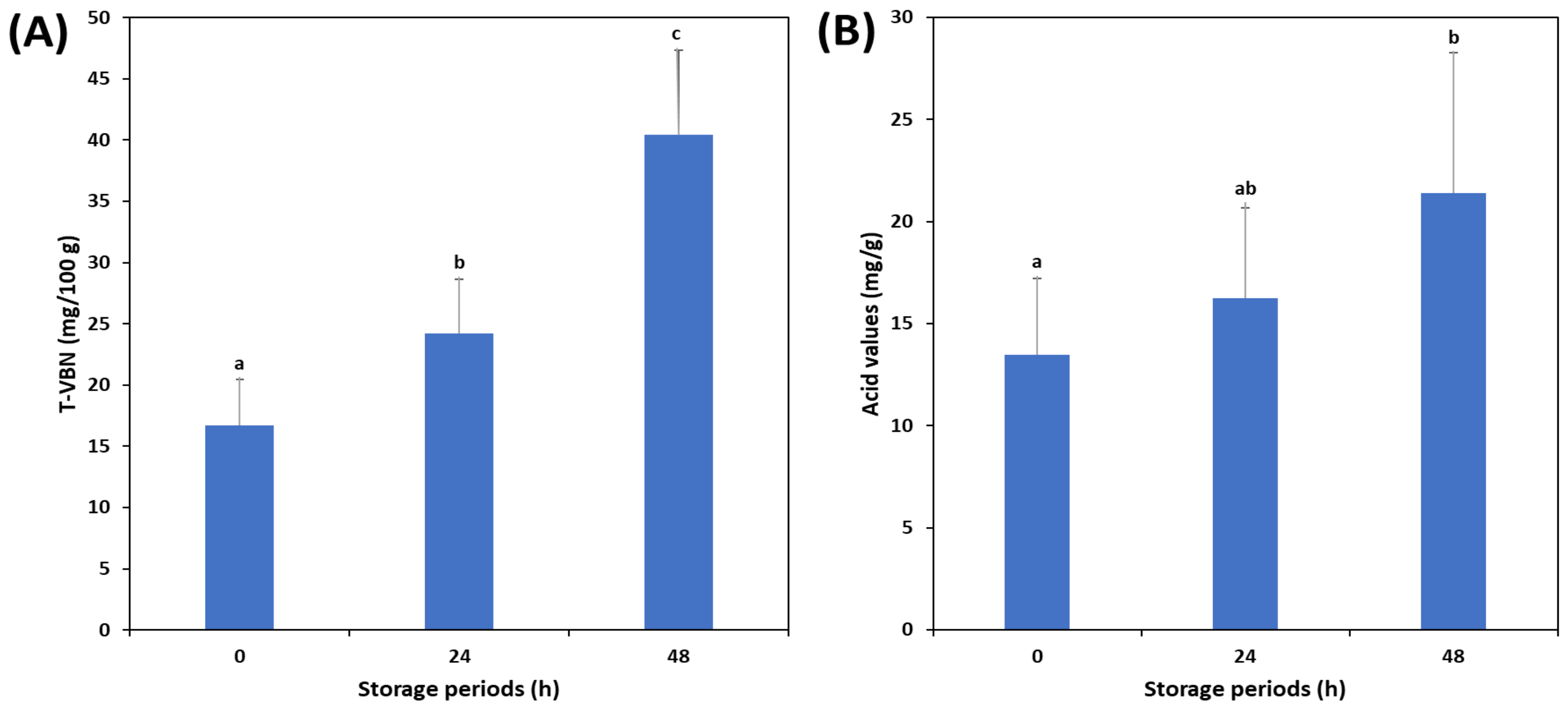
3.1. Changes in TVB-N and Acid Values of Mackerel According to the Freshness
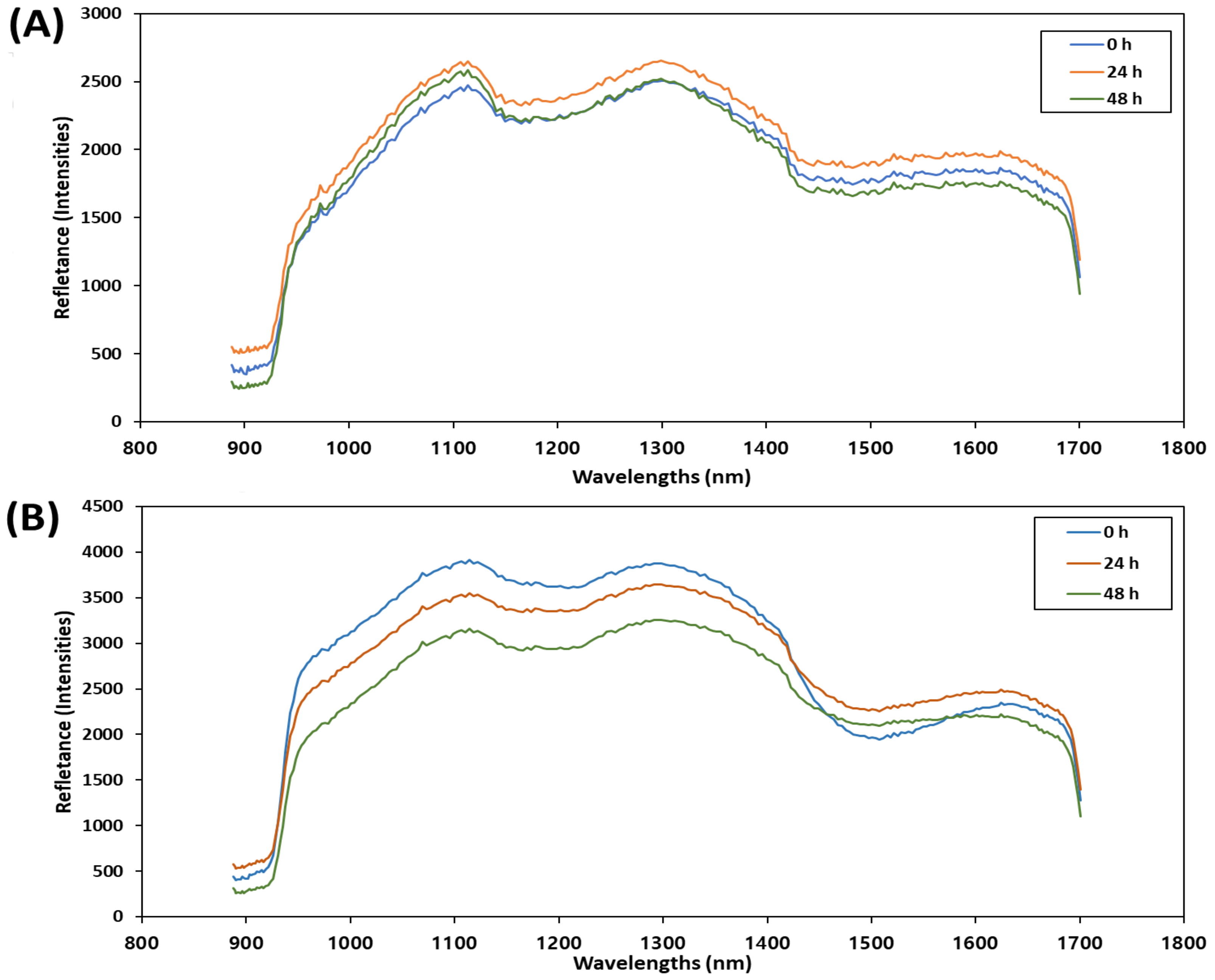
3.2. Spectral Features of Mean Spectra of Mackerel
3.3. PLS Score Plots for Classification of the Freshness of Mackerel
3.4. Classification Accuracy According to the Spectral Preprocessing
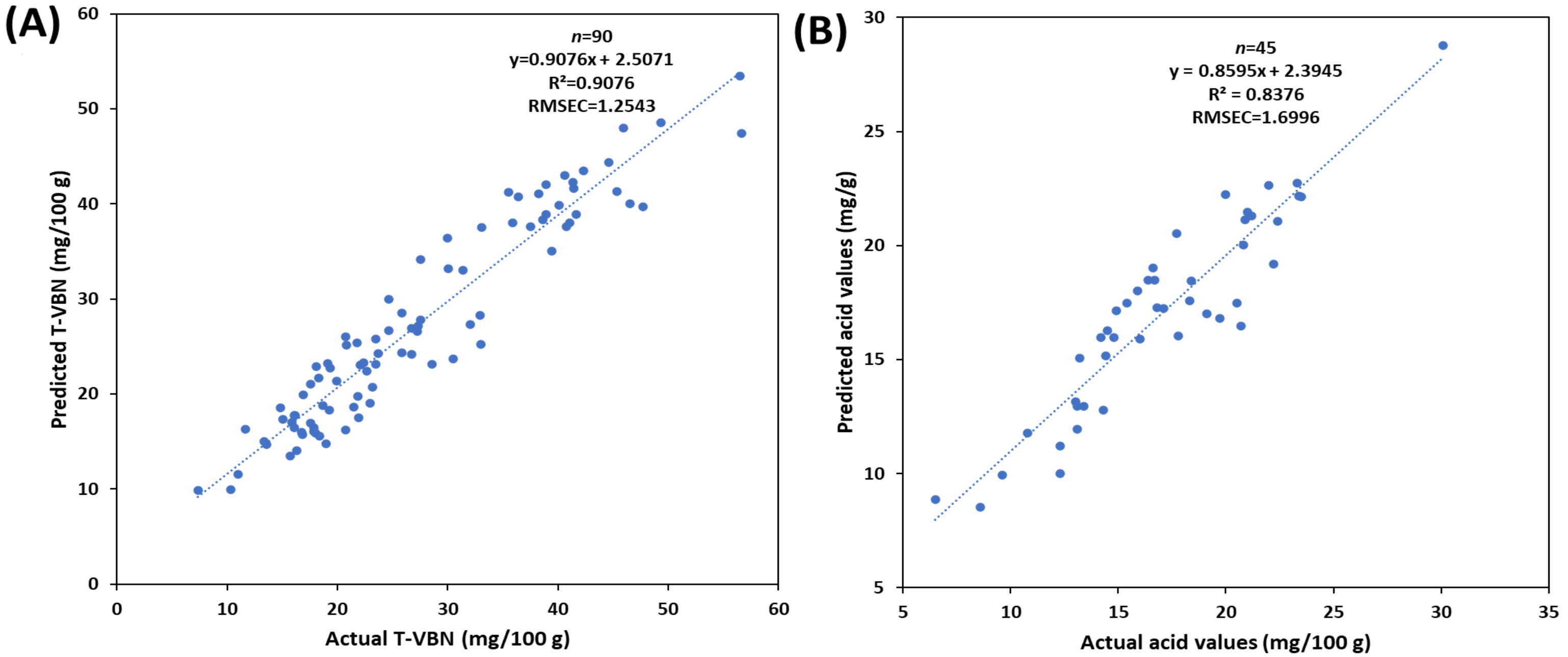
3.5. PLS Regression for Prediction of TVB-N and Acid Value
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weichselbaum, E.; Coe, S.; Buttriss, J.; Stanner, S. Fish in the diet: A review. Nutr. Bull. 2013, 38, 128–177. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Yim, D.-G.; Kim, G.; Jo, C. Hyperspectral Imaging Coupled with Multivariate Analyses for Efficient Prediction of Chemical, Biological and Physical Properties of Seafood Products. Food Eng. Rev. 2023, 15, 41–55. [Google Scholar] [CrossRef]
- Sone, I.; Skåra, T.; Olsen, S.H. Factors influencing post-mortem quality, safety and storage stability of mackerel species: A review. Eur. Food Res. Technol. 2019, 245, 775–791. [Google Scholar] [CrossRef]
- Anders, N.; Breen, M.; Skåra, T.; Roth, B.; Sone, I. Effects of capture-related stress and pre-freezing holding in refrigerated sea water (RSW) on the muscle quality and storage stability of Atlantic mackerel (Scomber scombrus) during subsequent frozen storage. Food Chem. 2023, 405, 134819. [Google Scholar] [CrossRef] [PubMed]
- Karoui, R.; Hassoun, A. Efficiency of rosemary and basil essential oils on the shelf-life extension of Atlantic mackerel (Scomber scombrus) fillets stored at 2 C. J. AOAC Int. 2017, 100, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaro, B.; Hernández, I.; Baliño-Zuazo, L.; Barranco, A. Quality changes of Atlantic horse mackerel fillets (Trachurus trachurus) packed in a modified atmosphere at different storage temperatures. J. Sci. Food Agric. 2013, 93, 2179–2187. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Quality Evaluation of Fish and Other Seafood by Traditional and Nondestructive Instrumental Methods: Advantages and Limitations. Crit. Rev. Food Sci. Nutr. 2015, 57, 1976–1998. [Google Scholar] [CrossRef]
- Hyldig, G.; Green-Petersen, D.M.B. Quality Index Method—An Objective Tool for Determination of Sensory Quality. J. Aquat. Food Prod. Technol. 2005, 13, 71–80. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Zeng, X.-A.; Pu, H.-B. Non-destructive and rapid determination of TVB-N content for freshness evaluation of grass carp (Ctenopharyngodon idella) by hyperspectral imaging. Innov. Food Sci. Emerg. Technol. 2014, 21, 179–187. [Google Scholar] [CrossRef]
- Goddard, J.M.; McClements, D.J.; Decker, E.A. Innovative technologies in the control of lipid oxidation. Lipid Technol. 2012, 24, 275–277. [Google Scholar] [CrossRef]
- Hasanah, U.; Setyowati, M.; Efendi, R.; Muslem, M.; Md Sani, N.D.; Safitri, E.; Yook Heng, L.; Idroes, R. Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring. Biosensors 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, E.J.; Kim, Y.; Xu, Y.; Na, Y.; Giaccia, A.J.; Lee, J.H. Evaluation of Salmon, Tuna, and Beef Freshness Using a Portable Spectrometer. Sensors 2020, 20, 4299. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kondo, N.; Ogawa, Y.; Suzuki, T.; Shirataki, Y.; Wakita, Y. Prediction of K value for fish flesh based on ultraviolet–visible spectroscopy of fish eye fluid using partial least squares regression. Comput. Electron. Agric. 2015, 117, 149–153. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Chen, Q.; Zhang, Y. Nondestructive measurement of total volatile basic nitrogen (TVB-N) in pork meat by integrating near infrared spectroscopy, computer vision and electronic nose techniques. Food Chem. 2014, 145, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, M.S.; Lee, W.-H.; Cho, B.-K. Determination of the total volatile basic nitrogen (TVB-N) content in pork meat using hyperspectral fluorescence imaging. Sens. Actuators B Chem. 2018, 259, 532–539. [Google Scholar] [CrossRef]
- Bockisch, M. Fats and Oils Handbook (Nahrungsfette Und Öle); Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Yagi, K. Lipid peroxides and human diseases. Chem. Phys. Lipids 1987, 45, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Dae-young, K. Comparison of Quality Control and Hygiene Management for Mackerels in Korea and Japan. J. Fish. Bus. Adm. 2019, 50, 17–29. [Google Scholar]
- Zheng, R.; Xu, X.; Xing, J.; Cheng, H.; Zhang, S.; Shen, J.; Li, H. Quality evaluation and characterization of specific spoilage organisms of Spanish mackerel by high-throughput sequencing during 0 C cold chain logistics. Foods 2020, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Amigo, J.M.; Babamoradi, H.; Elcoroaristizabal, S. Hyperspectral image analysis. A tutorial. Anal. Chim. Acta 2015, 896, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Khan, H.S.; Yousaf, A.; Khurshid, K.; Abbas, A. Modern Trends in Hyperspectral Image Analysis: A Review. IEEE Access 2018, 6, 14118–14129. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Khoshnoudi-Nia, S.; Azimifar, Z.; Kamyab, S. Evaluation of the total volatile basic nitrogen (TVB-N) content in fish fillets using hyperspectral imaging coupled with deep learning neural network and meta-analysis. Sci. Rep. 2021, 11, 5094. [Google Scholar] [CrossRef]
- Dale, L.M.; Thewis, A.; Boudry, C.; Rotar, I.; Dardenne, P.; Baeten, V.; Pierna, J.A.F. Hyperspectral imaging applications in agriculture and agro-food product quality and safety control: A review. Appl. Spectrosc. Rev. 2013, 48, 142–159. [Google Scholar] [CrossRef]
- Franceschelli, L.; Berardinelli, A.; Dabbou, S.; Ragni, L.; Tartagni, M. Sensing Technology for Fish Freshness and Safety: A Review. Sensors 2021, 21, 1373. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Moosavi-Nasab, M. Prediction of various freshness indicators in fish fillets by one multispectral imaging system. Sci. Rep. 2019, 9, 14704. [Google Scholar] [CrossRef] [Green Version]
- Khoshnoudi-Nia, S.; Moosavi-Nasab, M. Comparison of various chemometric analysis for rapid prediction of thiobarbituric acid reactive substances in rainbow trout fillets by hyperspectral imaging technique. Food Sci. Nutr. 2019, 7, 1875–1883. [Google Scholar] [CrossRef]
- Sone, I.; Olsen, R.L.; Sivertsen, A.H.; Eilertsen, G.; Heia, K. Classification of fresh Atlantic salmon (Salmo salar L.) fillets stored under different atmospheres by hyperspectral imaging. J. Food Eng. 2012, 109, 482–489. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Pu, H.-B.; Chen, X.; Liu, Y.; Zhang, H.; Li, J.-L. Integration of classifiers analysis and hyperspectral imaging for rapid discrimination of fresh from cold-stored and frozen-thawed fish fillets. J. Food Eng. 2015, 161, 33–39. [Google Scholar] [CrossRef]
- Qin, J.; Vasefi, F.; Hellberg, R.S.; Akhbardeh, A.; Isaacs, R.B.; Yilmaz, A.G.; Hwang, C.; Baek, I.; Schmidt, W.F.; Kim, M.S. Detection of fish fillet substitution and mislabeling using multimode hyperspectral imaging techniques. Food Control 2020, 114, 107234. [Google Scholar] [CrossRef]
- Shao, Y.; Shi, Y.; Wang, K.; Li, F.; Zhou, G.; Xuan, G. Detection of small yellow croaker freshness by hyperspectral imaging. J. Food Compos. Anal. 2023, 115, 104980. [Google Scholar] [CrossRef]
- Conway, E.J.; O’Malley, E. Microdiffusion methods. Ammonia and urea using buffered absorbents (revised methods for ranges greater than 10μg. N). Biochem. J. 1942, 36, 655–661. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Nicolai, B.; Sun, D.-W. Hyperspectral imaging with multivariate analysis for technological parameters prediction and classification of muscle foods: A review. Meat Sci. 2017, 123, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pu, H.; Sun, D.-W.; Gao, W.; Qu, J.-H.; Ma, K.-Y. Application of Vis–NIR hyperspectral imaging in classification between fresh and frozen-thawed pork Longissimus Dorsi muscles. Int. J. Refrig. 2015, 50, 10–18. [Google Scholar] [CrossRef]
- Xing, J.; De Baerdemaeker, J. Bruise detection on ‘Jonagold’ apples using hyperspectral imaging. Postharvest Biol. Technol. 2005, 37, 152–162. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part II: Applications. Innov. Food Sci. Emerg. Technol. 2013, 19, 15–28. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X.; Chen, Y.; Wang, P.; Wei, X.; Tao, F. Dynamic variability of the heading–flowering stages of single rice in China based on field observations and NDVI estimations. Int. J. Biometeorol. 2015, 59, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-N.; Lee, D.-G.; Han, S.-W.; Yoon, H.-K.; Hwang, I.-K. Quality changes of salted and semi-dried mackerel fillets by UV treatment during refrigerated storage. Korean J. Food Cook. Sci. 2005, 21, 662–668. [Google Scholar]
- Heu, M.-S.; Kim, H.-J.; Yoon, M.-S.; Park, D.-Y.; Park, K.-H.; Kim, J.-S. Food component characterization of muscle from salmon frame. J. Korean Soc. Food Sci. Nutr. 2008, 37, 1452–1456. [Google Scholar] [CrossRef]
- Gordon I, V.C.; Rainey, C.C.; Studmire, W.C. Validation of the Free Fatty Acid Test Kit for the Measurement of the Free Fatty Acid Content of Vegetable Oils, Fish Oils, Animal Fats (Tallows), Meat and Fish Meals, and Potato Chips and Grain-Based Snack Products: AOAC Performance Tested MethodSM 052004. J. AOAC Int. 2021, 104, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Khodabux, K.; L’Omelette, M.S.S.; Jhaumeer-Laulloo, S.; Ramasami, P.; Rondeau, P. Chemical and near-infrared determination of moisture, fat and protein in tuna fishes. Food Chem. 2007, 102, 669–675. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Q.; Zhang, M.; Zhu, Q. Prediction of color and moisture content for vegetable soybean during drying using hyperspectral imaging technology. J. Food Eng. 2014, 128, 24–30. [Google Scholar] [CrossRef]
- Wu, D.; Shi, H.; Wang, S.; He, Y.; Bao, Y.; Liu, K. Rapid prediction of moisture content of dehydrated prawns using online hyperspectral imaging system. Anal. Chim. Acta 2012, 726, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, D.-W.; Pu, H. Spectral absorption index in hyperspectral image analysis for predicting moisture contents in pork longissimus dorsi muscles. Food Chem. 2016, 197, 848–854. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.-W.; Pu, H.; Xie, A.; Han, Z.; Luo, M. Non-destructive prediction of thiobarbituricacid reactive substances (TBARS) value for freshness evaluation of chicken meat using hyperspectral imaging. Food Chem. 2015, 179, 175–181. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W. Rapid Quantification Analysis and Visualization of Escherichia coli Loads in Grass Carp Fish Flesh by Hyperspectral Imaging Method. Food Bioprocess Technol. 2015, 8, 951–959. [Google Scholar] [CrossRef]
- Özdoğan, G.; Lin, X.; Sun, D.-W. Rapid and noninvasive sensory analyses of food products by hyperspectral imaging: Recent application developments. Trends Food Sci. Technol. 2021, 111, 151–165. [Google Scholar] [CrossRef]
- Kuswandi, B.; Jayus; Larasati, T.S.; Abdullah, A.; Heng, L.Y. Real-Time Monitoring of Shrimp Spoilage Using On-Package Sticker Sensor Based on Natural Dye of Curcumin. Food Anal. Methods 2012, 5, 881–889. [Google Scholar] [CrossRef]
- Okpala, C.O.R.; Choo, W.S.; Dykes, G.A. Quality and shelf life assessment of Pacific white shrimp (Litopenaeus vannamei) freshly harvested and stored on ice. LWT Food Sci. Technol. 2014, 55, 110–116. [Google Scholar] [CrossRef]
- Cui, K.; Liu, N.; Sun, Y.; Sun, G.; Wang, S.; Yang, M.; Wang, X.; Zhou, D.; Ge, Y.; Wang, D.; et al. Effect of drying processes on the occurrence of lipid oxidation-derived 4-hydroxy-2-hexenal and 4-hydroxy-2-nonenal in Spanish mackerel (Scomberomorus niphonius). Food Sci. Nutr. 2023, 11, 1013–1023. [Google Scholar] [CrossRef]
- Zhang, F.; Kang, T.; Sun, J.; Wang, J.; Zhao, W.; Gao, S.; Wang, W.; Ma, Q. Improving TVB-N prediction in pork using portable spectroscopy with just-in-time learning model updating method. Meat Sci. 2022, 188, 108801. [Google Scholar] [CrossRef]

| Data Preprocessing (1) | Eye | Whole Body | ||
|---|---|---|---|---|
| n | Accuracy (%) | n | Accuracy (%) | |
| RAW | 71 | 81.7 | 71 | 80.3 |
| SNV | 71 | 77.5 | 71 | 78.9 |
| MSC | 71 | 80.3 | 71 | 90.1 |
| SG-1 | 71 | 80.3 | 71 | 78.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.-S.; Choi, B.; Lim, J.-H.; Choi, J.H.; Yun, D.-Y.; Park, S.-K.; Lee, G.; Park, K.-J.; Lee, J. Determination of Freshness of Mackerel (Scomber japonicus) Using Shortwave Infrared Hyperspectral Imaging. Foods 2023, 12, 2305. https://doi.org/10.3390/foods12122305
Cho J-S, Choi B, Lim J-H, Choi JH, Yun D-Y, Park S-K, Lee G, Park K-J, Lee J. Determination of Freshness of Mackerel (Scomber japonicus) Using Shortwave Infrared Hyperspectral Imaging. Foods. 2023; 12(12):2305. https://doi.org/10.3390/foods12122305
Chicago/Turabian StyleCho, Jeong-Seok, Byungho Choi, Jeong-Ho Lim, Jeong Hee Choi, Dae-Yong Yun, Seul-Ki Park, Gyuseok Lee, Kee-Jai Park, and Jihyun Lee. 2023. "Determination of Freshness of Mackerel (Scomber japonicus) Using Shortwave Infrared Hyperspectral Imaging" Foods 12, no. 12: 2305. https://doi.org/10.3390/foods12122305
APA StyleCho, J.-S., Choi, B., Lim, J.-H., Choi, J. H., Yun, D.-Y., Park, S.-K., Lee, G., Park, K.-J., & Lee, J. (2023). Determination of Freshness of Mackerel (Scomber japonicus) Using Shortwave Infrared Hyperspectral Imaging. Foods, 12(12), 2305. https://doi.org/10.3390/foods12122305







