Preparation of pH-Responsive Films from Polyvinyl Alcohol/Agar Containing Cochineal for Monitoring the Freshness of Pork
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Amylopectin and Cochineal-Loaded Starch Particles
2.3. Characterization of Amylopectin and Cochineal-Loaded Starch Particles
2.3.1. Encapsulation Efficiency (EE) of Cochineal
2.3.2. Morphological Structures and Particle Sizes of Amylopectin and Cochineal-Loaded Starch Particles
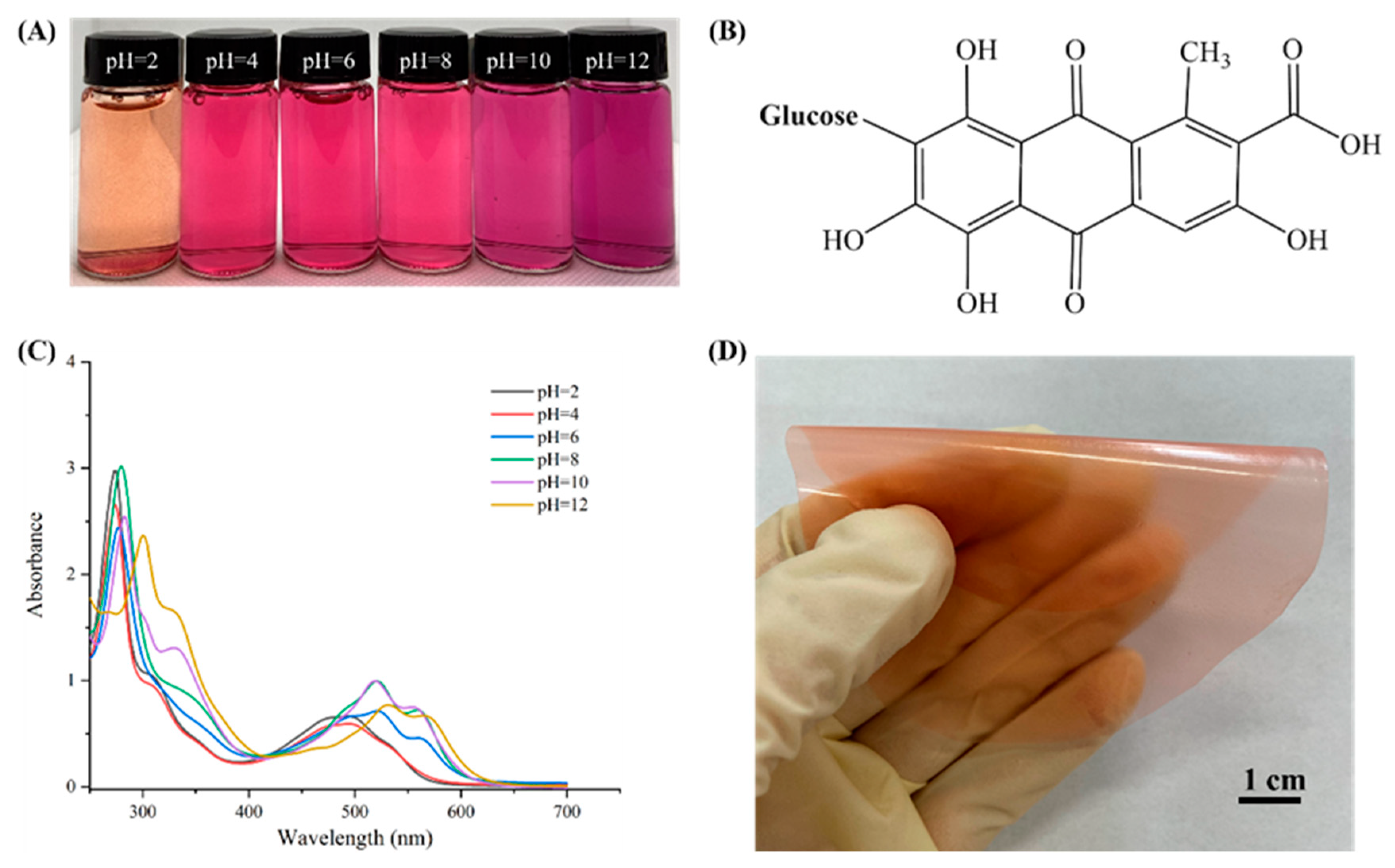
2.4. pH-Sensitive Property and Spectral Characterization of Cochineal Solutions
2.5. Fabrication of the Films
2.6. Structural Characterization of the Films
2.7. Physical Characterization of the Films
2.7.1. Optical Properties of the Films
2.7.2. Swelling Index (SI) and Water Solubility (WS)
2.7.3. Water Vapor Permeability (WVP)
2.7.4. Water Contact Angle (WCA)
2.8. Functional Characterization of the Films
2.8.1. Antioxidant Activities
2.8.2. Antibacterial Activities
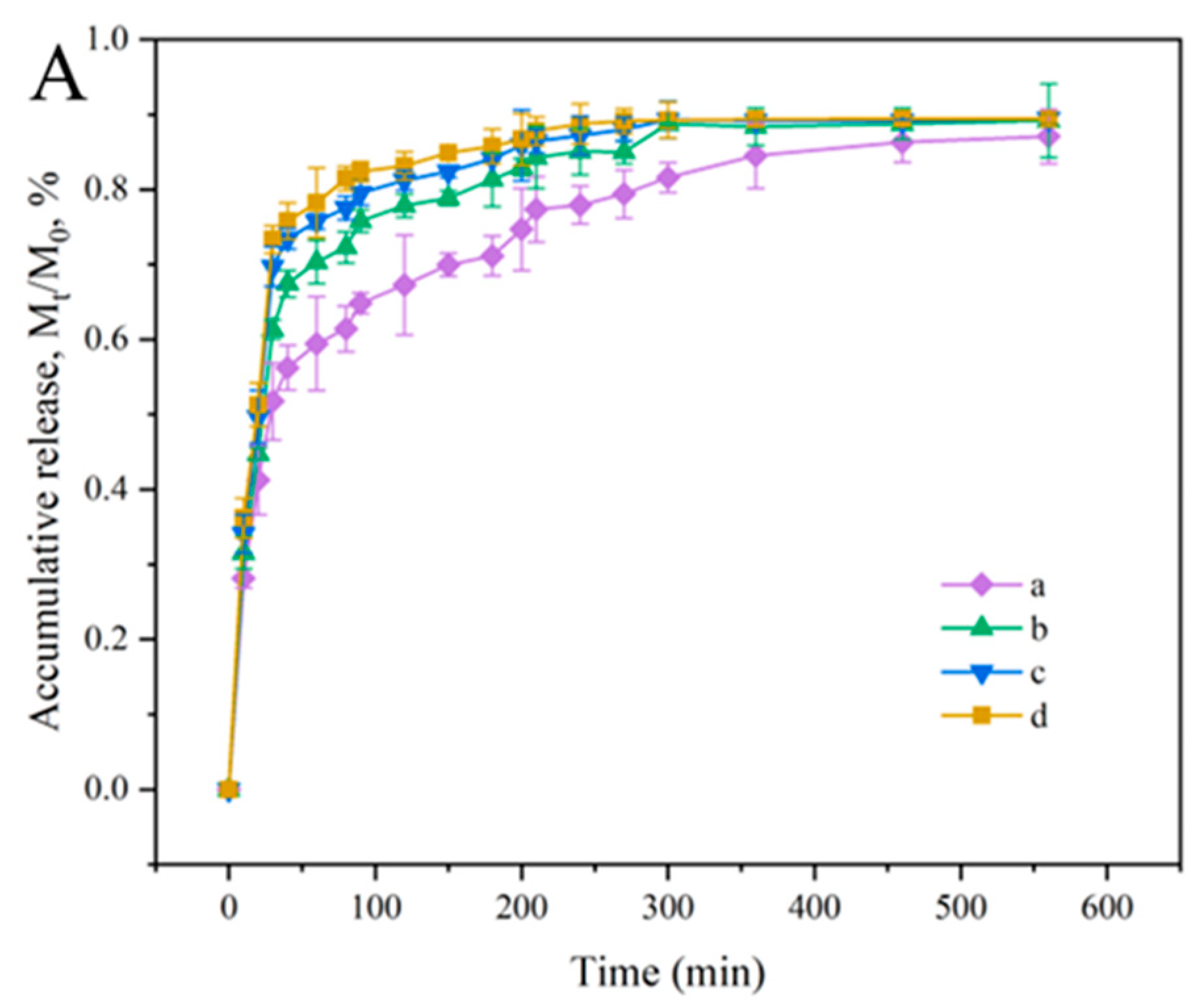
2.9. Release Behavior
2.10. Color Stability of the Films
2.11. Sensitivity to Ammonia of the Films
2.12. Application of the Films
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Particles
3.2. Color and UV-Vis Spectra of Cochineal Solutions in Different Buffer Solutions
3.3. Swelling Properties
3.4. Water Vapor Permeability and Water Contact Angle
3.5. Morphology and Structure of the Films
3.5.1. Scanning Electron Microscopy (SEM)
3.5.2. X-ray Diffraction Spectra (XRD)
3.5.3. FTIR Analysis
3.6. Color Parameters and Stability of the Films
3.7. Release Behavior Analysis of the Films
3.8. Functional Properties of the Films
3.8.1. Antibacterial Activities
3.8.2. Antioxidant Activities
3.8.3. Sensitivity of the pH-Responsive Films to Ammonia
3.9. Effectiveness of the Films to Monitor the Freshness of Pork
3.9.1. pH, TVB-N and TVC Contents
3.9.2. Color Variations of the Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, L.; Liu, L.; Yu, J.; Shao, P. Novel trends and applications of natural pH-responsive indicator film in food packaging for improved quality monitoring. Food Control 2022, 134, 108769. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Pu, Y.; Chen, S.; Liu, L.; Cui, Z.; Zhong, Y. Recent Advances in pH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness. Foods 2022, 11, 1884. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, J.G.; Cavalcante Braga, A.R.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Cano Pereira, V.A.; Egea, M.B. The potential of anthocyanins in smart, active, and bioactive eco-friendly polymer-based films: A review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of Echium amoenum anthocyanins into bacterial cellulose film: A novel colorimetric pH indicator for freshness/spoilage monitoring of shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]
- Goodarzi, M.M.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Development of an easy-to-use colorimetric pH label with starch and carrot anthocyanins for milk shelf life assessment. Int. J. Biol. Macromol. 2020, 153, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. 2021, 61, 2297–2325. [Google Scholar] [CrossRef]
- Aghaei, Z.; Ghorani, B.; Emadzadeh, B.; Kadkhodaee, R.; Tucker, N. Protein-based halochromic electrospun nanosensor for monitoring trout fish freshness. Food Control 2020, 111, 107065. [Google Scholar] [CrossRef]
- Huang, S.; Xiong, Y.; Zou, Y.; Dong, Q.; Ding, F.; Liu, X.; Li, H. A novel colorimetric indicator based on agar incorporated with Arnebia euchroma root extracts for monitoring fish freshness. Food Hydrocoll. 2019, 90, 198–205. [Google Scholar] [CrossRef]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Phan, T.D.; Debeaufort, F.; Luu, D.; Voilley, A. Functional properties of edible agar-based and starch-based films for food quality preservation. J. Agric. Food Chem. 2005, 53, 973–981. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Gautam, L.; Warkar, S.G.; Ahmad, S.I.; Kant, R.; Jain, M. A review on carboxylic acid cross-linked polyvinyl alcohol: Properties and applications. Polym. Eng. Sci. 2022, 62, 225–246. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, L. An active and pH-responsive film developed by sodium carboxymethyl cellulose/polyvinyl alcohol doped with rose anthocyanin extracts. Food Chem. 2022, 373 (Pt B), 131367. [Google Scholar] [CrossRef]
- Stathopoulou, K.; Valianou, L.; Skaltsounis, A.-L.; Karapanagiotis, I.; Magiatis, P. Structure elucidation and chromatographic identification of anthraquinone components of cochineal (Dactylopius coccus) detected in historical objects. Anal. Chim. Acta 2013, 804, 264–272. [Google Scholar] [CrossRef]
- Kannangara, R.; Siukstaite, L.; Borch-Jensen, J.; Madsen, B.; Kongstad, K.T.; Staerk, D.; Bennedsen, M.; Okkels, F.T.; Rasmussen, S.A.; Larsen, T.O.; et al. Characterization of a membrane-bound C-glucosyltransferase responsible for carminic acid biosynthesis in Dactylopius coccus Costa. Nat. Commun. 2017, 8, 1987. [Google Scholar] [CrossRef] [Green Version]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Tech. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Koop, B.L.; da Silva, M.N.; da Silva, F.D.; dos Santos Lima, K.T.; Soares, L.S.; de Andrade, C.J.; Valencia, G.A.; Monteiro, A.R. Flavonoids, anthocyanins, betalains, curcumin, and carotenoids: Sources, classification and enhanced stabilization by encapsulation and adsorption. Food Res. Int. 2022, 153, 110929. [Google Scholar] [CrossRef]
- Wang, F.; Chang, R.; Ma, R.; Qiu, H.; Tian, Y. Eco-Friendly and pH-Responsive Nano-Starch-Based Superhydrophobic Coatings for Liquid-Food Residue Reduction and Freshness Monitoring. ACS Sustain. Chem. Eng. 2021, 9, 10142–10153. [Google Scholar] [CrossRef]
- Escobar-Puentes, A.A.; Garcia-Gurrola, A.; Rincon, S.; Zepeda, A.; Martinez-Bustos, F. Effect of amylose/amylopectin content and succinylation on properties of corn starch nanoparticles as encapsulants of anthocyanins. Carbohydr. Polym. 2020, 250, 116972. [Google Scholar] [CrossRef] [PubMed]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control. 2021, 126, 108039. [Google Scholar] [CrossRef]
- Zheng, T.; Tang, P.; Li, G. Development of a pH-sensitive film based on collagen/chitosan/ZnO nanoparticles and mulberry extract for pork freshness monitoring. Food Chem. 2023, 402, 134428. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@ Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.F.; Pang, S.C.; Tay, S.H. Size controlled synthesis of starch nanoparticles by a simple nanoprecipitation method. Carbohydr. Polym. 2011, 86, 1817–1819. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Wang, Q. Nanoparticles Synthesized from Soy Protein: Preparation, Characterization, and Application for Nutraceutical Encapsulation. J. Agric. Food Chem. 2012, 60, 2712–2720. [Google Scholar] [CrossRef]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, H.; Li, L.; Wang, Q.; Jiang, S.; Chen, M.; Li, X.; Jiang, S. pH-responsive antibacterial film based polyvinyl alcohol/poly (acrylic acid) incorporated with aminoethyl-phloretin and application to pork preservation. Food Res. Int. 2021, 147, 110532. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ma, P.; Zhang, Z.; Xu, W.; Teng, Z.; Luo, Y.; Gong, C.; Wang, Q. Integrated Portable Shrimp-Freshness Prediction Platform Based on Ice-Templated Metal-Organic Framework Colorimetric Combinatorics and Deep Convolutional Neural Networks. ACS Sustain. Chem. Eng. 2021, 9, 16926–16936. [Google Scholar] [CrossRef]
- Alamdari, N.G.; Forghani, S.; Salmasi, S.; Almasi, H.; Moradi, M.; Molaei, R. Ixiolirion tataricum anthocyanins-loaded biocellulose label: Characterization and application for food freshness monitoring. Int. J. Biol. Macromol. 2022, 200, 87–98. [Google Scholar] [CrossRef]
- Qiu, C.; Qin, Y.; Zhang, S.; Xiong, L.; Sun, Q. A comparative study of size-controlled worm-like amylopectin nanoparticles and spherical amylose nanoparticles: Their characteristics and the adsorption properties of polyphenols. Food Chem. 2016, 213, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Montazer, M.; Parvinzadeh, M.; Kiumarsi, A. Colorimetric properties of wool dyed with natural dyes after treatment with ammonia. Color Technol. 2004, 120, 161–166. [Google Scholar] [CrossRef]
- Chairat, M.; Rattanaphani, V.; Bremner, J.B.; Rattanaphani, S.; Perkins, D.F. An absorption spectroscopic investigation of the interaction of lac dyes with metal ions. Dye. Pigment. 2004, 63, 141–150. [Google Scholar] [CrossRef]
- Alves, V.D.; Mali, S.; Beléia, A.; Grossmann, M.V.E. Effect of glycerol and amylose enrichment on cassava starch film properties. J. Food Eng. 2007, 78, 941–946. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J.; Qin, Y.; Bai, R.; Zhang, X.; Liu, J. Antioxidant and pH-sensitive films developed by incorporating purple and black rice extracts into chitosan matrix. Int. J. Biol. Macromol. 2019, 137, 307–316. [Google Scholar] [CrossRef]
- Hashim, S.B.; Tahir, H.E.; Liu, L.; Zhang, J.; Zhai, X.; Mahdi, A.A.; Awad, F.; Hassan, M.M.; Zou, X.; Shi, J. Intelligent colorimetric pH sensoring packaging films based on sugarcane wax/agar integrated with butterfly pea flower extract for optical tracking of shrimp freshness. Food Chem. 2022, 373, 131514. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a visual pH-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Sukhija, S.; Singh, S.; Riar, C.S. Analyzing the effect of whey protein concentrate and psyllium husk on various characteristics of biodegradable film from lotus (Nelumbo nucifera) rhizome starch. Food Hydrocoll. 2016, 60, 128–137. [Google Scholar] [CrossRef]
- Bayer, G.; Shayganpour, A.; Zia, J.; Bayer, I.S. Polyvinyl alcohol-based films plasticized with an edible sweetened gel enriched with antioxidant carminic acid. J. Food Eng. 2022, 323, 111000. [Google Scholar] [CrossRef]
- Qin, Y.; Yun, D.; Xu, F.; Chen, D.; Kan, J.; Liu, J. Smart packaging films based on starch/polyvinyl alcohol and Lycium ruthenicum anthocyanins-loaded nano-complexes: Functionality, stability and application. Food Hydrocoll. 2021, 119, 106850. [Google Scholar] [CrossRef]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Wang, H.; Wang, Q.; Chen, M.; Li, L.; Li, X.; Jiang, S. Intelligent double-layer fiber mats with high colorimetric response sensitivity for food freshness monitoring and preservation. Food Hydrocoll. 2020, 101, 105468. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (PVA)/nano silver hydrogels. Colloid Surf. B 2020, 188, 110757. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microb. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Pu, Y.; Chen, S.; Li, H.; Zhong, Y. Novel colorimetric films based on polyvinyl alcohol/sodium carboxymethyl cellulose doped with anthocyanins and betacyanins to monitor pork freshness. Food Chem. 2022, 404 Pt A, 134426. [Google Scholar] [CrossRef]
- Ran, R.; Chen, S.; Su, Y.; Wang, L.; He, S.; He, B.; Li, C.; Wang, C.; Liu, Y. Preparation of pH-colorimetric films based on soy protein isolate/ZnO nanoparticles and grape-skin red for monitoring pork freshness. Food Control 2022, 137, 108958. [Google Scholar] [CrossRef]









| Physical Properties | Cochineal | PVA/GG Film | PVA/GG-2 Film | PVA/GG-4 Film | PVA/GG-6 Film | PVA/GG-8 Film |
|---|---|---|---|---|---|---|
| L | 17.33 ± 1.63 d | 66.17 ± 0.75 a | 59.17 ± 0.75 b | 57.83 ± 4.07 c | 56.00 ± 1.09 c | 51.50 ± 1.22 c |
| a | 13.00 ± 0.63 c | −0.50 ± 0.55 e | 7.67 ± 0.52 d | 21.00 ± 1.26 b | 21.50 ± 0.84 b | 28.83 ± 1.17 a |
| b | 3.00 ± 1.27 f | 7.50 ± 0.55 e | 10.50 ± 1.22 d | 23.17 ± 0.75 b | 25.00 ± 0.89 b | 29.67 ± 1.51 a |
| ΔE* | 75.48 ± 1.69 a | 7.89 ± 0.55 e | 13.29 ± 1.01 d | 31.38 ± 1.02 c | 33.37 ± 2.12 c | 42.43 ± 1.89 b |
| SI (%) | \ | 372.32 ± 23.58 a | 326.67 ± 19.54 c | 366.12 ± 29.18 a | 365.24 ± 22.26 a | 361.43 ± 28.84 b |
| WS (%) | \ | 38.76 ± 1.57 a | 36.84 ± 1.93 b | 36.51 ± 1.81 b | 36.07 ± 2.18 b | 36.43 ± 2.32 b |
| WVP (×10−11 g m−1 s−1 Pa−1) | \ | 13.27 ± 0.46 a | 12.83 ± 0.32 b | 12.67 ± 0.27 b | 12.46 ± 0.13 b | 12.39 ± 0.23 b |
| WCA (°) | \ | 59.72 ± 1.62 a | 58.33 ± 1.32 b | 57.96 ± 1.03 b | 57.81 ± 1.01 b | 57.39 ± 1.23 b |
| Time (D) | pH | TVB-N (mg/100 g) | TVC (log10 CFU/g) | Color Variations of the pH-Responsive Films (4 °C) | ||
|---|---|---|---|---|---|---|
| PVA/GG-2 | PVA/GG-4 | PVA/GG-6 | ||||
| 0 | 5.11 ± 0.09 e | 3.84 ± 0.08 h | 2.95 ± 0.21 h |  |  |  |
| 1 | 5.48 ± 0.14 d | 5.80 ± 0.09 g | 3.41 ± 0.33 g |  |  |  |
| 2 | 5.82 ± 0.09 b | 7.03 ± 0.18 f | 3.88 ± 0.15 f |  |  |  |
| 3 | 5.64 ± 0.15 c | 8.78 ± 0.19 e | 4.32 ± 0.23 e |  |  |  |
| 4 | 6.01 ± 0.05 b | 10.86 ± 0.31 d | 4.82 ± 0.21 d |  |  |  |
| 5 | 6.15 ± 0.06 a | 12.75 ± 0.39 c | 5.07 ± 0.19 c |  |  |  |
| 6 | 6.20 ± 0.13 a | 18.71 ± 0.58 b | 6.08 ± 0.22 b |  |  |  |
| 7 | 6.26 ± 0.12 a | 23.45 ± 0.42 a | 7.02 ± 0.17 a |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhong, Y.; Pu, Y.; Li, X.; Chen, S.; Zhang, C. Preparation of pH-Responsive Films from Polyvinyl Alcohol/Agar Containing Cochineal for Monitoring the Freshness of Pork. Foods 2023, 12, 2316. https://doi.org/10.3390/foods12122316
Liu D, Zhong Y, Pu Y, Li X, Chen S, Zhang C. Preparation of pH-Responsive Films from Polyvinyl Alcohol/Agar Containing Cochineal for Monitoring the Freshness of Pork. Foods. 2023; 12(12):2316. https://doi.org/10.3390/foods12122316
Chicago/Turabian StyleLiu, Danfei, Yunfei Zhong, Yumei Pu, Xiaoxuan Li, Siyuan Chen, and Changfan Zhang. 2023. "Preparation of pH-Responsive Films from Polyvinyl Alcohol/Agar Containing Cochineal for Monitoring the Freshness of Pork" Foods 12, no. 12: 2316. https://doi.org/10.3390/foods12122316