Biological Activities and Solubilization Methodologies of Naringin
Abstract
:1. Introduction
2. The Biological Activities of Naringin
2.1. Anti-Inflammatory
2.2. Anti-Diabetes
2.3. Hepatoprotective Activity
2.4. Neuroprotective Activity
2.5. The Drug Absorption Enhancer
| Physiological Activities | Constituent | Dose | Animal Model | Potential Mechanisms | References |
|---|---|---|---|---|---|
| Anti-inflammatory | NG | 100 mg/kg | HFD-induced obesity mice | Decrease: Mac-2, MCP-1, JNK phosphorylation | [45] |
| 36.8 mg/kg | CS-induced chronic bronchitis in guinea pigs | Increase: Activities of SOD and LXA4 Decrease: IL-8, LTB4, TNF-α, BALF, and myeloperoxidase activity | [46] | ||
| 60 mg/kg | LPS-induced endotoxin shock in mice | Decrease: NO, TNF-α, IL-6, iNOS, COX-2 and transcriptional activity of NF-κB | [47] | ||
| 3 mg | LPS/D-galactosamine-induced liver injury mice | Decrease: AST, ALT, CK, TNF-α | [48] | ||
| Anti-diabetes | NG | 30 mg/kg | STZ-induced diabetic mice | Increase: Activity of hexokinase Decrease: Activities of glucose-6-phosphatase and fructose-1,6-bisphosphatase in the liver and kidney | [49] |
| 200 mg/kg | C57BL/KsJ-db/db mice (Diabetic mouse model) | Increase: Hepatic glucokinase activity and glycogen concentration Decrease: Activity of hepatic G6-P and phosphoenolpyruvate carboxykinase | [50] | ||
| naringenin | 50 mg/kg | STZ-nicotinamide–induced diabetes mice | Increase: Serum insulin concentrations Decrease: Activities of ALT, AST, ALP, and LDH in serum, Concentrations of fasting blood glucose, Glycosylated hemoglobin | [51] | |
| NG | 50 mg/kg | HFD/STZ-nicotinamide–induced diabetes mice | Increase: G6Pase, Glycogen phosphorylase, FBPase, Insulin release Decrease: MDA, NO, TNF-α, IL-2 | [52] | |
| Hepatoprotective activity | NG | 80 mg/kg (Nickel) and 50 mg/kg (Cadmium) | Nickel and Cadmium-induced hepatotoxicity in mice | Increase: SOD, CAT, GPx, GST, GST, GSH, vitamin C, and vitamin E Decrease: AST, ALT, ALP, LDH, GGT, TB, The liver nickel concentration, Lipid peroxidation indices, and protein carbonyl contents | [34,53] |
| naringenin | 50 mg/kg | DMN-induced liver injury mice | Increase: Body weight, Serum albumin, and total protein levels Decrease: ALT, AST, ALP, and bilirubin levels, MDA, Hepatic stellate cell activation | [35] | |
| NG | 20 mg/kg | APAP induced in male Wistar mice | Increase: Albumin, IL-4, GSH, SOD, GST, GPx, Bcl-2 Decrease: AST, ALT, ALP, LDH, GGT bilirubin, lipid, TNF-α, lipid peroxidation p53, Bax, CASP-3 | [54] | |
| naringenin | 25 mg/L | 2% ethanol-induced larvae of zebrafish | Increase: Cyp2y3 and Fabp10α, Histological injury severity, Apoptotic cell death, and SOD radical levels | [55] | |
| NG | 100 mg/kg | 5-fluorouracil induced liver and kidney toxicity in mice | Increase: GSH, SOD Decrease: ALT, AST, ALP, MDA, IL-1α, TNF-α, IL-6 | [56] | |
| Neuroprotective Activity | NG | 80 mg/kg | 3-NP-induced neurodegenerative disease in mice | Increase: Nuclear translocation of Nrf2, Induce phase II genes such as HO-1, NQO-1, GST-P1 and γ-GCL expression Decrease: TNF-α, COX-2, and iNOS mRNA expression | [36] |
| 80 mg/kg | KA-induced neurodegenerative disease in mice | Increase: Protected hippocampal CA1 neurons, the expression of LC3 Decrease: TNF-α, Occurrence of SRS | [57] | ||
| 100 mg/kg | Aβ-induced AD mice | Increase: CaMKII activity, Phosphorylation of AMPA, Improved long-term learning and memory ability Decrease: GSK-3β activity | [58] | ||
| 200 mg/kg | ICV-STZ-induced AD mice | Increase: CAT, SOD, GSH, Mitochondrial complex (I, II, and IV) Decrease: Cholinesterase activity, MDA, nitrate level, TNF-α, IL-1β | [59] | ||
| The drug Absorption Enhancer | PLH/Naringin-G | PLH 40 mg/kg/NG 80 mg/kg | Male Sprague–Dawley mice | Increase: PLH solubility and absorption | [20] |
| NG | 15 mg/kg | in-situ rat models | Increase: Candesartan absorption, AUC value, and Cmaxvalue Decrease: tmaxvalue, the release of protein and ALP | [44] | |
| Preparation of GGTN composite with NG | 10 mg/mL | Rabbit skull defect model | Increase: Bone regeneration, bone conduction activity, new bone growth, wound healing | [60] |
3. The Methods of Solubilization
3.1. Structure Modification
3.1.1. Acylation

3.1.2. Glycosylation
3.2. Solid Dispersion
3.3. Inclusion Compound and Polymeric Micelle
3.3.1. Cyclodextrin Inclusion Compound

3.3.2. Polymeric Micelle
3.4. Liposome and Nanoparticles
3.4.1. Naringin Liposome
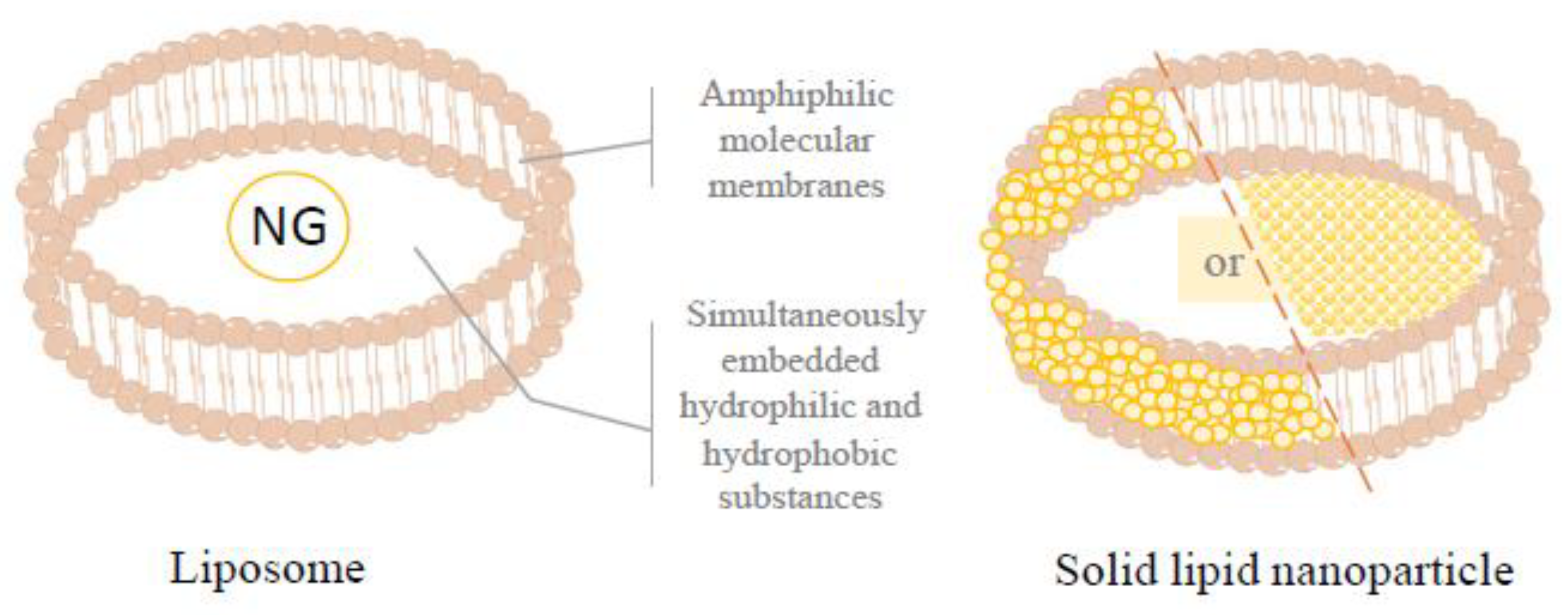
3.4.2. Solid Lipid Nanoparticle
3.4.3. Chitosan Nanoparticle
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A systematic review of the preventive and therapeutic effects of naringin against human malignancies. Front. Pharmacol. 2021, 12, 639840. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, M.d.L.M.; Andrés-Lacueva, C.; Jáuregui, O.; Lamuela-Raventos, R.M. Determination of flavonoids in a Citrus fruit extract by LC–DAD and LC–MS. Food Chem. 2007, 101, 1742–1747. [Google Scholar]
- Matsuo, Y.; Akita, K.; Taguchi, H.; Fujii, S.; Yoshie-Stark, Y.; Araki, T. Utilization and evaluation of Citrus natsudaidai peel waste as a source of natural food additives. Food Chem. 2022, 373, 131464. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Jayaprakasha, G.; Jifon, J.; Patil, B.S. Optimization of flavanones extraction by modulating differential solvent densities and centrifuge temperatures. Talanta 2011, 85, 353–362. [Google Scholar] [CrossRef]
- Hartonen, K.; Parshintsev, J.; Sandberg, K.; Bergelin, E.; Nisula, L.; Riekkola, M.-L. Isolation of flavonoids from aspen knotwood by pressurized hot water extraction and comparison with other extraction techniques. Talanta 2007, 74, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Singla, G.; Singh, U.; Sangwan, R.S.; Panesar, P.S.; Krishania, M. Comparative study of various processes used for removal of bitterness from kinnow pomace and kinnow pulp residue. Food Chem. 2021, 335, 127643. [Google Scholar] [CrossRef]
- Vilet, N.Z.; Gué, E.; Servent, A.; Delalonde, M.; Wisniewski, C. Filtration-compression step as downstream process for flavonoids extraction from citrus peels: Performances and flavonoids dispersion state in the filtrate. Food Bioprod. Process. 2020, 120, 104–113. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Kan, H.; Chen, S.-X.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Comparison of phenolic compounds extracted from Diaphragma juglandis fructus, walnut pellicle, and flowers of Juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem. 2020, 321, 126672. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of naringin and naringenin extraction from Citrus × paradisi L. using hydrolysis and excipients as adsorbent. Pharmaceutics 2022, 14, 890. [Google Scholar] [CrossRef]
- Çiçek, S.S. Structure-dependent activity of plant-derived sweeteners. Molecules 2020, 25, 1946. [Google Scholar] [CrossRef] [Green Version]
- Dai, K.-R.; Yan, S.-G.; Yan, W.-Q.; Chen, D.-Q.; Xu, Z.-W. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur. J. Pharmacol. 2009, 607, 1–5. [Google Scholar]
- Ribeiro, M.H. Naringinases: Occurrence, characteristics, and applications. Appl. Microbiol. Biotechnol. 2011, 90, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Andrades, N.E.; Paulino, N.; Sawaya, A.C.; Eberlin, M.N.; Marcucci, M.C.; Favero, G.M.; Novak, E.M.; Bydlowski, S.P. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules 2007, 12, 1352–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wu, H.; Chen, P.; Su, W.; Peng, W.; Li, P. Fertility and early embryonic development toxicity assessment of naringin in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2021, 123, 104938. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Zheng, Y.; He, Y.; He, Y.; Rao, H.; Peng, W.; Yao, H. Pharmacokinetics, Tissue Distribution, Metabolism, and Excretion of Naringin in Aged Rats. Front Pharm. 2019, 10, 34. [Google Scholar] [CrossRef]
- Dangre, P.V.; Korekar, P.P.; Borkar, M.R.; Chaturvedi, K.K.; Borikar, S.P.; Pethe, A.M. Tailoring Deep Eutectic Solvents to Provoke Solubility and Bioavailability of Naringin: Implications of a Computational Approach. ACS Omega 2023, 8, 12820–12829. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhu, Y.; Yu, J.N.; Xu, X.M. Advanced in solubilization methods of water-insoluble natural drugs. Zhongguo Zhong Yao Za Zhi 2014, 39, 3226–3231. [Google Scholar]
- Choi, J.-S.; Shin, S.-C. Enhanced paclitaxel bioavailability after oral coadministration of paclitaxel prodrug with naringin to rats. Int. J. Pharm. 2005, 292, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Alhalmi, A.; Amin, S.; Khan, Z.; Beg, S.; Al Kamaly, O.; Saleh, A.; Kohli, K. Nanostructured Lipid Carrier-Based Codelivery of Raloxifene and Naringin: Formulation, Optimization, In Vitro, Ex Vivo, In Vivo Assessment, and Acute Toxicity Studies. Pharmaceutics 2022, 14, 1771. [Google Scholar] [CrossRef]
- Uchiyama, H.; Kadota, K.; Nakanishi, A.; Tandia, M.; Tozuka, Y. A simple blending with α-glycosylated naringin produces enhanced solubility and absorption of pranlukast hemihydrate. Int. J. Pharm. 2019, 567, 118490. [Google Scholar] [CrossRef]
- Lai, C.-S.; Wu, J.-C.; Ho, C.-T.; Pan, M.-H. Disease chemopreventive effects and molecular mechanisms of hydroxylated polymethoxyflavones. BioFactors 2015, 41, 301–313. [Google Scholar] [CrossRef]
- Bharti, S.; Rani, N.; Krishnamurthy, B.; Arya, D.S. Preclinical evidence for the pharmacological actions of naringin: A review. Planta Med. 2014, 80, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-κB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, H.; Nie, Y.-C.; Chen, J.-L.; Su, W.-W.; Li, P.-B. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-κB pathway. Int. Immunopharmacol. 2011, 11, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.Á.; Ramos, S. Dietary Flavonoids and Insulin Signaling in Diabetes and Obesity. Cells 2021, 10, 1474. [Google Scholar] [CrossRef]
- Sandeep, M.S.; Nandini, C.D. Influence of quercetin, naringenin and berberine on glucose transporters and insulin signalling molecules in brain of streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2017, 94, 605–611. [Google Scholar] [CrossRef]
- Dayarathne, L.A.; Ranaweera, S.S.; Natraj, P.; Rajan, P.; Lee, Y.J.; Han, C.-H. The effects of naringenin and naringin on the glucose uptake and AMPK phosphorylation in high glucose treated HepG2 cells. J. Vet. Sci. 2021, 22, e92. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Li, H.; Chen, F.; Shi, J. Naringin ameliorates diabetic nephropathy by inhibiting NADPH oxidase 4. Eur. J. Pharm. 2017, 804, 1–6. [Google Scholar] [CrossRef]
- Bugianesi, R.; Catasta, G.; Spigno, P.; D’Uva, A.; Maiani, G. Naringenin from cooked tomato paste is bioavailable in men. J. Nutr. 2002, 132, 3349–3352. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.B.; Liu, D.X.; Liu, D.K.; Wu, G. Application of solid dispersion technique to improve solubility and sustain release of emamectin benzoate. Molecules 2019, 24, 4315. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Zhou, Z.; Zhong, W.; Huang, P.; Ma, N.; Zhang, Y.; Zhou, C.; Lai, Y.; Huang, S.; An, H.; et al. Naringenin inhibits alcoholic injury by improving lipid metabolism and reducing apoptosis in zebrafish larvae. Oncol. Rep. 2017, 38, 2877–2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirani, K.; Yousefsani, B.S.; Shirani, M.; Karimi, G. Protective effects of naringin against drugs and chemical toxins induced hepatotoxicity: A review. Phytother. Res. 2020, 34, 1734–1744. [Google Scholar] [CrossRef]
- Adwas, A.A.; Elsayed, A.; Azab, A.; Quwaydir, F. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar]
- Pari, L.; Amudha, K. Hepatoprotective role of naringin on nickel-induced toxicity in male Wistar rats. Eur. J. Pharmacol. 2011, 650, 364–370. [Google Scholar] [CrossRef]
- Lee, M.-H.; Yoon, S.; Moon, J.-O. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol. Pharm. Bull. 2004, 27, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, K.; Sudhandiran, G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 2012, 227, 134–143. [Google Scholar] [CrossRef]
- Mani, V.M.; Sadiq, A.M.M. Flavonoid naringin inhibits microglial activation and exerts neuroprotection against deltamethrin induced neurotoxicity through Nrf2/ARE signaling in the cortex and hippocampus of rats. World J. Pharm. Sci. 2015, 3, 2292–2514. [Google Scholar]
- Ramful-Baboolall, D.; Neergheen-Bhujun, V.; Bahorun, T. Prophylactic propensity of citrus phytochemicals: Action and mechanisms. Mol. Phylogeny Antioxid. Prop. Med. Uses 2014, 95, 95–98. [Google Scholar]
- Budel, R.G.; da Silva, D.A.; Moreira, M.P.; Dalcin, A.J.F.; da Silva, A.F.; Nazario, L.R.; Majolo, J.H.; Lopes, L.Q.S.; Santos, R.C.V.; Soares, F.A.A. Toxicological evaluation of naringin-loaded nanocapsules in vitro and in vivo. Colloids Surf. B Biointerfaces 2020, 188, 110754. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Y.; Chen, J.; Xuan, A.; Wang, X. Microglia and immunotherapy in Alzheimer’s disease. Acta Neurol. Scand. 2022, 145, 273–278. [Google Scholar] [CrossRef]
- Ruan, Q.; Ruan, J.; Zhang, W.; Qian, F.; Yu, Z. Targeting NAD+ degradation: The therapeutic potential of flavonoids for Alzheimer’s disease and cognitive frailty. Pharmacol. Res. 2018, 128, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Sparreboom, A.; Van Asperen, J.; Mayer, U.; Schinkel, A.H.; Smit, J.W.; Meijer, D.K.; Borst, P.; Nooijen, W.J.; Beijnen, J.H.; Van Tellingen, O. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. USA 1997, 94, 2031–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, P.; Mosher, S.J.; Quilliam, M.A.; Montague, T.J. Species comparison of pharmacokinetics and metabolism of diltiazem in humans, dogs, rabbits, and rats. Drug Metab. Dispos. 1990, 18, 1055–1059. [Google Scholar] [PubMed]
- Surampalli, G.; Nanjwade, B.K.; Patil, P. Corroboration of naringin effects on the intestinal absorption and pharmacokinetic behavior of candesartan cilexetil solid dispersions using in-situ rat models. Drug Dev. Ind. Pharm. 2015, 41, 1057–1065. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, H.; Ishida, A.; Watanabe, W.; Narumi, K.; Atsumi, T.; Sugita, C.; Kurokawa, M. Naringenin suppresses macrophage infiltration into adipose tissue in an early phase of high-fat diet-induced obesity. Biochem. Biophys. Res. Commun. 2014, 454, 95–101. [Google Scholar] [CrossRef]
- Luo, Y.-L.; Zhang, C.-C.; Li, P.-B.; Nie, Y.-C.; Wu, H.; Shen, J.-G.; Su, W.-W. Naringin attenuates enhanced cough, airway hyperresponsiveness and airway inflammation in a guinea pig model of chronic bronchitis induced by cigarette smoke. Int. Immunopharmacol. 2012, 13, 301–307. [Google Scholar] [CrossRef]
- Kanno, S.-i.; Shouji, A.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Ujibe, M.; Obara, Y.; Nakahata, N.; Ishikawa, M. Inhibitory effect of naringin on lipopolysaccharide (LPS)-induced endotoxin shock in mice and nitric oxide production in RAW 264.7 macrophages. Life Sci. 2006, 78, 673–681. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Kikuchi, S.; Hasegawa, H.; Maruyama, H.; Morita, H.; Kumazawa, Y. Suppression of lipopolysaccharide-induced tumor necrosis factor-release and liver injury in mice by naringin. Eur. J. Pharmacol. 1999, 368, 245–250. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [Green Version]
- Jung, U.J.; Lee, M.-K.; Jeong, K.-S.; Choi, M.-S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004, 134, 2499–2503. [Google Scholar] [CrossRef] [Green Version]
- Annadurai, T.; Muralidharan, A.R.; Joseph, T.; Hsu, M.; Thomas, P.; Geraldine, P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin–nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012, 68, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Its Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; Fahim, H.I.; Ahmed, H.Y.; Al-Muzafar, H.M.; Ahmed, R.R.; Amin, K.A.; El-Nahass, E.S.; Abdelazeem, W.H. The Preventive Effects and the Mechanisms of Action of Navel Orange Peel Hydroethanolic Extract, Naringin, and Naringenin in N-Acetyl-p-aminophenol-Induced Liver Injury in Wistar Rats. Oxid. Med. Cell. Longev. 2019, 2019, 2745352. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Lai, Y.; Huang, P.; Xie, L.; Lin, H.; Zhou, Z.; Mo, C.; Deng, G.; Yan, W.; Gao, Z.; et al. Naringin attenuates alcoholic liver injury by reducing lipid accumulation and oxidative stress. Life Sci. 2019, 216, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Gelen, V.; Şengül, E.; Yıldırım, S.; Atila, G. The protective effects of naringin against 5-fluorouracil-induced hepatotoxicity and nephrotoxicity in rats. Iran. J. Basic Med. Sci. 2018, 21, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Jung, U.J.; Kim, S.R. Naringin Attenuates Autophagic Stress and Neuroinflammation in Kainic Acid-Treated Hippocampus In Vivo. Evid. Based Complement. Altern. Med. 2015, 2015, 354326. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Yang, Y.J.; Zhang, L.; Zhang, X.; Guan, F.F.; Zhang, L.F. Naringin Enhances CaMKII Activity and Improves Long-Term Memory in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2013, 14, 5576–5586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachdeva, A.K.; Kuhad, A.; Chopra, K. Naringin ameliorates memory deficits in experimental paradigm of Alzheimer’s disease by attenuating mitochondrial dysfunction. Pharm. Biochem. Behav. 2014, 127, 101–110. [Google Scholar] [CrossRef]
- Chen, K.Y.; Lin, K.C.; Chen, Y.S.; Yao, C.H. A novel porous gelatin composite containing naringin for bone repair. Evid. Based Complement. Altern. Med. 2013, 2013, 283941. [Google Scholar] [CrossRef] [Green Version]
- Shaik, B.F.; Gandhodi, G.; Kondupalli, D.; Payyala, R.; Nelson, V.K.; Kumar, V.; Nelson, V.K. Coumarin modulates the pharmacological activity via structural modification. Int. J. Innov. Pharm. Sci. Res. 2019, 7, 31–46. [Google Scholar] [CrossRef]
- Amin, T.; Naik, H.; Hussain, S.Z.; Naseer, B. Functional Foods: Bioavailability, Structure, and Nutritional Properties. In Health Benefits of Secondary Phytocompounds from Plant and Marine Sources; Apple Academic Press: New York, NY, USA, 2021; pp. 3–38. [Google Scholar]
- Alseekh, S.; de Souza, L.P.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Pozzo, T.; Liu, J.; Gulshan Ara, K.Z.; Turner, C.; Nordberg Karlsson, E. Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J. Agric. Food Chem. 2014, 62, 3321–3333. [Google Scholar] [CrossRef] [PubMed]
- Shokri, Z.; Seidi, F.; Saeb, M.R.; Jin, Y.; Li, C.; Xiao, H. Elucidating the impact of enzymatic modifications on the structure, properties, and applications of cellulose, chitosan, starch and their derivatives: A review. Mater. Today Chem. 2022, 24, 100780. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.M.; Branco, C.R.; Assis, S.A.; Vieira, I.J.; Braz-Filho, R.; Branco, A. Synthesis of naringin 6″-ricinoleate using immobilized lipase. Chem. Cent. J. 2012, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Kometani, T.; Nishimura, T.; Nakae, T.; Takii, H.; Okada, S. Synthesis of neohesperidin glycosides and naringin glycosides by cyclodextrin glucano-transferase from an Alkalophilic Bacillus Species. Biosci. Biotechnol. Biochem. 1996, 60, 645–649. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Kim, J.-C.; Kim, M.J.; Kitaoka, M.; Park, C.S.; Lee, S.Y.; Ra, M.-J.; Moon, T.W.; Robyt, J.F.; Park, K.H. Transglycosylation of naringin by Bacillus stearothermophilus maltogenic amylase to give glycosylated naringin. J. Agric. Food Chem. 1999, 47, 3669–3674. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure-activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef]
- Mohapatra, D.; Agrawal, A.K.; Sahu, A.N. Exploring the potential of solid dispersion for improving solubility, dissolution & bioavailability of herbal extracts, enriched fractions, and bioactives. J. Microencapsul. 2021, 38, 594–612. [Google Scholar]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Enhanced dissolution and bioavailability of grapefruit flavonoid Naringenin by solid dispersion utilizing fourth generation carrier. Drug Dev. Ind. Pharm. 2015, 41, 772–779. [Google Scholar] [CrossRef]
- Tekade, A.R.; Yadav, J.N. A review on solid dispersion and carriers used therein for solubility enhancement of poorly water soluble drugs. Adv. Pharm. Bull. 2020, 10, 359. [Google Scholar] [CrossRef]
- Sinha, S.; Ali, M.; Baboota, S.; Ahuja, A.; Kumar, A.; Ali, J. Solid dispersion as an approach for bioavailability enhancement of poorly water-soluble drug ritonavir. AAPS PharmSciTech 2010, 11, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of poorly soluble drugs: Drug bioavailability and physicochemical stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ye, X.; Lin, S.; Liu, H.; Qiang, Y.; Chen, H.; Jiang, Z.; Zhang, K.; Duan, X.; Xu, Y. Preparation, characterization and in vitro and in vivo evaluation of a solid dispersion of Naringin. Drug Dev. Ind. Pharm. 2018, 44, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: A comparative study. J. Appl. Polym. Sci. 2006, 102, 460–471. [Google Scholar] [CrossRef]
- Wu, R.; Dong, Q.; Che, Z.; Wang, H.; Cao, J.; Cao, F.; Su, E. Green and Efficient Simultaneous Enrichment and Separation of Multiple Valuable Bioactive Compounds from Agricultural Waste Ginkgo biloba Exocarp Using a Two-Phase Deep Eutectic Solvent System. ACS Sustain. Chem. Eng. 2022, 10, 16958–16968. [Google Scholar] [CrossRef]
- Manogna, K.; Nagaveni, P.; Thyagaraju, K. Enhancement of solubility of poorly soluble drugs by solid dispersion: An overview. Indian J. Pharm. Biol. Res. 2017, 5, 17–23. [Google Scholar] [CrossRef]
- Nair, A.R.; Lakshman, Y.D.; Anand, V.S.K.; Sree, K.N.; Bhat, K.; Dengale, S.J. Overview of extensively employed polymeric carriers in solid dispersion technology. AAPS PharmSciTech 2020, 21, 309. [Google Scholar] [CrossRef]
- Boel, E.; Smeets, A.; Vergaelen, M.; Victor, R.; Hoogenboom, R.; Van den Mooter, G. Comparative study of the potential of poly (2-ethyl-2-oxazoline) as carrier in the formulation of amorphous solid dispersions of poorly soluble drugs. Eur. J. Pharm. Biopharm. 2019, 144, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Serajuddin, A.T. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 1999, 88, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef]
- Rahimi, S.K.; O’Donnell, K.; Haight, B.; Machado, A.; Martin, C.; Meng, F.; Listro, T.; Zhang, F. Supercritical-CO2 Foam Extrusion of Hydroxypropyl Methyl Cellulose Acetate Succinate/Itraconazole Amorphous Solid Dispersions: Processing-Structure-Property Relations. J. Pharm. Sci. 2021, 110, 1444–1456. [Google Scholar] [CrossRef]
- Obaidat, R.M.; Tashtoush, B.M.; Awad, A.A.; Al Bustami, R.T. Using Supercritical Fluid Technology (SFT) in Preparation of Tacrolimus Solid Dispersions. AAPS PharmSciTech 2017, 18, 481–493. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Ashogbon, A.O.; Singh, A.; Chaudhary, V.; Whiteside, W.S. Enzymatic modification of starch: A green approach for starch applications. Carbohydr. Polym. 2022, 287, 119265. [Google Scholar] [CrossRef]
- Xiang, L.; Lu, S.; Quek, S.Y.; Liu, Z.; Wang, L.; Zheng, M.; Tang, W.; Yang, Y. Exploring the effect of OSA-esterified waxy corn starch on naringin solubility and the interactions in their self-assembled aggregates. Food Chem. 2021, 342, 128226. [Google Scholar] [CrossRef]
- Wang, S.; Wei, Y.; Wang, Y.; Cheng, Y. Cyclodextrin regulated natural polysaccharide hydrogels for biomedical applications—A review. Carbohydr. Polym. 2023, 313, 120760. [Google Scholar] [CrossRef]
- Matshetshe, K.I. Synthesis and Characterization of Cyclodextrin Based Chitosan Nanoparticles for Drug Delivery of Essential Oil; University of Johannesburg: Johannesburg, South Africa, 2017. [Google Scholar]
- Zhao, Y.; Cai, Y.; Wang, Y.; Xu, S. A win-win strategy of β-cyclodextrin and ion-doped polypyrrole composite nanomaterials for asymmetric capacitive deionization. Sep. Purif. Technol. 2021, 259, 118175. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Gupta, S.S.; Serajuddin, A. Investigation of polymer-surfactant and polymer-drug-surfactant miscibility for solid dispersion. AAPS J. 2016, 18, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, C.; Gaardahl, K.; Houston, B. Drug Solubilization by Mixtures of Cyclodextrins: Additive and Synergistic Effects. Mol. Pharm. 2019, 16, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Zhang, Z.H.; Sun, E.; Jia, X.B. Effect of β-Cyclodextrin Complexation on Solubility and Enzymatic Conversion of Naringin. Int. J. Mol. Sci. 2012, 13, 14251–14261. [Google Scholar] [CrossRef] [Green Version]
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-β-cyclodextrin. PLoS ONE 2011, 6, e18033. [Google Scholar] [CrossRef] [Green Version]
- Stasiłowicz-Krzemień, A.; Gołębiewski, M.; Płazińska, A.; Płaziński, W.; Miklaszewski, A.; Żarowski, M.; Adamska-Jernaś, Z.; Cielecka-Piontek, J. The Systems of Naringenin with Solubilizers Expand Its Capability to Prevent Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 755. [Google Scholar] [CrossRef] [PubMed]
- Abasian, P.; Shakibi, S.; Maniati, M.S.; Nouri Khorasani, S.; Khalili, S. Targeted delivery, drug release strategies, and toxicity study of polymeric drug nanocarriers. Polym. Adv. Technol. 2021, 32, 931–944. [Google Scholar] [CrossRef]
- Qu, X.; Zou, Y.; He, C.; Zhou, Y.; Jin, Y.; Deng, Y.; Wang, Z.; Li, X.; Zhou, Y.; Liu, Y. Improved intestinal absorption of paclitaxel by mixed micelles self-assembled from vitamin E succinate-based amphiphilic polymers and their transcellular transport mechanism and intracellular trafficking routes. Drug Deliv. 2018, 25, 210–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Chen, F.; Xiao, Q.; Cai, M.; Yang, Q.; Weng, H.; Xiao, A. Structure and physicochemical properties of amphiphilic agar modified with octenyl succinic anhydride. Carbohydr. Polym. 2021, 251, 117031. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, P.; Zhou, L.; Mo, F.; Jin, Z.; Ma, J.; Lin, R.; Liu, Y.; Zhang, J. Naringin-loaded polymeric micelles as buccal tablets: Formulation, characterization, invitro release, cytotoxicity and histopathology studies. Pharm. Dev. Technol. 2020, 25, 547–555. [Google Scholar] [CrossRef]
- Percebom, A.M.; Costa, L.H.M. Formation and assembly of amphiphilic Janus nanoparticles promoted by polymer interactions. Adv. Colloid Interface Sci. 2019, 269, 256–269. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-u. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Egharevba, H.O. Chemical properties of starch and its application in the food industry. In Chemical Properties of Starch; IntechOpen: London, UK, 2019; Volume 9. [Google Scholar]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y. New perspective on natural plant protein-based nanocarriers for bioactive ingredients delivery. Foods 2022, 11, 1701. [Google Scholar] [CrossRef]
- Magri, A.; Petriccione, M.; Cerqueira, M.A.; Gutiérrez, T.J. Self-assembled lipids for food applications: A review. Adv. Colloid Interface Sci. 2020, 285, 102279. [Google Scholar] [CrossRef] [PubMed]
- Varma, L.T.; Singh, N.; Gorain, B.; Choudhury, H.; Tambuwala, M.M.; Kesharwani, P.; Shukla, R. Recent advances in self-assembled nanoparticles for drug delivery. Curr. Drug Deliv. 2020, 17, 279–291. [Google Scholar] [CrossRef]
- Tao, J.; Zhu, Q.; Qin, F.; Wang, M.; Chen, J.; Zheng, Z.-P. Preparation of steppogenin and ascorbic acid, vitamin E, butylated hydroxytoluene oil-in-water microemulsions: Characterization, stability, and antibrowning effects for fresh apple juice. Food Chem. 2017, 224, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Osorno, L.L.; Brandley, A.N.; Maldonado, D.E.; Yiantsos, A.; Mosley, R.J.; Byrne, M.E. Review of contemporary self-assembled systems for the controlled delivery of therapeutics in medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef]
- Villa, C.C.; Correa, N.M.; Silber, J.J.; Moyano, F.; Falcone, R.D. Singularities in the physicochemical properties of spontaneous AOT-BHD unilamellar vesicles in comparison with DOPC vesicles. Phys. Chem. Chem. Phys. 2015, 17, 17112–17121. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: Recent advances. Biotechnol. Adv. 2021, 48, 107727. [Google Scholar] [CrossRef]
- Löffler, P.M.; Rabe, A.; Vogel, S. Lipid-modified peptide nucleic acids: Synthesis and application to programmable liposome fusion. Pept. Nucleic Acids Methods Protoc. 2020, 2015, 75–96. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive loaded novel nano-formulations for targeted drug delivery and their therapeutic potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef]
- Kobanenko, M.K.; Tretiakova, D.S.; Shchegravina, E.S.; Antipova, N.V.; Boldyrev, I.A.; Fedorov, A.Y.; Vodovozova, E.L.; Onishchenko, N.R. Liposomal Formulation of a PLA2-Sensitive Phospholipid–Allocolchicinoid Conjugate: Stability and Activity Studies In Vitro. Int. J. Mol. Sci. 2022, 23, 1034. [Google Scholar] [CrossRef]
- Awwad, S.; Angkawinitwong, U. Overview of antibody drug delivery. Pharmaceutics 2018, 10, 83. [Google Scholar] [CrossRef] [Green Version]
- Ruano, M.; Mateos-Maroto, A.; Ortega, F.; Ritacco, H.; Rubio, J.E.; Guzmán, E.; Rubio, R.G. Fabrication of robust capsules by sequential assembly of polyelectrolytes onto charged liposomes. Langmuir 2021, 37, 6189–6200. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911–925. [Google Scholar] [CrossRef] [Green Version]
- Elkhoury, K.; Sanchez-Gonzalez, L.; Lavrador, P.; Rui, A.; Mano, J.F. Gelatin Methacryloyl (GelMA) Nanocomposite Hydrogels Embedding Bioactive Naringin Liposomes. Polymers 2020, 12, 2944. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Mir-Palomo, S.; Díez-Sales, O.; Buso, M.; Sauri, A.R.; Nácher, A. A novel ultradeformable liposomes of Naringin for anti-inflammatory therapy. Colloids Surf. B Biointerfaces 2017, 162, 265. [Google Scholar] [CrossRef]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Yang, F.; Hu, S.; Sheng, X.; Liu, Y. Naringenin loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in hepatic fibrosis. Biomed. Microdevices 2020, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.G.; Shirsat, N.S.; Mishra, A.C.; Waghulde, S.O.; Kale, M.K. A review on chitosan nanoparticles applications in drug delivery. J. Pharm. Phytochem. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Koroleva, M.; Portnaya, I.; Mischenko, E.; Abutbul-Ionita, I.; Kolik-Shmuel, L.; Danino, D. Solid lipid nanoparticles and nanoemulsions with solid shell: Physical and thermal stability. J. Colloid Interface Sci. 2022, 610, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Montoto, S.S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, S.; Chavoshi, H.; Mohammadi, M.; Ghorbani, M.; Sabzichi, M.; Ramezani, F. Naringenin-loaded nano-structured lipid carrier fortifies oxaliplatin-dependent apoptosis in HT-29 cell line. Process Biochem. 2019, 83, 168–175. [Google Scholar] [CrossRef]
- Akpan, E.; Gbenebor, O.; Adeosun, S.; Cletus, O. Solubility, degree of acetylation, and distribution of acetyl groups in chitosan. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–164. [Google Scholar]
- Saheed, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Maurizzi, E.; Bigi, F.; Quartieri, A.; De Leo, R.; Volpelli, L.A.; Pulvirenti, A. The Green Era of Food Packaging: General Considerations and New Trends. Polymers 2022, 14, 4257. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Malathy, S.; Iyer, P.R. Naringin Loaded Chitosan Nanoparticle for Bone Regeneration: A Preliminary in vitro Study. J. Nanomed. Nanotechnol. 2018, 9, 4172. [Google Scholar]
- Mhe, A.; Hsb, C.; Std, D.; Nrk, E.; Nm, F.; Mss, G.; Mshi, J. Peripheral nerve regeneration in rats by chitosan/alginate hydrogel composited with Berberine and Naringin nanoparticles: In vitro and in vivo study. J. Mol. Liq. 2020, 318, 114226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Zhang, M.; Lin, X.; Zheng, X.; Qi, H.; Chen, J.; Zeng, X.; Bai, W.; Xiao, G. Biological Activities and Solubilization Methodologies of Naringin. Foods 2023, 12, 2327. https://doi.org/10.3390/foods12122327
Jiang H, Zhang M, Lin X, Zheng X, Qi H, Chen J, Zeng X, Bai W, Xiao G. Biological Activities and Solubilization Methodologies of Naringin. Foods. 2023; 12(12):2327. https://doi.org/10.3390/foods12122327
Chicago/Turabian StyleJiang, Hao, Mutang Zhang, Xiaoling Lin, Xiaoqing Zheng, Heming Qi, Junping Chen, Xiaofang Zeng, Weidong Bai, and Gengsheng Xiao. 2023. "Biological Activities and Solubilization Methodologies of Naringin" Foods 12, no. 12: 2327. https://doi.org/10.3390/foods12122327
APA StyleJiang, H., Zhang, M., Lin, X., Zheng, X., Qi, H., Chen, J., Zeng, X., Bai, W., & Xiao, G. (2023). Biological Activities and Solubilization Methodologies of Naringin. Foods, 12(12), 2327. https://doi.org/10.3390/foods12122327






