Inhibitory-like Substances Produced by Yeasts Isolated from Andean Blueberries: Prospective Food Antimicrobials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Antimicrobial Activity Assay
2.3. Effect of Medium Composition on Antimicrobial Activity
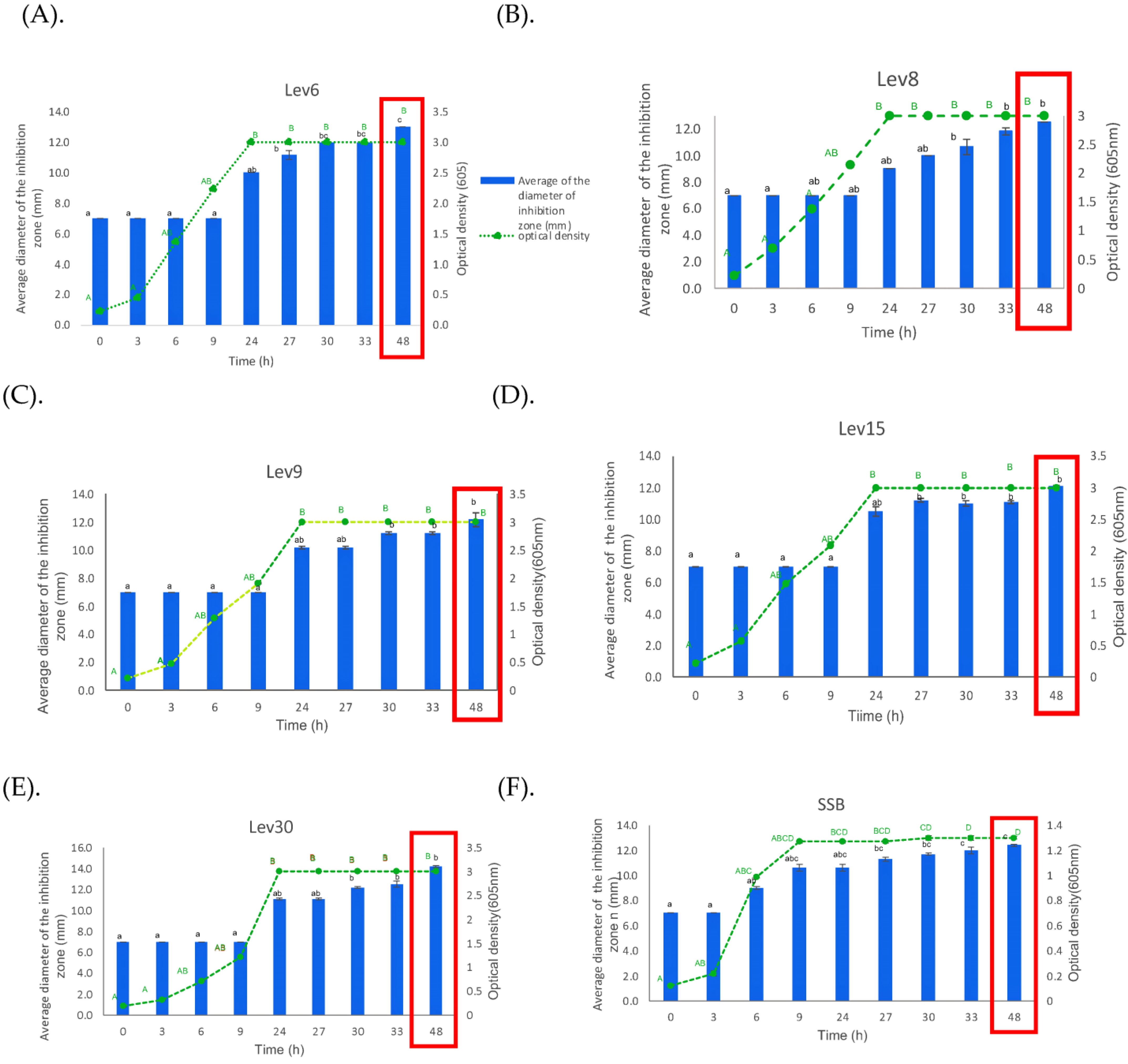
2.4. Yeast-Growth Kinetics and Production of Antimicrobial Substances
2.5. Partial Characterization of Antimicrobial Substances and In Vitro Stability
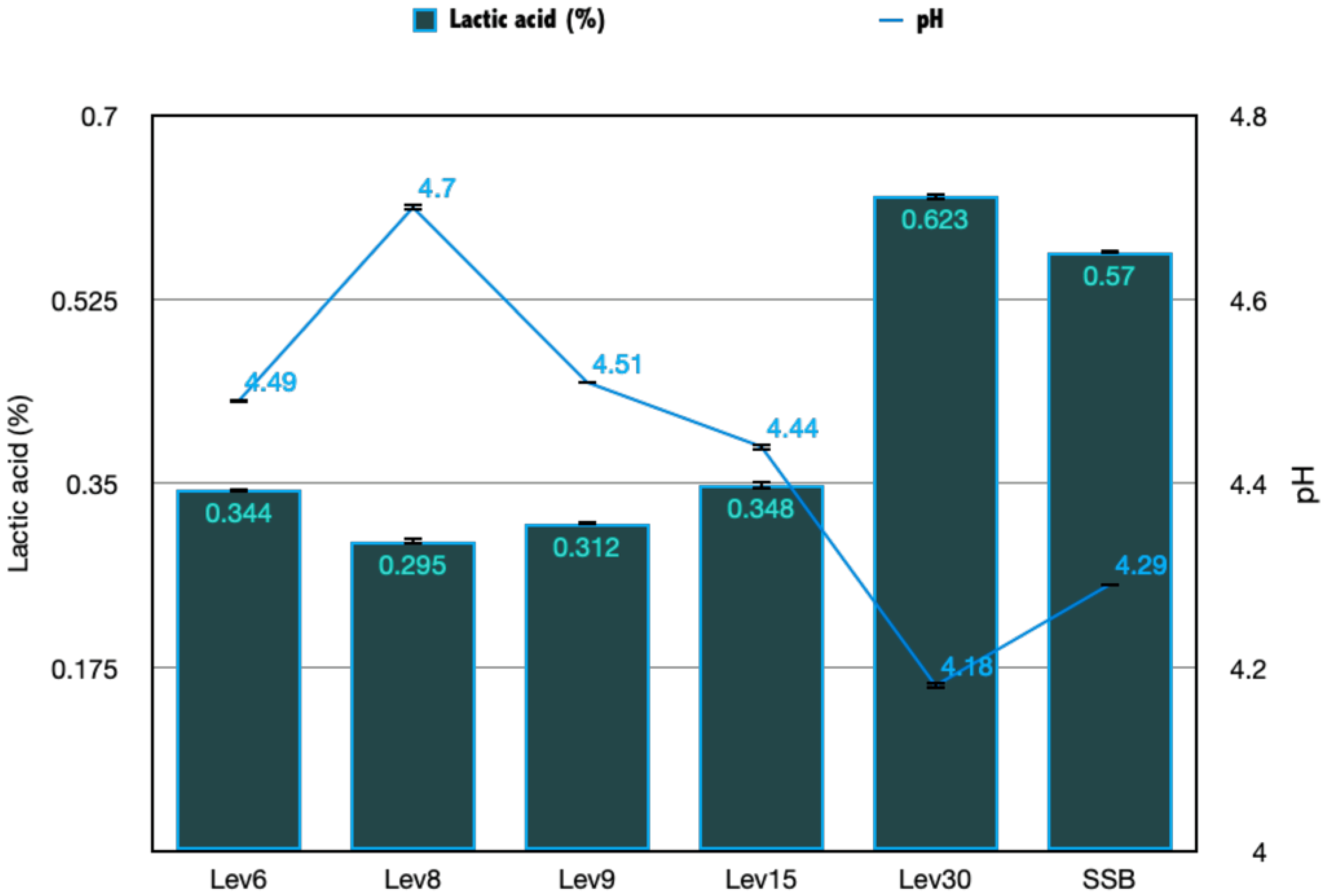
2.5.1. Titrable Acidity and pH
2.5.2. Enzymatic Sensitivity
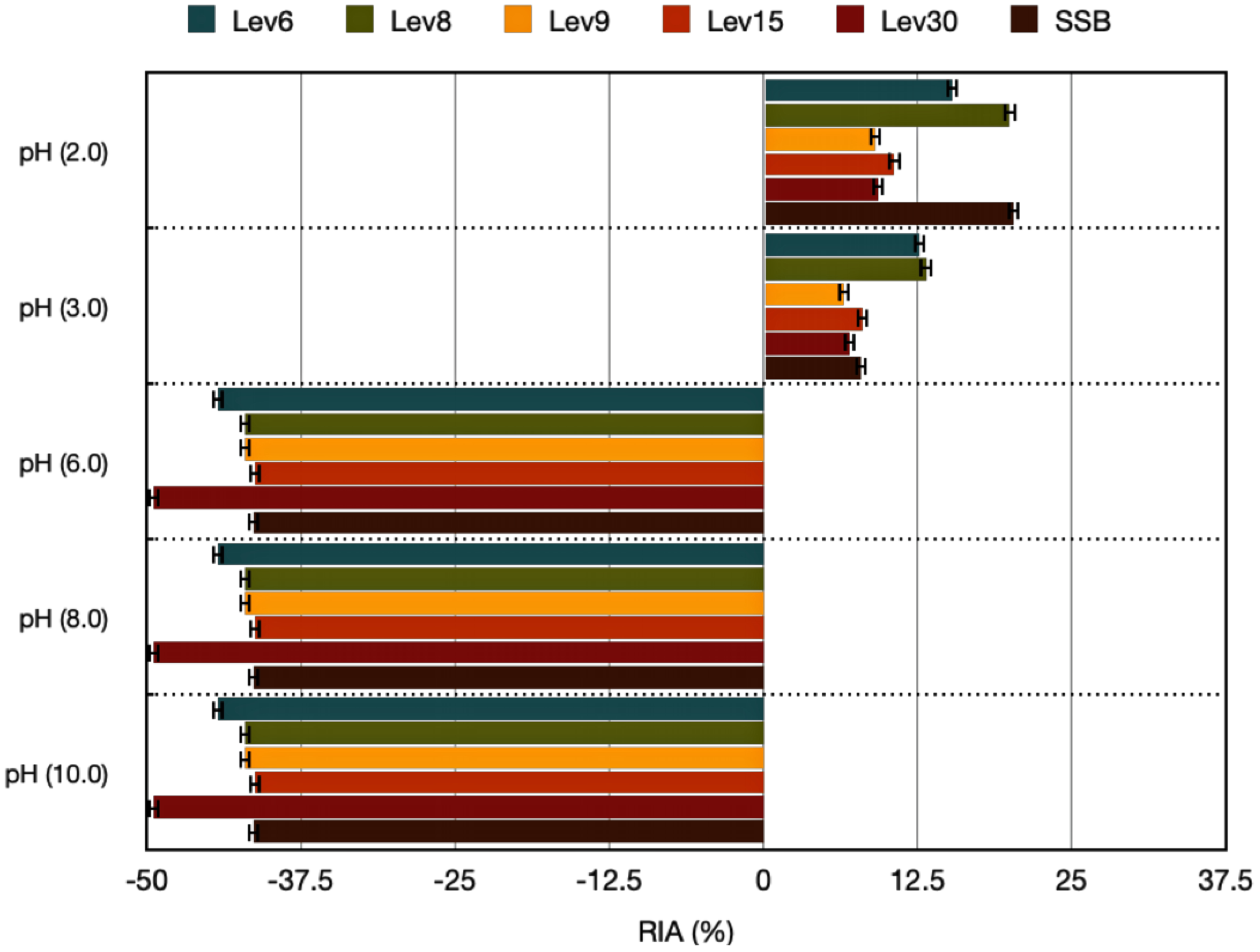
2.5.3. pH, Heat, and Detergent Sensitivity
2.6. Inhibitory Spectrum
2.7. Solvent Concentration and Partial Purification
2.8. Statistical Analysis
3. Results and Discussion
3.1. Medium Composition and Antimicrobial Activity
3.2. Growth Kinetics and Antimicrobial Activity
3.3. Evaluation of Antimicrobial Substances and Partial Characterization
3.3.1. Acidity and pH Evaluation
3.3.2. Enzyme Sensitivity
3.3.3. Effect of pH, Heat, and Detergent Exposure on Antimicrobial Activity
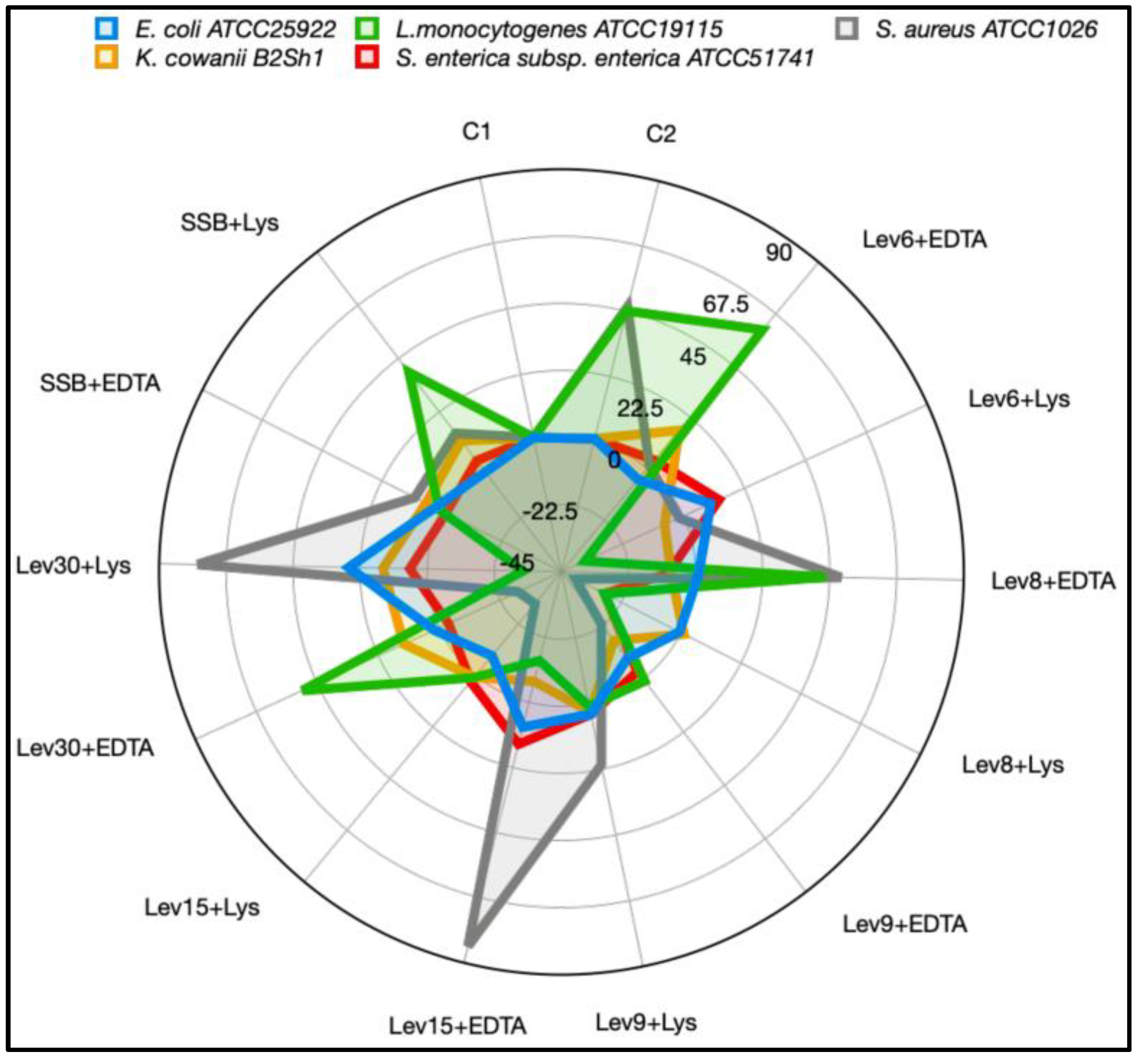
3.4. High Heat, EDTA, and Lys-Enhanced Antimicrobial Action against Some Food Pathogens
3.5. Estimation of Molecular Weight and Antimicrobial Activity of Concentrated and Partially Purified Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acuña-Fontecilla, A.; Silva-Moreno, E.; Ganga, M.A.; Godoy, L. Evaluation of antimicrobial activity from native wine yeast against food industry pathogenic microorganisms. CyTA J. Food 2017, 5, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.L. Natural antibiotic resistance and contamination by antibiotic resistance determinants: The two ages in the evolution of resistance to antimicrobials. Front. Microbiol. 2012, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wu, M.; Qin, X.; Dong, Q.; Li, Z. Antimicrobial function of yeast against pathogenic and spoilage microorganisms via either antagonism or encapsulation: A review. Food Microbiol. 2023, 112, 104242. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef]
- Naimah, A.K.; Al-Manhel, A.J.A.; Al-Shawi, M.J. Isolation, purification and characterization of antimicrobial peptides produced from Saccharomyces boulardii. Int. J. Pept. Res. Ther. 2018, 24, 455–461. [Google Scholar] [CrossRef]
- Peña, R.; Ganga, M.A. Novel antimicrobial peptides produced by Candida intermedia LAMAP1790 active against the wine-spoilage yeast Brettanomyces bruxellensis. Antonie Van Leeuwenhoek 2019, 112, 297–304. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G.; Altemimi, A.B.; Al-Manhel, A.J.A.; Niamah, A.K.; Lakhssassi, N.; Ibrahim, S.A. Purification of Bioactive Peptide with Antimicrobial Properties Produced by Saccharomyces cerevisiae. Food 2020, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Younis, G.; Awad, A.; Dawod, R.E.; Yousef, N.E. Antimicrobial activity of yeasts against some pathogenic bacteria. Vet. World 2017, 10, 979–983. [Google Scholar] [CrossRef] [Green Version]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and Potentially Probiotic Yeasts-Characteristics and Food Application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Muhammad Ali, G.; Elasbali, A.M.; Alharethi, S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wisniewski, M.; Droby, S.; Liu, J. Responses of yeast biocontrol agents to environmental stress. Appl. Environ. Microbiol. 2015, 81, 2968–2975. [Google Scholar] [CrossRef] [Green Version]
- Breinig, F.; Sendzik, T.; Eisfeld, K.; Schmitt, M.J. Dissecting toxin immunity in virus-infected killer yeast uncovers an intrinsic strategy of self-protection. Proc. Natl. Acad. Sci. USA 2006, 103, 3810–3815. [Google Scholar] [CrossRef] [Green Version]
- Rajkowska, K.; Kunicka-Styczyńska, A.; Rygala, A. Probiotic activity of Saccharomyces cerevisiae var. boulardii against human pathogens. Food Technol. Biotechnol. 2012, 50, 230–236. [Google Scholar]
- Cosme, F.; Inês, A.; Vilela, A. Consumer’s acceptability and health consciousness of probiotic and prebiotic of non-dairy products. Food Res. Int. 2022, 151, 110842. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yu, H.H.; Park, Y.J.; Lee, N.; Paik, H. Anti-Biofilm Activity of Cell-Free Supernatant of Saccharomyces cerevisiae against Staphylococcus aureus. J. Microbiol. Biotechnol. 2020, 20, 1854–1861. [Google Scholar] [CrossRef]
- Pereira, P.R.; Freitas, C.S.; Paschoalin, V.M.F. Saccharomyces cerevisiae biomass as a source of next-generation food preservatives: Evaluating potential proteins as a source of antimicrobial peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4450–4479. [Google Scholar] [CrossRef]
- Hernández-Lauzardo, A.N.; Bautista-Baños, S.; Velázquez-Del Valle, M.G.; Hernández-Rodríguez, A. Uso de Microorganismos Antagonistas en el Control de Enfermedades Postcosecha en Frutos. Rev. Mex. De Fitopatol. 2007, 25, 66–74. [Google Scholar]
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis-Opportunism Versus Pathogenicity: A Review of the Virulence Traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef]
- Garg, M.; Priyanka Chatterjee, M. Isolation, characterization and antibacterial effect of biosurfactant from Candida parapsilosis. Biotechnol. Rep. 2018, 18, e00251. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Guo, H.; Guo, X.; Zhu, Y.; Dong, K. Bactericidal activity against meticillin-resistant Staphylococcus aureus of a novel eukaryotic therapeutic recombinant antimicrobial peptide. Int. J. Antimicrob. Agents 2012, 39, 496–499. [Google Scholar] [CrossRef]
- Shruthi, B.; Deepa, N.; Somashekaraiah, R.; Adithi, G.; Divyashree, S.; Sreenivasa, M.Y. Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnol. Rep. 2022, 34, e00716. [Google Scholar] [CrossRef]
- Tenea, G.N.; Veintimilla, F. Potential Use of Native Yeasts to Produce Bioethanol and Other Byproducts from Black Sugarcane, an Alternative to Increment the Subsistence Farming in Northern Ecuador. Sustainability 2021, 13, 10924. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 17th ed.; Association of Official Analytical Chemistry: Washington, DC, USA, 2003; p. 24. [Google Scholar]
- EFSA. Scientific opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed. EFSA J. 2012, 10, 3020. [Google Scholar] [CrossRef] [Green Version]
- Canché-Collí, C.; Barahona, F.; Medina-Medina, L.A.; Canto, A. The effect of sugar concentration on yeast growth associated with floral nectar and honey. Sci. Fungorum 2021, 52, e1288. [Google Scholar] [CrossRef]
- Czerucka, D.; Rampal, P. Diversity of Saccharomyces boulardii CNCM I-745 mechanisms of action against intestinal infections. World J. Gastroenterol. 2019, 25, 2188–2203. [Google Scholar] [CrossRef]
- Sen, S.; Mansell, T.J. Yeasts as probiotics: Mechanisms, outcomes, and future potential. Fungal Genet. Biol. 2020, 137, 103333. [Google Scholar] [CrossRef]
- Fadahunsi, I.F.; Olubodun, S. Antagonistic pattern of yeast species against some selected food-borne pathogens. Bull. Natl. Res. Cent. 2021, 45, 34. [Google Scholar] [CrossRef]
- Olivares-Marin, I.K.; González-Hernández, J.C.; Regalado-Gonzalez, C.; Madrigal-Perez, L.A. Saccharomyces cerevisiae Exponential Growth Kinetics in Batch Culture to Analyze Respiratory and Fermentative Metabolism. J. Vis. Exp. JoVE 2018, 139, 58192. [Google Scholar] [CrossRef] [Green Version]
- Male, O.; Nesvadba, H. Studies on proteolytic activities of a series of yeasts by means of aminoacid-beta-naphthylamidesubstrates. Antonie Van Leeuwenhoek 1969, 35, I27–I28. [Google Scholar]
- El-Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Ultrastructural and Cytochemical Aspects of the Biological Control of Botrytis cinerea by Candida saitoana in Apple Fruit. Phytopathology 1998, 88, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Wen, P.; Yang, H.; Li, N.; Lou, W.Y.; Zong, M.H.; Wu, H. Enhancement of the antimicrobial activity of cinnamon essential oil-loaded electrospun nanofilm by the incorporation of lysozyme. RSC Adv. 2017, 7, 1572–1580. [Google Scholar] [CrossRef] [Green Version]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef] [Green Version]
- Vilcacundo, R.; Méndez, P.; Reyes, W.; Romero, H.; Pinto, A.; Carrillo, W. Antibacterial activity of hen egg white lysozyme denatured by thermal and chemical treatments. Sci. Pharm. 2018, 86, 48. [Google Scholar] [CrossRef] [Green Version]
- Davidson, P.M.; Taylor, T.M.; David, J.R. Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Khorshidian, N.; Khanniri, E.; Koushki, M.R.; Sohrabvandi, S.; Yousefi, M. An Overview of Antimicrobial Activity of Lysozyme and Its Functionality in Cheese. Front. Nutr. 2022, 9, 833618. [Google Scholar] [CrossRef]
- Hiramoto, S.; Itoh, K.; Shizuuchi, S.; Kawachi, Y.; Morishita, Y.; Nagase, M.; Suzuki, Y.; Nobuta, Y.; Sudou, Y.; Nakamura, O.; et al. Melanoidin, a food protein-derived advanced Maillard reaction product, suppresses Helicobacter pylori in vitro and in vivo. Helicobacter 2004, 9, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Garzon, K.; Ortega, C.; Tenea, G.N. Characterization of bacteriocin-producing lactic acid bacteria isolated from native fruits of Ecuadorian Amazon. Pol. J. Microbiol. 2017, 66, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatoum, R.; Labrie, S.; Fliss, I. Antimicrobial and probiotic properties of yeasts: From fundamental to novel applications. Front. Microbiol. 2012, 3, 421. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.H.; Pan, T.M. Characterization of an antimicrobial substance produced by Lactobacillus plantarum NTU 102. J. Microbiol. Immunol. Infect. 2019, 52, 409–417. [Google Scholar] [CrossRef]
- Kukuminato, S.; Koyama, K.; Koseki, S. Antibacterial properties of melanoidins produced from various combinations of Maillard reaction against pathogenic bacteria. Microbiol. Spectr. 2021, 9, e0114221. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Zou, H.; Liu, Y.; Ruan, R. Effects of microwave heating on the protein structure, digestion properties and Maillard products of gluten. J. Food Sci. Technol. 2020, 57, 2139–2149. [Google Scholar] [CrossRef]
- Goktas, H.; Dertli, E.; Sagdic, O. Comparison of functional characteristics of distinct Saccharomyces boulardii strains isolated from commercial food supplements. LWT 2021, 136, 110340. [Google Scholar] [CrossRef]
- Kim, S.W.; Kang, S.I.; Shin, D.H.; Oh, S.Y.; Lee, C.W.; Yang, Y.; Son, Y.K.; Yang, H.S.; Lee, B.H.; An, H.J.; et al. Potential of Cell-Free Supernatant from Lactobacillus plantarum NIBR97, Including Novel Bacteriocins, as a Natural Alternative to Chemical Disinfectants. Pharmaceuticals 2020, 13, 266. [Google Scholar] [CrossRef]
- Hatoum, R.; Labrie, S.; Fliss, I. Identification and Partial Characterization of Antilisterial Compounds Produced by Dairy Yeasts. Probiotics Antimicrob. Proteins 2013, 5, 8–17. [Google Scholar] [CrossRef]
- Finnegan, S.; Percival, S.L. EDTA: An Antimicrobial and Antibiofilm Agent for Use in Wound Care. Adv. Wound Care 2015, 4, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Roostita, L.B.; Fleet, G.H.; Wendry, S.P.; Apon, Z.M.; Gemilang, L.U. Determination of yeasts antimicrobial activity in milk and meat products. Adv. J. Food Sci. Technol. 2011, 3, 442–445. [Google Scholar]
- Chial, H.J.; Splittgerber, A.G. A comparison of the binding of Coomassie brilliant blue to proteins at low and neutral pH. Anal. Biochem. 1993, 213, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Mehlomakulu, N.N.; Prior, K.J.; Setati, M.E.; Divol, B. Candida pyralidae killer toxin disrupts the cell wall of Brettanomyces bruxellensis in red grape juice. J. Appl. Microbiol. 2017, 122, 747–758. [Google Scholar] [CrossRef]
- Santos, M.F.D.S.; Freitas, C.S.; Verissimo da Costa, G.C.; Pereira, P.R.; Paschoalin, V.M.F. Identification of Antibacterial Peptide Candidates Encrypted in Stress-Related and Metabolic Saccharomyces cerevisiae Proteins. Pharmaceuticals 2022, 15, 163. [Google Scholar] [CrossRef] [PubMed]

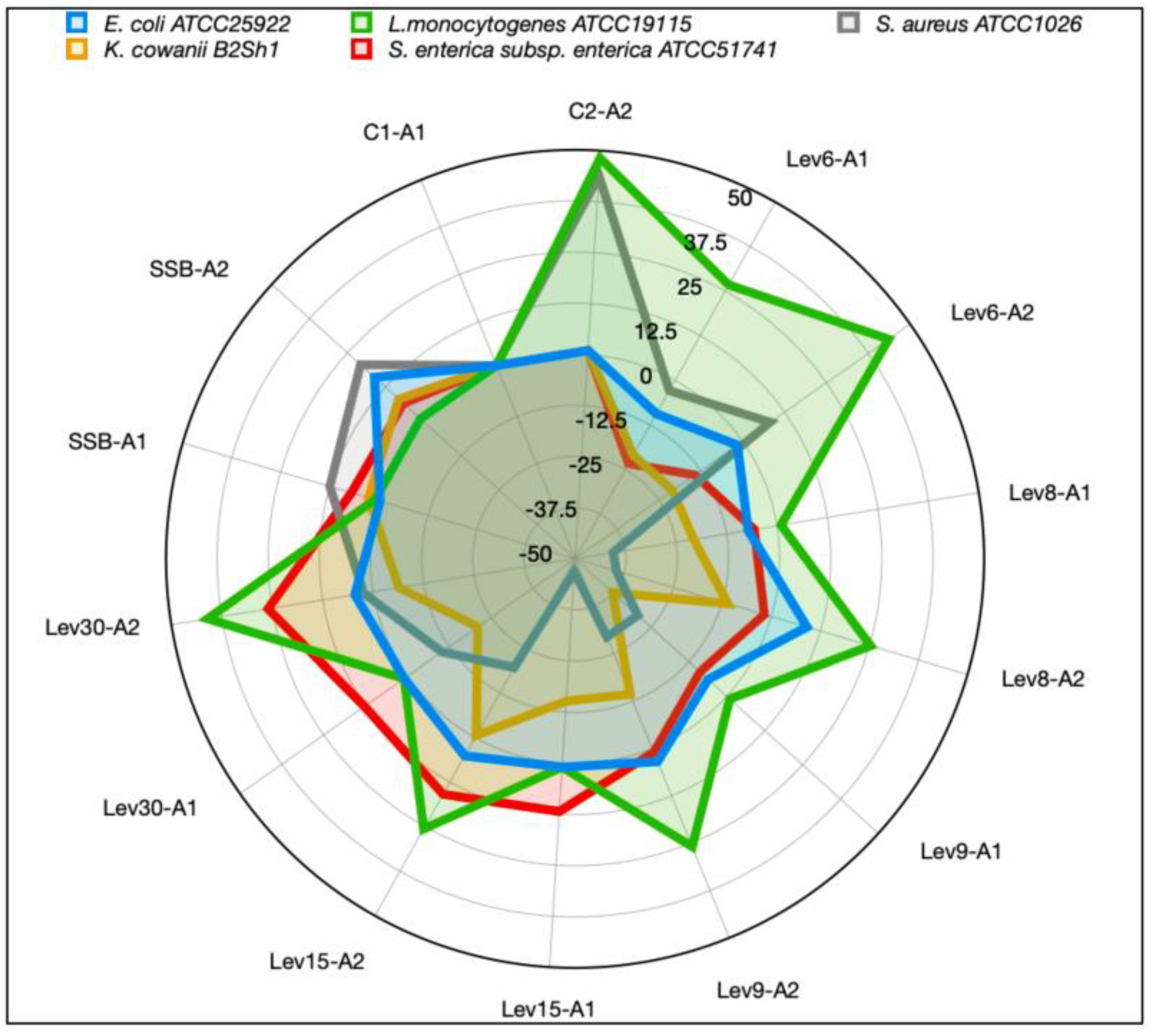

| Strain | Treatment | E. coli ATCC25922 | L. monocytogenes ATCC19115 | S. aureus ATCC1026 | K. cowanii B2Sh1 | S. enterica subsp. enterica ATCC51741 |
|---|---|---|---|---|---|---|
| Lev6 | CFS (RT) | 12.83 ± 0.20 e | 7.02 ± 0.10 c | 9.02 ± 0.02 g | 10.67 ± 0.45 f | 11.01 ± 0.02 f |
| CFS (120 °C, 60 min) | 13.70 ± 0.29 cd | 7.02 ± 0.10 c | 12.50 ± 0.29 de | 11.67 ± 0.29 e | 12.10 ± 0.29 e | |
| PPm (120 °C, 60 min) | 13.80 ± 0.29 cd | 7.02 ± 0.10 c | 13.33 ± 0.29 d | 13.33 ± 0.29 cd | 13.80 ± 0.29 d | |
| Lev8 | CFS (RT) | 12.33 ± 0.29 ef | 7.02 ± 0.10 c | 12.01 ± 0.29 e | 11.51 ± 0.29 ef | 12.67 ± 0.45 de |
| CFS (120 °C, 60 min) | 13.51 ± 0.29 cd | 7.02 ± 0.10 c | 11.01 ± 0.29 ef | 12.51 ± 0.29 d | 13.51 ± 0.29 d | |
| PPm (120 °C, 60 min) | 14.81 ± 0.29 c | 7.02 ± 0.10 c | 14.81 ± 0.29 bc | 10.81 ± 0.29 f | 16.51 ± 0.29 b | |
| Lev9 | CFS (RT) | 12.17 ± 0.29 f | 7.02 ± 0.10 c | 11.83 ± 0.23 ef | 12.67 ± 0.25 d | 11.67 ± 0.15 ef |
| CFS (120 °C, 60 min) | 13.50 ± 0.20 cd | 7.02 ± 0.10 c | 11.01 ± 0.30 ef | 12.33 ± 0.29 de | 13.51 ± 0.29 d | |
| PPm (120 °C, 60 min) | 13.50 ± 0.20 cd | 7.02 ± 0.10 c | 14.81 ± 0.30 bc | 15.33 ± 0.29 b | 16.51 ± 0.29 b | |
| Lev15 | CFS (RT) | 12.16 ± 0.20 f | 7.02 ± 0.10 c | 12.83 ± 0.36 d | 11.02 ± 0.10 d | 11.01 ± 0.01 f |
| CFS (120 °C, 60 min) | 13.16 ± 0.15 d | 7.02 ± 0.10 c | 12.16 ± 0.29 de | 13.33 ± 0.51 cd | 12.66 ± 0.45 de | |
| PPm (120 °C, 60 min) | 13.16 ± 0.15 d | 7.02 ± 0.10 c | 14.31 ± 0.29 c | 13.22 ± 0.51 cd | 15.51 ± 0.28 c | |
| Lev30 | CFS (RT) | 14.17 ± 0.29 c | 7.02 ± 0.10 c | 12.33 ± 0.58 de | 13.67 ± 0.29 c | 11.33 ± 0.58 ef |
| CFS (120 °C, 60 min) | 15.67 ± 0.29 b | 7.02 ± 0.10 c | 12.83 ± 0.28 de | 12.01 ± 0.30 de | 17.66 ± 0.29 a | |
| PPm (120 °C, 60 min) | 15.67 ± 0.29 b | 8.02 ± 0.10 c | 16.21 ± 0.28 b | 15.21 ± 0.28 b | 16.66 ± 0.29 b | |
| SSB | CFS (RT) | 12.67 ± 0.17 ef | 7.02 ± 0.10 c | 10.61 ± 0.30 f | 11.64 ± 0.25 e | 11.33 ± 0.10 ef |
| CFS (120 °C, 60 min) | 13.01 ± 0.17 e | 11.02 ± 0.10 a | 12.51 ± 0.28 e | 11.21 ± 0.29 ef | 13.51 ± 0.28 e | |
| PPm (120 °C, 60 min) | 18.01 ± 0.29 a | 9.71 ± 0.29 b | 19.01 ± 0.28 a | 16.51 ± 0.17 a | 17.21 ± 0.17 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenea, G.N.; Anrango Cajas, B.; Carlosama Sanchez, B. Inhibitory-like Substances Produced by Yeasts Isolated from Andean Blueberries: Prospective Food Antimicrobials. Foods 2023, 12, 2435. https://doi.org/10.3390/foods12132435
Tenea GN, Anrango Cajas B, Carlosama Sanchez B. Inhibitory-like Substances Produced by Yeasts Isolated from Andean Blueberries: Prospective Food Antimicrobials. Foods. 2023; 12(13):2435. https://doi.org/10.3390/foods12132435
Chicago/Turabian StyleTenea, Gabriela N., Blanca Anrango Cajas, and Bladimir Carlosama Sanchez. 2023. "Inhibitory-like Substances Produced by Yeasts Isolated from Andean Blueberries: Prospective Food Antimicrobials" Foods 12, no. 13: 2435. https://doi.org/10.3390/foods12132435
APA StyleTenea, G. N., Anrango Cajas, B., & Carlosama Sanchez, B. (2023). Inhibitory-like Substances Produced by Yeasts Isolated from Andean Blueberries: Prospective Food Antimicrobials. Foods, 12(13), 2435. https://doi.org/10.3390/foods12132435






