Isolation and Characterization of Yeast with Benzenemethanethiol Synthesis Ability Isolated from Baijiu Daqu
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Instruments and Equipment
2.3. Sample Collection
2.4. Yeast Isolation and Identification
2.4.1. Polymerase Chain Reaction Amplification of 5.8S rDNA
2.4.2. Purification and Sequencing of PCR Product
2.5. Fermentation Conditions
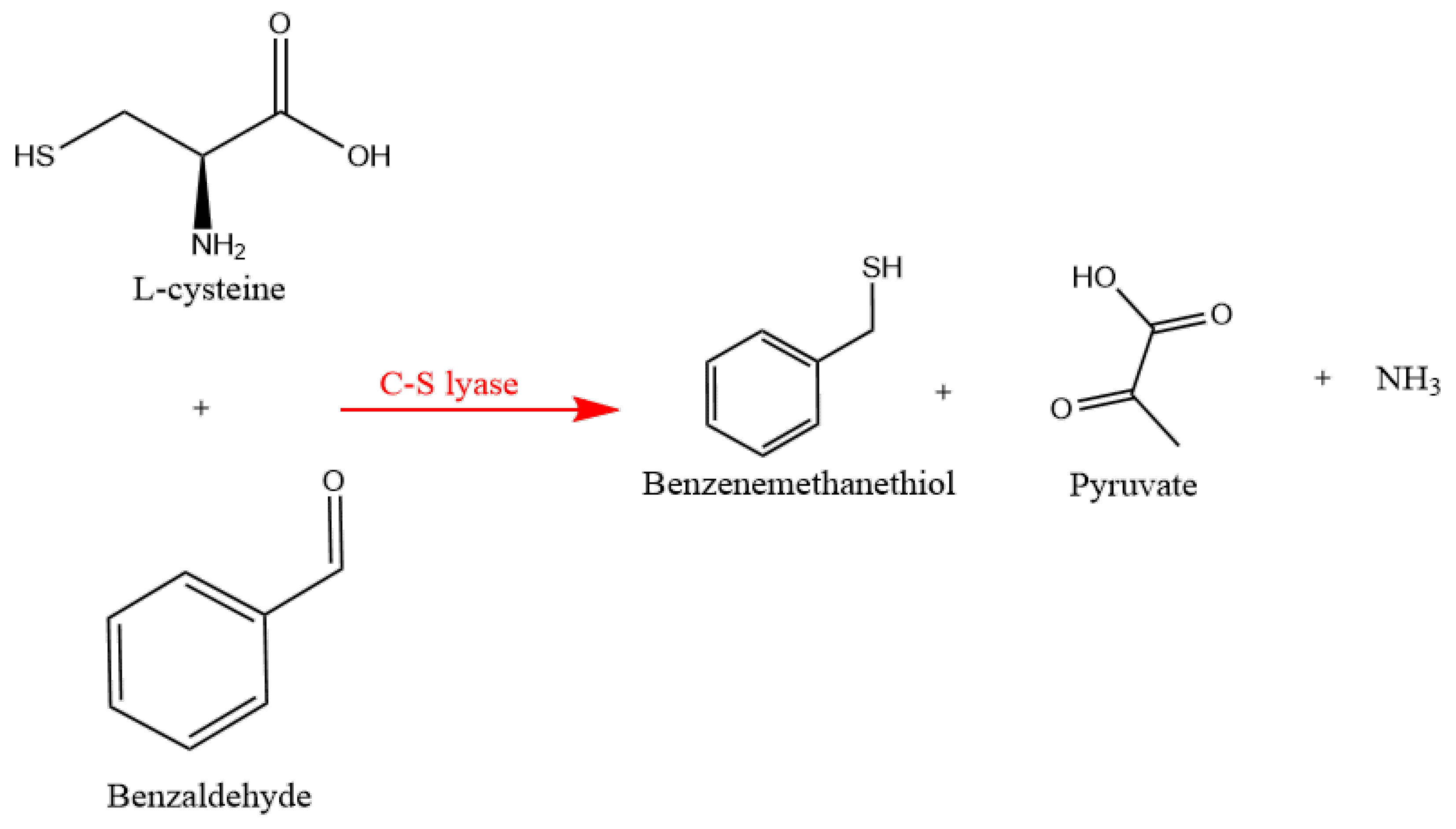
2.6. Quantitation of Benzenemethanethiol in the Fermentation Broth
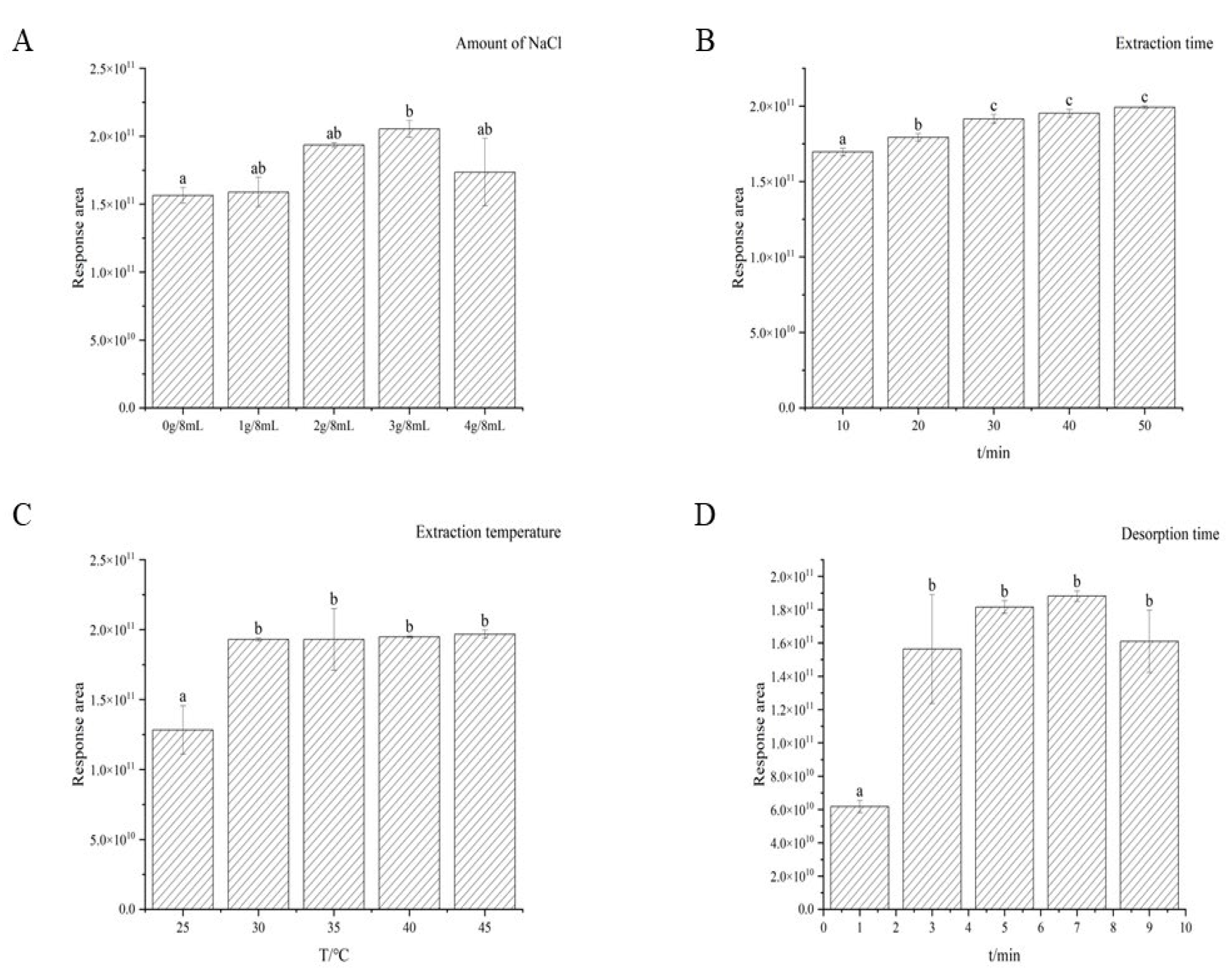
2.6.1. Optimization of Extraction Condition
2.6.2. HS-SPME Parameter Optimization
2.6.3. GC-SCD Analysis
2.7. Optimization of Benzenemethanethiol Production Conditions
3. Results and Discussions
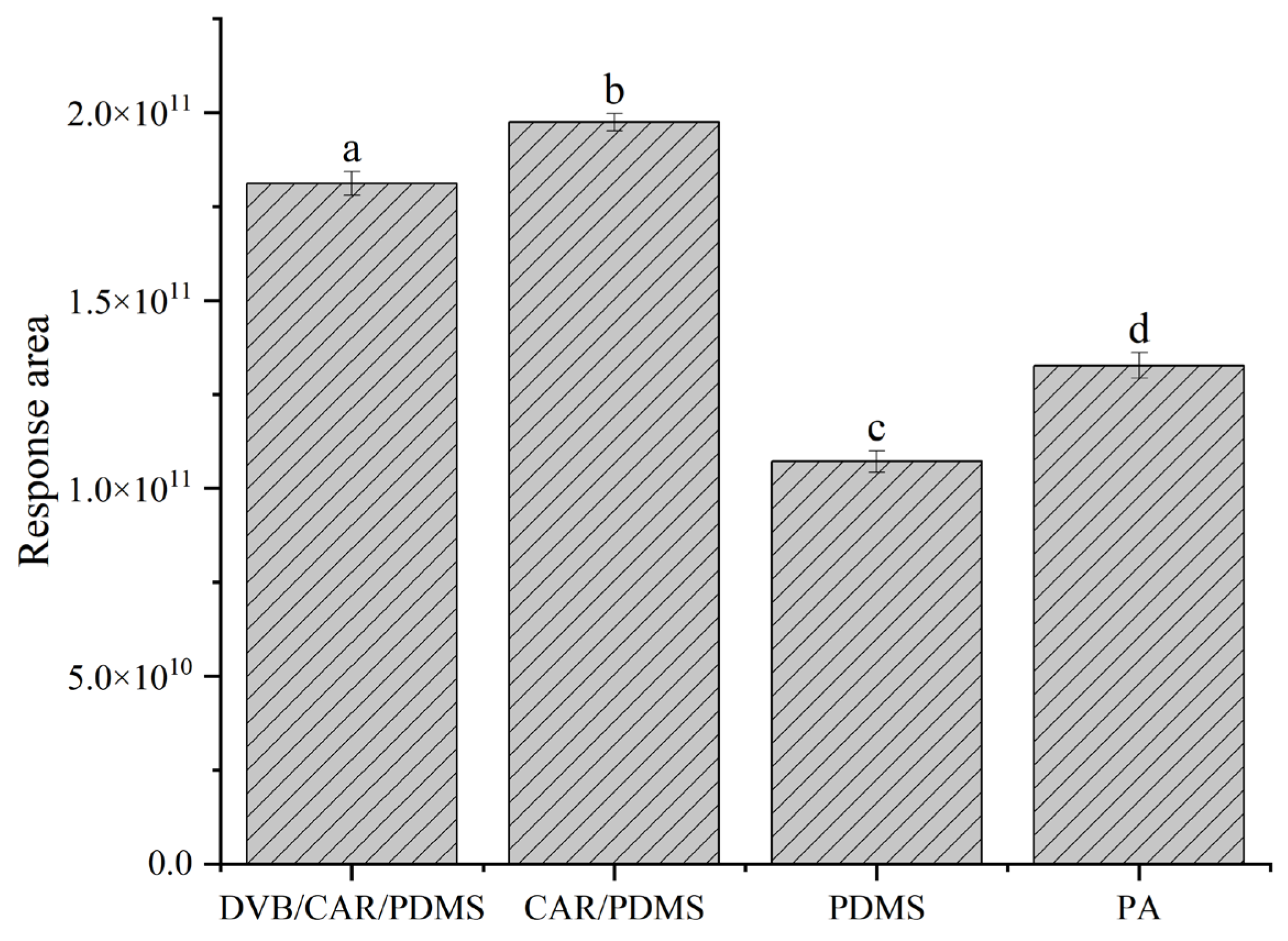
3.1. Optimization of HS-SPME Extraction Conditions
3.2. Determination of Benzenemethanethiol Biosynthesis in Yeast Isolates
3.3. Identification of High Carbon–Sulfur-Lyase-Producing Strains
3.3.1. Electrophoresis of PCR Product
3.3.2. Construction of the Evolutionary Tree
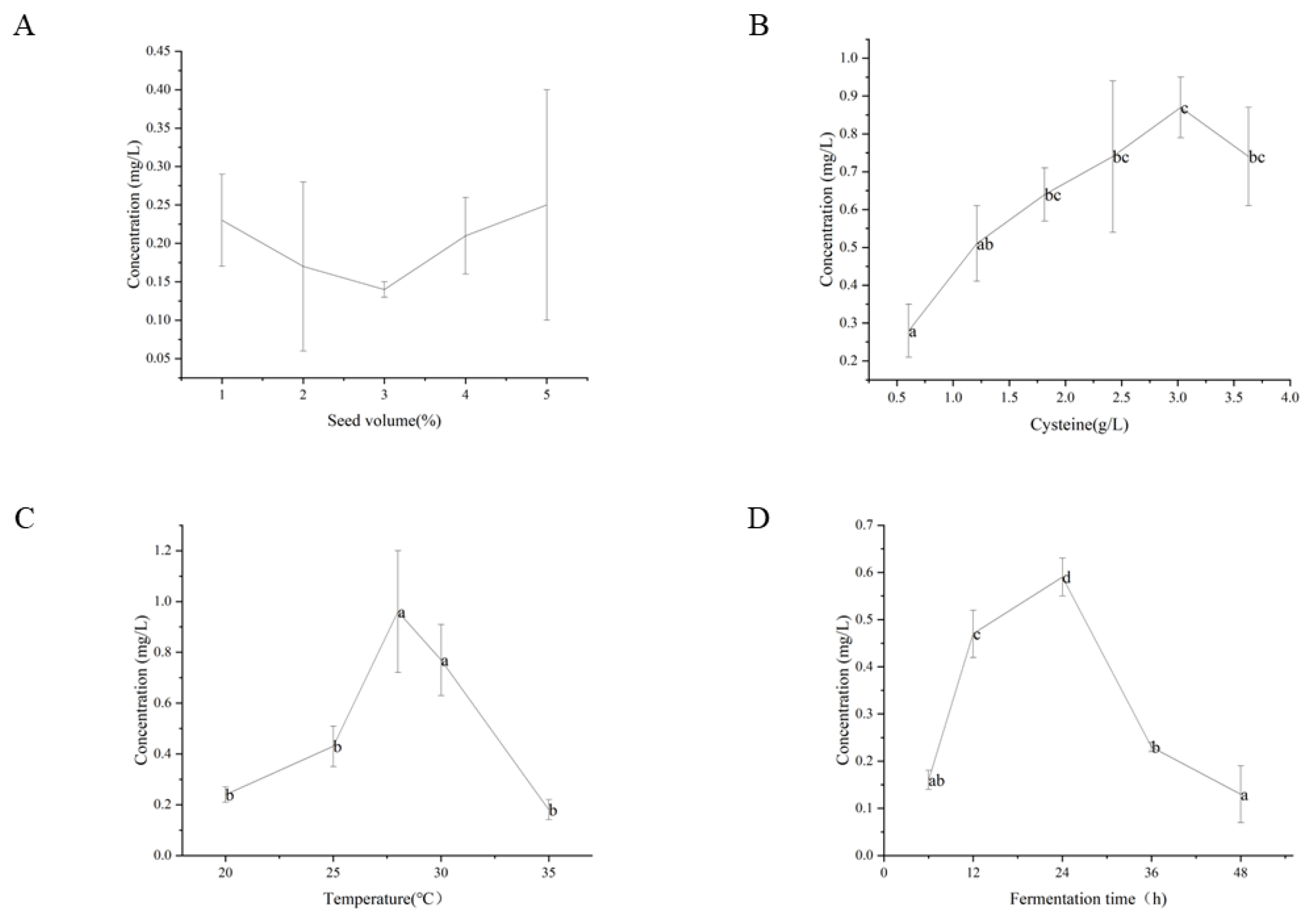
3.4. Optimization of Fermentation Condition
3.4.1. Effect of Seed Volume
3.4.2. Effect of L-Cysteine Supplementation Amount
3.4.3. Effect of Fermentation Temperature
3.4.4. Effect of Fermentation Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, H.; Lu, H.; Wu, M.; Lin, M.; Zhang, C.; Zhao, Z.; Li, W.; Zhang, C.; Li, X.; et al. Characterization of an Aspergillus niger for Efficient Fatty Acid Ethyl Ester Synthesis in Aqueous Phase and the Molecular Mechanism. Front. Microbiol. 2022, 12, 820380. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Shan, Y.; Wang, J.; Sun, B.; Xu, Y.; Zhang, W.; Zhang, Y. Recent trends in aroma release and perception during food oral processing: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, Y.; Chen, H.; Wang, J.; Zhang, C.; Zhao, Z.; Ao, R.; Huang, H.; Hong, J.; Zhao, D.; et al. “Key Factor” for Baijiu Quality: Research Progress on Acid Substances in Baijiu. Foods 2022, 11, 2959. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, M.; Niu, J.; Huang, H.; Nie, Z.; Fu, Z.; Zhang, C.; Zhao, Z.; Lu, H.; Li, X.; et al. Clostridium btbubcensis BJN0001, a potentially new species isolated from the cellar mud of Chinese strong-flavor baijiu, produces ethanol, acetic acid and butyric acid from glucose. 3 Biotech 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, M.; Niu, J.; Lin, M.; Zhu, H.; Wang, K.; Li, X.; Sun, B. Characteristics and correlation of the microbial communities and flavor compounds during the first three rounds of fermentation in Chinese Sauce-Flavor Baijiu. Foods 2023, 12, 207. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Sun, B. Low Quantity but Critical Contribution to Flavor: Review of the current understanding of volatile Sulfur-containing Compounds in Baijiu. J. Food Compos. Anal. 2021, 103, 104079. [Google Scholar] [CrossRef]
- Zhu, L.; Song, X.; Li, X.; Geng, X.; Zheng, F.; Li, H.; Sun, J.; Huang, M.; Sun, B. Interactions between kafirin and pickle-like odorants in soy sauce flavor Baijiu: Aroma profile change and binding mechanism. Food Chem. 2023, 400, 133854. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, G.; Xu, L.; Duan, J.; Li, H.; Sun, J.; Sun, B. Investigation on the interaction between 1,3-dimethyltrisulfide and aroma-active compounds in sesame-flavor baijiu by Feller Additive Model, Odor Activity Value and Partition Coefficient. Food Chem. 2023, 410, 135451. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, J.; Nie, Y.; Li, C.; Chen, S.; Xu, Y. Characterization of volatile thiols in Chinese liquor (Baijiu) by ultraperformance liquid chromatography–mass spectrometry and ultraperformance liquid chromatography-quadrupole-time-of-flight mass spectrometry. Front. Nutr. 2022, 9, 1022600. [Google Scholar] [CrossRef]
- Li, H.; Qin, D.; Wu, Z.; Sun, B.; Sun, X.; Huang, M.; Sun, J.; Zheng, F. Characterization of key aroma compounds in Chinese Guojing sesame-flavor Baijiu by means of molecular sensory science. Food Chem. 2019, 284, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, L.; Wang, X.; Zheng, F.; Zhao, M.; Liu, Y.; Li, H.; Zhang, F.; Zhang, Y.; Chen, F. Characterization of key aroma-active sulfur-containing compounds in Chinese Laobaigan Baijiu by gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography coupled with sulfur chemiluminescence detection. Food Chem. 2019, 297, 124959. [Google Scholar] [CrossRef]
- Wu, Z.; Qin, D.; Duan, J.; Li, H.; Sun, J.; Huang, M.; Sun, B. Characterization of benzenemethanethiol in sesame-flavour baijiu by high-performance liquid chromatography-mass spectrometry and sensory science. Food Chem. 2021, 364, 130345. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, Y.; Wu, J.; Yu, B.; Zhao, H.; Huang, M.; Zheng, F. Sensory-directed decoding of key aroma compounds from Jiugui-series Baijiu, the representative of Fuyu-flavor-type Baijiu (FFTB). J. Food Compos. Anal. 2022, 114, 104799. [Google Scholar] [CrossRef]
- Huynh-Ba, T.; Matthey-Doret, W.; Fay, L.B.; Bel Rhlid, R. Generation of Thiols by biotransformation of Cysteine-Aldehyde Conjugates with Baker’s Yeast. J. Agric. Food Chem. 2003, 51, 3629–3635. [Google Scholar] [CrossRef] [PubMed]
- Neiers, F.; Gourrat, K.; Canon, F.; Schwartz, M. Metabolism of Cysteine Conjugates and production of flavor sulfur compounds by a carbon–sulfur lyase from the oral anaerobe fusobacterium nucleatum. J. Agric. Food Chem. 2022, 70, 9969–9979. [Google Scholar] [CrossRef]
- Rudden, M.; Herman, R.; Rose, M.; Bawdon, D.; Cox, D.S.; Dodson, E.; Holden, M.T.G.; Wilkinson, A.J.; James, A.G.; Thomas, G.H. The molecular basis of thioalcohol production in human body odour. Sci. Rep. 2020, 10, 12500. [Google Scholar] [CrossRef]
- Tominaga, T.; Guimbertau, G.; Dubourdieu, D. Contribution of Benzenemethanethiol to Smoky Aroma of CertainVitis vinifera L. Wines. J. Agric. Food Chem. 2003, 51, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Sun, B.; Yin, S.; Mehmood, A.; Cheng, L.; Wang, C. Generation of 2-Furfurylthiol by carbon–sulfur lyase from the Baijiu Yeast Saccharomyces cerevisiae G20. J. Agric. Food Chem. 2018, 66, 2114–2120. [Google Scholar] [CrossRef]
- Zha, M.; Yin, S.; Sun, B.; Wang, X.; Wang, C. STR3 and CYS3 Contribute to 2-Furfurylthiol biosynthesis in Chinese Sesame-Flavored Baijiu Yeast. J. Agric. Food Chem. 2017, 65, 5503–5511. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, W.; Xu, Y. Chameleon-like microbes promote microecological differentiation of Daqu. Food Microbiol. 2023, 109, 104144. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sha, S.; Qian, M.; Xu, Y. Characterization of volatile sulfur compounds in Moutai Liquors by Headspace Solid-Phase Microextraction Gas Chromatography-Pulsed Flame Photometric Detection and Odor Activity Value. J. Food Sci. 2017, 82, 2816–2822. [Google Scholar] [CrossRef] [PubMed]
- Spietelun, A.; Kloskowski, A.; Chrzanowski, W.; Namieśnik, J. Understanding Solid-Phase Microextraction: Key factors influencing the extraction process and trends in improving the technique. Chem. Rev. 2013, 113, 1667–1685. [Google Scholar] [CrossRef]
- Qin, D.; Duan, J.; Li, H.; Zheng, F.; Cheng, H.; Ye, X.; Sun, B. Characterization and comparison of the aroma-active compounds on different grades of sesame-flavor Baijiu by headspace solid-phase microextraction and gas chromatography-olfactometry-mass spectrometry. Food Sci. Human Wellness 2023, 12, 79–88. [Google Scholar] [CrossRef]
- Sun, Z.; Cui, H.; Yang, N.; Ayed, C.; Zhang, X.; D Fisk, I. Enhancement of coffee brew aroma through control of the aroma staling pathway of 2-furfurylthiol. Food Chem. 2020, 322, 126754. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Hui, T.; Fang, F.; Zhang, D. New insight into the formation mechanism of 2-furfurylthiol in the glucose-cysteine reaction with ribose. Food Res. Int. 2021, 143, 110295. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Natera, R.; Durán, E.; García-Barroso, C. Application of solid phase extraction techniques to analyse volatile compounds in wines and other enological products. Eur. Food Res. Technol. 2008, 228, 1–18. [Google Scholar] [CrossRef]
- Blank, I.; Pascual, E.C.; Devaud, S.; Fay, L.B.; Stadler, R.H.; Yeretzian, C.; Goodman, B.A. Degradation of the Coffee flavor compound furfuryl mercaptan in model Fenton-type reaction systems. J. Agric. Food Chem. 2002, 50, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Czerny, M.; Grosch, W.E. Sensory study of the character impact aroma compounds of a coffee beverage. Eur. Food Res. Technol. 2000, 211, 272–276. [Google Scholar] [CrossRef]
- Shi, W.; Chai, L.; Fang, G.; Mei, J.; Lu, Z.; Zhang, X.; Xiao, C.; Wang, S.; Shen, C.; Shi, J.; et al. Spatial heterogeneity of the microbiome and metabolome profiles of high-temperature Daqu in the same workshop. Food Res. Int. 2022, 156, 111298. [Google Scholar] [CrossRef]
- Yang, L.; Xian, C.; Li, P.; Wang, X.; Song, D.; Zhao, L.; Zhang, C. The spatio-temporal diversity and succession of microbial community and its environment driving factors during stacking fermentation of Maotai-flavor baijiu. Food Res. Int. 2023, 169, 112892. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, M.; Zhao, D.; Zheng, J.; Dai, M.; Li, X.; Li, W.; Zhang, C.; Sun, B. Simulated fermentation of strong-flavor Baijiu through functional microbial combination to realize the stable synthesis of important flavor chemicals. Foods 2023, 12, 644. [Google Scholar] [CrossRef]
- Miao, Z.; Hao, H.; Yan, R.; Wang, X.; Wang, B.; Sun, J.; Li, Z.; Zhang, Y.; Sun, B. Individualization of Chinese alcoholic beverages: Feasibility towards a regulation of organic acids. LWT Food Sci. Technol. 2022, 172, 114168. [Google Scholar] [CrossRef]
- Yang, L.; Fan, W.; Xu, Y. GC × GC-TOF/MS and UPLC-Q-TOF/MS based untargeted metabolomics coupled with physicochemical properties to reveal the characteristics of different type daqus for making soy sauce aroma and flavor type baijiu. LWT Food Sci. Technol. 2021, 146, 111416. [Google Scholar] [CrossRef]
- Du, R.; Liu, J.; Jiang, J.; Wang, Y.; Ji, X.; Yang, N.; Wu, Q.; Xu, Y. Construction of a synthetic microbial community for the biosynthesis of volatile sulfur compound by multi-module division of labor. Food Chem. 2021, 347, 129036. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Q.; Wang, P.; Lin, J.; Huang, L.; Xu, Y. Synergistic Effect in core microbiota associated with sulfur metabolism in spontaneous Chinese Liquor fermentation. Appl. Environ. Microbiol. 2017, 83, e1417–e1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, M.; Zimmer, A.; Thibon, C.; Marullo, P. Enhancement of volatile thiol release of Saccharomyces cerevisiae strains using molecular breeding. Appl. Microbiol. Biotechnol. 2013, 97, 5893–5905. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Gibney, P.; Cheng, L.; Liu, S.; Peck, G. Yeast assimilable nitrogen concentrations influence yeast gene expression and hydrogen sulfide production during cider fermentation. Front. Microbiol. 2020, 11, 1264. [Google Scholar] [CrossRef]
- Shen, T.; Liu, J.; Wu, Q.; Xu, Y. Increasing 2-furfurylthiol content in Chinese sesame-flavored Baijiu via inoculating the producer of precursor l-cysteine in Baijiu fermentation. Food Res. Int. 2020, 138, 109757. [Google Scholar] [CrossRef]
- Geffroy, O.; Morère, M.; Lopez, R.; Pasquier, G.; Condoret, J. Investigating the aroma of Syrah wines from the northern Rhone valley using supercritical CO2-Dearomatized Wine as a matrix for reconstitution studies. J. Agric. Food Chem. 2020, 68, 11512–11523. [Google Scholar] [CrossRef] [PubMed]
- Münch, P.; Hofmann, T.; Schieberle, P. Comparison of key odorants generated by thermal treatment of commercial and self-prepared yeast extracts: Influence of the amino acid composition on odorant formation. J. Agric. Food Chem. 1997, 45, 1338–1344. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, X.; Xiao, W.; Shi, J.; Xu, Z. Production of L-serine and its derivative L-cysteine from renewable feedstocks using Corynebacterium glutamicum: Advances and perspectives. Crit. Rev. Biotechnol. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Elyasi Far, B.; Dilmaghani, A.; Yari Khosroushahi, A. In Silico Study and Optimization of Bacillus megaterium alpha-Amylases production obtained from Honey Sources. Curr. Microbiol. 2020, 77, 2593–2601. [Google Scholar] [CrossRef] [PubMed]

| Instrument | Manufacturer |
|---|---|
| PCR instrument (MG96+) | LongGene Scientific Instruments, Inc. (Hangzhou, China) |
| Gel imager (JY04S-3C) | Junyi Electrophoresis Co., Ltd. (Beijing, China) |
| JY300C ligand electrophoresis system | Junyi Electrophoresis Co., Ltd. (Beijing, China) |
| Medical centrifuge (H1650-W) | Hunan Xiangyi Co., Ltd. (Hunan, China) |
| 3730XL sequencer (625-0020) | Applied Biosystems (Waltham, MA, USA) |
| Reactant | Volume |
|---|---|
| 10 × Ex Taq buffer | 5.0 μL |
| 2.5 mM dNTP Mix | 4.0 μL |
| 10p Primer 1 | 2.0 μL |
| 10p Primer 2 | 2.0 μL |
| 5u Ex Taq | 0.5 μL |
| Template | 2.0 μL |
| ddH2O | 34.5 μL |
| Total volume | 50 μL |
| Temperature (°C) | Time | Cycles | |
|---|---|---|---|
| Initial denaturation | 94 | 3 min | 1 |
| Denaturation | 94 | 30 s | 24 |
| Primer annealing | 54 | 30 s | |
| Primer extension | 72 | 1 min 30 s | |
| Final extension | 72 | 10 min | 1 |
| Name | Concentration of BM (mg/L) | Name | Concentration of BM (mg/L) |
|---|---|---|---|
| J2 a | 0.12 ± 0.04 | J14 d | 0.50 ± 0.07 |
| J5 bcd | 0.34 ± 0.16 | J15 bcd | 0.33 ± 0.04 |
| J6 abc | 0.27 ± 0.11 | J19 a | 0.13 ± 0.04 |
| J9 bcd | 0.33 ± 0.04 | J21 a | 0.11 ± 0.02 |
| J10 ab | 0.21 ± 0.06 | J23 cd | 0.43 ± 0.12 |
| J13 cd | 0.41 ± 0.08 | J27 ab | 0.20 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Xiao, P.; Xu, Y.; Li, H.; Li, H.; Sun, J.; Sun, B. Isolation and Characterization of Yeast with Benzenemethanethiol Synthesis Ability Isolated from Baijiu Daqu. Foods 2023, 12, 2464. https://doi.org/10.3390/foods12132464
Zhang G, Xiao P, Xu Y, Li H, Li H, Sun J, Sun B. Isolation and Characterization of Yeast with Benzenemethanethiol Synthesis Ability Isolated from Baijiu Daqu. Foods. 2023; 12(13):2464. https://doi.org/10.3390/foods12132464
Chicago/Turabian StyleZhang, Guihu, Peng Xiao, Youqiang Xu, Honghua Li, Hehe Li, Jinyuan Sun, and Baoguo Sun. 2023. "Isolation and Characterization of Yeast with Benzenemethanethiol Synthesis Ability Isolated from Baijiu Daqu" Foods 12, no. 13: 2464. https://doi.org/10.3390/foods12132464






