Influence of a Commercial Synbiotic Administered In Ovo and In-Water on Broiler Chicken Performance and Meat Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Birds and Experimental Design
2.3. Slaughter Surveys and Physico-Chemical Analyses
2.4. Total Lipids and Fatty Acid Profile
2.5. Statistical Analyses
3. Results and Discussion
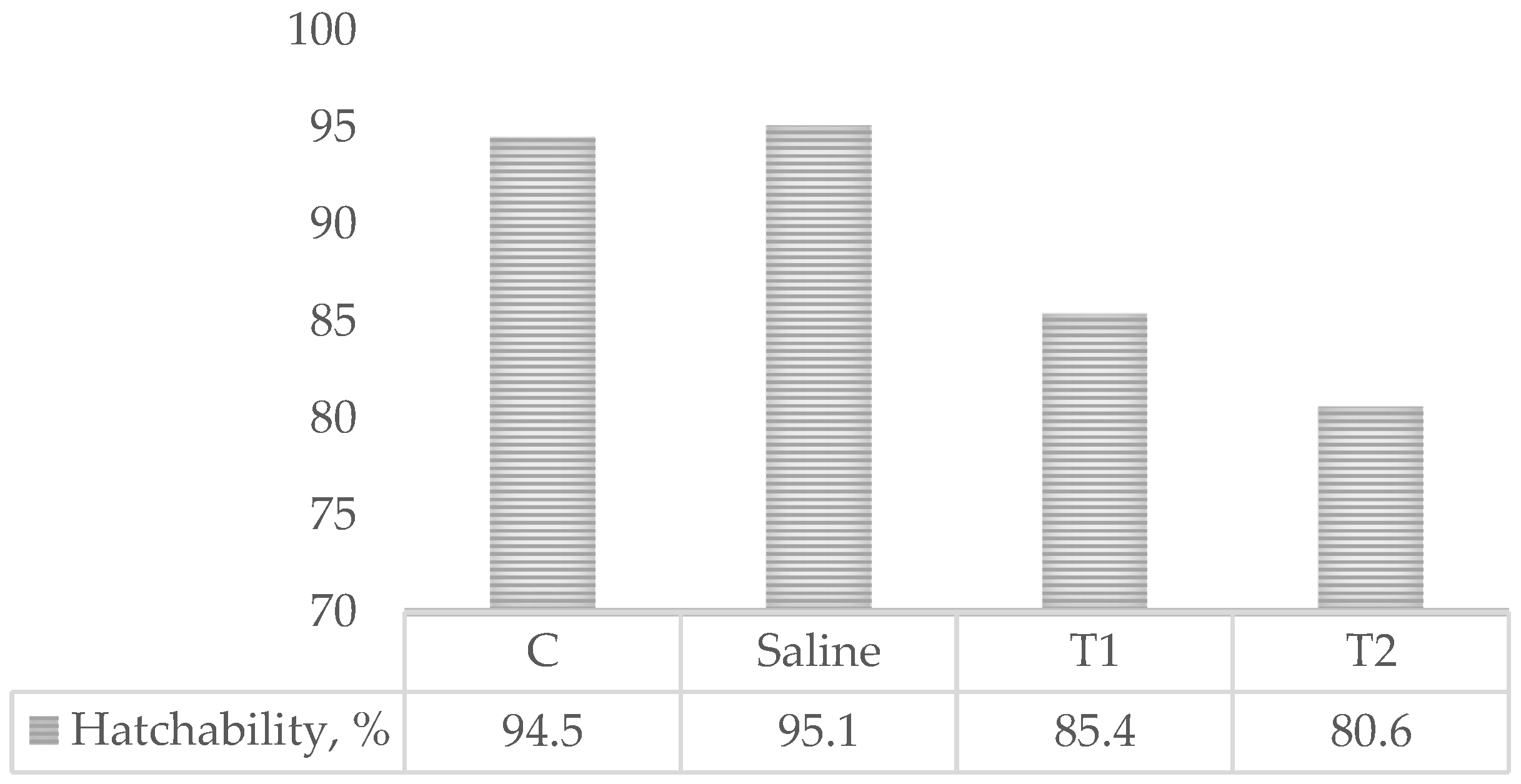
3.1. Hatchability
3.2. Growth Performance
3.3. Carcass Traits and Physico-Chemical Properties of Breast Muscle
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Leão, A.P.A.; Alvarenga, R.R.; Zangeronimo, M.G. In ovo inoculation of probiotics for broiler chickens: Systematic review and meta-analysis. Anim. Feed Sci. Technol. 2021, 280, 115080. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Urbanowski, M.; Gulewicz, P.; Kasperczyk, K.; Maiorano, G.; Szwaczkowski, T. Field and in vitro study on prebiotic effect of raffinose family oligosaccharides in chickens. Bull. Vet. Inst. Pulawy 2011, 55, 465–469. [Google Scholar]
- Bednarczyk, M.; Stadnicka, K.; Kozłowska, I.; Abiuso, C.; Tavaniello, S.; Dankowiakowska, A.; Sławińska, A.; Maiorano, G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal 2016, 10, 1271–1279. [Google Scholar] [CrossRef] [Green Version]
- Dunislawska, A.; Slawinska, A.; Stadnicka, K.; Bednarczyk, M.; Gulewicz, P.; Jozefiak, D.; Siwek, M. Synbiotics for broiler chickens—In vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS ONE 2017, 12, e0168587. [Google Scholar] [CrossRef] [Green Version]
- Siwek, M.; Slawinska, A.; Stadnicka, K.; Bogucka, J.; Dunislawska, A.; Bednarczyk, M. Prebiotics and synbiotics—In ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018, 14, 402. [Google Scholar] [CrossRef]
- El-Moneim, A.E.M.E.A.; El-Wardany, I.; Abu-Taleb, A.M.; Wakwak, M.M.; Ebeid, T.A.; Saleh, A.A. Assessment of in ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal histomorphometry, blood hematological, and biochemical parameters of broilers. Probiotics Antimicrob. Proteins 2020, 12, 439–450. [Google Scholar] [CrossRef]
- Sobolewska, A.; Bogucka, J.; Dankowiakowska, A.; Elminowska-Wenda, G.; Stadnicka, K.; Bednarczyk, M. The impact of synbiotic administration through in ovo technology on the microstructure of a broiler chicken small intestine tissue on the 1st and 42nd day of rearing. J. Anim. Sci. Biotechnol. 2017, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Slawinska, A.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Bertocchi, M.; Tavaniello, S.; Maiorano, G. Impact of galactooligosaccharides delivered in ovo on mitigating negative effects of heat stress on performance and welfare of broilers. Poult. Sci. 2020, 99, 407–415. [Google Scholar] [CrossRef]
- Maiorano, G.; Sobolewska, A.; Cianciullo, D.; Walasik, K.; Elminowska-Wenda, G.; Sławińska, A.; Tavaniello, S.; Zylinska, J.; Bardowski, J.; Bednarczyk, M. Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 2012, 91, 2963–2969. [Google Scholar] [CrossRef]
- Bogucka, J.; Dankowiakowska, A.; Elminowska-Wenda, G.; Sobolewska, A.; Szczerba, A.; Bednarczyk, M. Effects of prebiotics and synbiotics delivered in ovo on broiler small intestine histomorphology during the first days after hatching. Folia Biol. 2016, 64, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Dankowiakowska, A.; Bogucka, J.; Sobolewska, A.; Tavaniello, S.; Maiorano, G.; Bednarczyk, M. Effects of in ovo injection of prebiotics and synbiotics on the productive performance and microstructural features of the superficial pectoral muscle in broiler chickens. Poult. Sci. 2019, 98, 5157–5165. [Google Scholar] [CrossRef]
- Tavaniello, S.; Mucci, R.; Stadnicka, K.; Acaye, O.; Bednarczyk, M.; Maiorano, G. Effect of in ovo administration of different synbiotics on carcass and meat quality traits in broiler chickens. Poult. Sci. 2019, 98, 464–472. [Google Scholar] [CrossRef]
- Tavaniello, S.; Slawinska, A.; Prioriello, D.; Petrecca, V.; Bertocchi, M.; Zampiga, M.; Salvatori, G.; Maiorano, G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020, 99, 612–619. [Google Scholar] [CrossRef]
- Bogucka, J.; Dankowiakowska, A.; Stanek, M.; Stadnicka, K.; Kirkiłło-Stacewicz, K. Effect of synbiotics administered in ovo on microvascularization and histopathological changes in pectoral muscle and the biochemical profile of broiler chicken blood. Poult. Sci. 2022, 101, 101628. [Google Scholar] [CrossRef]
- Madej, J.P.; Bednarczyk, M. Effect of in ovo-delivered prebiotics and synbiotics on the morphology and specific immune cell composition in the gut-associated lymphoid tissue. Poult. Sci. 2016, 95, 19–29. [Google Scholar] [CrossRef]
- Stefaniak, T.; Madej, J.P.; Graczyk, S.; Siwek, M.; Łukaszewicz, E.; Kowalczyk, A.; Sieńczyk, M.; Bednarczyk, M. Selected prebiotics and synbiotics administered in ovo can modify innate immunity in chicken broilers. BMC Vet. Res. 2019, 15, 105. [Google Scholar] [CrossRef]
- Pruszynska-Oszmalek, E.; Kolodziejski, P.A.; Stadnicka, K.; Sassek, M.; Chalupka, D.; Kuston, B.; Nogowski, L.; Mackowiak, P.; Maiorano, G.; Jankowski, J.; et al. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 2015, 94, 1909–1916. [Google Scholar] [CrossRef]
- Stadnicka, K.; Bogucka, J.; Stanek, M.; Graczyk, R.; Krajewski, K.; Maiorano, G.; Bednarczyk, M. Injection of raffinose family oligosaccharides at 12 days of egg incubation modulates the gut development and resistance to opportunistic pathogens in broiler chickens. Animals 2020, 10, 592. [Google Scholar] [CrossRef] [Green Version]
- Płowiec, A.; Sławińska, A.; Siwek, M.Z.; Bednarczyk, M.F. Effect of in ovo administration of inulin and Lactococcus lactis on immune-related gene expression in broiler chickens. Am. J. Vet. Res. 2015, 76, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Slawinska, A.; Mendes, S.; Dunislawska, A.; Siwek, M.; Zampiga, M.; Meluzzi, A.; Sirri, F.; Tavaniello, S.; Maiorano, G. Avian model to mitigate gut-derived immune response and oxidative stress during heat. Biosystems 2019, 178, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Dunislawska, A.; Herosimczyk, A.; Ozgo, M.; Lepczynski, A.; Ciechanowicz, A.K.; Bednarczyk, M.; Siwek, M. Proteome changes upon in ovo stimulation with Lactobacillus synbiotic in chicken liver. Poult. Sci. 2021, 100, 101449. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Yan, F.F.; Hu, J.Y.; Cheng, H.W.; Kim, Y.H.B. Effects of probiotics feeding on meat quality of chicken breast during postmortem storage. Poult. Sci. 2016, 95, 1457–1464. [Google Scholar] [CrossRef]
- Abdulhasan, S.D. Effect of Digestrom® and Poultry Star® on the body performance and immunity status of broiler chickens. Int. J. Poult. Sci. 2018, 17, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Shanmugasundaram, R.; Mortada, M.; Cosby, D.E.; Singh, M.; Applegate, T.J.; Syed, B.; Pender, C.M.; Curry, S.; Murugesan, G.R.; Selvaraj, R.K. Synbiotic supplementation to decrease Salmonella colonization in the intestine and carcass contamination in broiler birds. PLoS ONE 2019, 14, e0223577. [Google Scholar] [CrossRef]
- Prentza, Z.; Castellone, F.; Legnardi, M.; Antlinger, B.; Segura-Wang, M.; Kefalas, G.; Papaioannou, N.; Stylianaki, I.; Papatsiros, V.G.; Franzo, G.; et al. Administration of a multi-genus synbiotic to broilers: Effects on gut health, microbial composition and performance. Animals 2023, 13, 113. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Triplett, M.D.; Zhai, W.; Peebles, E.D.; McDaniel, C.D.; Kiess, A.S. Investigating commercial in ovo technology as a strategy for introducing probiotic bacteria to broiler embryos. Poult. Sci. 2018, 97, 658–666. [Google Scholar] [CrossRef]
- Stefaniak, T.; Madej, J.P.; Graczyk, S.; Siwek, M.; Lukaszewicz, E.; Kowalczyk, A.; Sienczyk, M.; Maiorano, G.; Bednarczyk, M. Impact of prebiotics and synbiotics administered in ovo on the immune response against experimental antigens in chicken broilers. Animals 2020, 10, 643. [Google Scholar] [CrossRef] [Green Version]
- Duan, A.Y.; Ju, A.Q.; Zhang, Y.N.; Qin, Y.J.; Xue, L.G.; Ma, X.; Luan, W.M.; Yang, S.B. The effects of in ovo injection of synbiotics on the early growth performance and intestinal health of chicks. Front. Vet. Sci. 2021, 8, 658301. [Google Scholar] [CrossRef]
- Calik, A.; Ceylan, A.; Ekim, B.; Adabi, S.G.; Dilber, F.; Bayraktaroglu, A.G.; Tekinay, T.; Özen, D.; Sacakli, P. The effect of intra-amniotic and posthatch dietary synbiotic administration on the performance, intestinal histomorphology, cecal microbial population, and short-chain fatty acid composition of broiler chickens. Poult. Sci. 2017, 96, 169–183. [Google Scholar] [CrossRef]
- Falaki, M.; Shargh, M.S.; Dastar, B.; Zerehdaran, S. Effects of different levels of probiotic and prebiotic on performance and carcass characteristics of broiler chickens. J. Anim. Vet. Adv. 2011, 9, 2390–2395. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Chen, Y.; Li, X.; Yang, W.; Wen, C.; Kang, Y.; Wang, A.; Zhou, Y. Effects of synbiotic supplementation on growth performance, carcass characteristics, meat quality and muscular antioxidant capacity and mineral contents in broilers. J. Sci. Food Agric. 2017, 97, 3699–3705. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Jacobs, J.A.; Murugesan, G.R.; Cheng, H.W. Effect of dietary synbiotic supplement on behavioral patterns and growth performance of broiler chickens reared under heat stress. Poult. Sci. 2018, 97, 1101–1108. [Google Scholar] [CrossRef]
- Luoma, A.; Markazi, A.; Shanmugasundaram, R.; Murugesan, G.R.; Mohnl, M.; Selvaraj, R. Effect of synbiotic supplementation on layer production and cecal Salmonella load during a Salmonella challenge. Poult. Sci. 2017, 96, 4208–4216. [Google Scholar] [CrossRef]
- Brugaletta, G.; De Cesare, A.; Zampiga, M.; Laghi, L.; Oliveri, C.; Zhu, C.; Manfreda, G.; Syed, B.; Valenzuela, L.; Sirri, F. Effects of alternative administration programs of a synbiotic supplement on broiler performance, foot pad dermatitis, caecal microbiota, and blood metabolites. Animals 2020, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Szczypka, M.; Suszko-Pawłowska, A.; Kuczkowski, M.; Gorczykowski, M.; Lis, M.; Kowalczyk, A.; Łukaszewicz, E.; Poradowski, D.; Zbyryt, I.; Bednarczyk, M.; et al. Effects of Selected Prebiotics or Synbiotics Administered in ovo on Lymphocyte Subsets in Bursa of the Fabricius, Thymus, and Spleen in Non-Immunized and Immunized Chicken Broilers. Animals 2021, 11, 476. [Google Scholar] [CrossRef]
- Fornazier, R.; Junior, V.R.; Albino, L.F.; Rodrigues, D.; Tavernari, F.D.; da Silva, D.L.; Rostagno, H.S.; Serafini, S. A symbiotic improves performance and carcass yield of broilers. J. Anim. Physiol. Anim. Nutr. 2019, 103, 41–52. [Google Scholar] [CrossRef]
- Barbut, S. Problem of pale soft exudative meat in broiler chickens. Br. Poult. Sci. 1997, 38, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Barbut, S. Rheological characteristics of fresh and frozen PSE, normal and DFD chicken breast meat. Br. Poult. Sci. 2005, 46, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, Y.; Chen, Y.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Effects of dietary synbiotic supplementation on growth performance, lipid metabolism, antioxidant status, and meat quality in Partridge shank chickens. J. Appl. Anim. Res. 2019, 47, 586–590. [Google Scholar] [CrossRef] [Green Version]
- Dev, K.; Begum, J.; Biswas, A.; Mir, N.A.; Singh, J.; Prakash, R.; Sonowal, J.; Bharali, K.; Tomar, S.; Kant, R.; et al. Hepatic transcriptome analysis reveals altered lipid metabolism and consequent health indices in chicken supplemented with dietary Bifidobacterium bifidum and mannan-oligosaccharides. Sci. Rep. 2021, 11, 17895. [Google Scholar] [CrossRef]
- Lei, J.; Dong, Y.; Hou, Q.; He, Y.; Lai, Y.; Liao, C.; Kawamura, Y.; Li, J.; Zhang, B. Intestinal microbiota regulate certain meat quality parameters in chicken. Front. Nutr. 2022, 9, 747705. [Google Scholar] [CrossRef]
- Poureslami, R.; Turchini, G.M.; Raes, K.; Huyghebaert, G.; De Smet, S. Effect of diet, sex and age on fatty acid metabolism in broiler chickens: SFA and MUFA. Br. J. Nutr. 2010, 104, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
| Period | |||
|---|---|---|---|
| Starter (0–14 d) | Grower (15–36 d) | Finisher (37–56 d) | |
| Dietary components, kg | |||
| Corn | 42.17 | 34.96 | 12.73 |
| White corn | 0.00 | 0.00 | 15.00 |
| Wheat | 10.00 | 20.00 | 25.01 |
| Sorghum | 0.00 | 0.00 | 5.00 |
| Soybean meal | 23.11 | 20.63 | 17.60 |
| Expanded soybean | 10.00 | 10.00 | 13.00 |
| Sunflower | 3.00 | 3.00 | 3.00 |
| Corn gluten | 4.00 | 3.00 | 0.00 |
| Soybean oil | 3.08 | 4.43 | 5.48 |
| Dicalcium phosphate | 1.52 | 1.20 | 0.57 |
| Calcium carbonate | 0.91 | 0.65 | 0.52 |
| Sodium bicarbonate | 0.15 | 0.10 | 0.15 |
| Salt | 0.27 | 0.27 | 0.25 |
| Choline chloride | 0.10 | 0.10 | 0.10 |
| Lysine sulfate | 0.59 | 0.55 | 0.46 |
| DL-methionine | 0.27 | 0.29 | 0.30 |
| Threonine | 0.15 | 0.14 | 0.14 |
| Enzyme-roxazyme G2g | 0.08 | 0.08 | 0.08 |
| Phytase 0.1% | 0.10 | 0.10 | 0.10 |
| Vitamin–mineral premix a | 0.50 | 0.50 | 0.50 |
| Calculated nutrient content | |||
| Dry matter, % | 88.57 | 88.65 | 88.64 |
| Crude protein, % | 22.70 | 21.49 | 19.74 |
| Lipid, % | 7.06 | 8.24 | 9.74 |
| Fiber, % | 3.08 | 3.04 | 3.07 |
| Ash, % | 5.85 | 5.17 | 4.49 |
| Lysine, % | 1.38 | 1.29 | 1.21 |
| Methionine, % | 0.67 | 0.62 | 0.59 |
| Methionine + cysteine, % | 1.03 | 0.97 | 0.91 |
| Calcium, % | 0.91 | 0.80 | 0.59 |
| Phosphate, % | 0.63 | 0.57 | 0.46 |
| Metabolizable energy, Kcal/kg | 3.076 | 3.168 | 3.264 |
| Group a | ||||||
|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | SEM | p-Value | |
| Body weight, g | no. 120 | no. 120 | no. 120 | no. 120 | ||
| d 0 | 50.81 | 51.21 | 50.97 | 50.68 | 0.17 | 0.799 |
| d 14 | 467.00 | 475.41 | 492.86 | 489.60 | 5.89 | 0.384 |
| d 36 | 2154.78 a | 2002.36 b | 2118.70 ab | 2206.81 a | 25.28 | 0.004 |
| d 56 | 4006.03 | 3977.78 | 4152.78 | 4297.22 | 50.56 | 0.061 |
| Daily weight gain, g/bird/day | ||||||
| d 0–14 | 29.73 | 30.30 | 31.56 | 31.35 | 0.42 | 0.390 |
| d 15–36 | 140.65 a | 127.25 b | 135.49 ab | 143.10 a | 2.00 | 0.002 |
| d 37–56 | 92.56 | 98.77 | 101.70 | 104.52 | 2.17 | 0.131 |
| d 0–56 | 70.63 | 70.12 | 73.25 | 75.83 | 0.90 | 0.060 |
| FCR b | ||||||
| d 0–14 | 1.28 | 1.28 | 1.30 | 1.29 | 0.00 | 0.481 |
| d 15–36 | 1.61 | 1.60 | 1.59 | 1.60 | 0.00 | 0.454 |
| d 37–56 | 2.00 | 1.97 | 2.00 | 1.98 | 0.01 | 0.301 |
| d 0–56 | 1.63 | 1.62 | 1.63 | 1.63 | 0.00 | 0.297 |
| Daily Feed intake, g/bird/day | ||||||
| d 0–14 | 38.15 | 38.89 | 40.91 | 40.46 | 0.56 | 0.217 |
| d 15–36 | 225.79 a | 203.78 b | 215.21 ab | 229.20 a | 3.33 | 0.003 |
| d 37–56 | 185.03 | 194.42 | 203.30 | 207.51 | 4.77 | 0.212 |
| d 0–56 | 115.05 | 113.40 | 119.24 | 123.31 | 1.58 | 0.068 |
| Group a | ||||||
|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | SEM | p-Value | |
| Final body weight, g | 3807.99 | 4108.88 | 4053.86 | 4136.70 | 69.72 | 0.333 |
| Carcass weight, g | 2755.67 | 2983.67 | 2939.17 | 2998.67 | 49.98 | 0.294 |
| Carcass yield, % | 72.39 | 72.62 | 72.53 | 72.49 | 0.07 | 0.695 |
| Breast weight, g | 795.50 | 848.42 | 854.83 | 880.50 | 17.34 | 0.375 |
| Breast yield, % | 28.84 | 28.39 | 29.08 | 29.32 | 0.25 | 0.618 |
| Legs weight, g | 770.17 | 826.67 | 798.00 | 811.17 | 13.60 | 0.521 |
| Legs yield, % | 27.93 | 27.74 | 27.29 | 27.00 | 0.18 | 0.261 |
| Group a | ||||||
|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | SEM | p-Value | |
| pH24 | 5.81 | 5.75 | 5.74 | 5.87 | 0.03 | 0.367 |
| Color 24 h | ||||||
| L * | 53.50 | 54.32 | 52.84 | 54.13 | 0.38 | 0.525 |
| a * | 1.43 | 2.05 | 1.45 | 2.03 | 0.16 | 0.367 |
| b * | 6.93 | 5.93 | 5.46 | 4.63 | 0.30 | 0.121 |
| Group a | ||||||
|---|---|---|---|---|---|---|
| C | T1 | T2 | T3 | SEM | p-Value | |
| Cholesterol, mg/100 g | 63.84 | 61.96 | 61.86 | 59.93 | 1.12 | 0.715 |
| Lipids, % | 1.83 | 1.52 | 1.68 | 1.63 | 0.07 | 0.492 |
| Fatty acids b, % of total fatty acids | ||||||
| C14:0 | 0.45 ab | 0.47 ab | 0.55 a | 0.40 b | 0.02 | 0.009 |
| C16:0 | 24.38 | 23.75 | 25.68 | 25.14 | 0.26 | 0.041 |
| C16:1 | 2.41 ab | 1.90 Bb | 3.09 Aa | 2.17 b | 0.12 | 0.001 |
| C17:0 | 0.04 | 0.04 | 0.03 | 0.04 | 0.00 | 0.098 |
| C17:1 | 0.06 | 0.06 | 0.05 | 0.05 | 0.00 | 0.697 |
| C18:0 | 9.41 ab | 10.29 a | 8.88 ab | 8.27 b | 0.20 | 0.002 |
| C18:1 n-9 | 31.04 | 30.72 | 31.14 | 33.03 | 0.39 | 0.137 |
| C18:2 n-6 | 24.46 | 24.13 | 23.31 | 24.01 | 0.22 | 0.313 |
| C18:3 n-3 | 1.08 | 1.13 | 1.09 | 1.09 | 0.02 | 0.863 |
| C18:3 n-6 | 0.06 | 0.05 | 0.05 | 0.06 | 0.00 | 0.278 |
| C20:0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.00 | 0.736 |
| C20:2 n-6 | 0.34 | 0.42 | 0.33 | 0.30 | 0.02 | 0.043 |
| C20:3 n-6 | 0.32 | 0.39 | 0.28 | 0.33 | 0.02 | 0.116 |
| C20:3 n-3 | 0.02 | 0.02 | 0.01 | 0.01 | 0.00 | 0.039 |
| C20:4 n-6 | 4.26 | 4.64 | 4.01 | 3.56 | 0.20 | 0.281 |
| C20:5 n-3 | 0.10 | 0.10 | 0.11 | 0.12 | 0.00 | 0.604 |
| C22:4 n-6 | 0.88 | 1.07 | 0.80 | 0.74 | 0.05 | 0.065 |
| C22:5 n-3 | 0.44 | 0.51 | 0.38 | 0.46 | 0.02 | 0.295 |
| C22:6 n-3 | 0.24 | 0.26 | 0.19 | 0.20 | 0.01 | 0.204 |
| ƩSFA | 34.30 | 34.59 | 35.16 | 33.87 | 0.28 | 0.425 |
| ƩMUFA | 33.50 | 32.68 | 34.29 | 35.25 | 0.45 | 0.219 |
| ƩPUFA | 32.20 | 32.73 | 30.55 | 30.88 | 0.37 | 0.113 |
| Ʃn-6 PUFA | 30.32 | 30.70 | 28.76 | 29.00 | 0.35 | 0.126 |
| Ʃn-3 PUFA | 1.88 | 2.03 | 1.79 | 1.88 | 0.03 | 0.079 |
| Nutritional indices c | ||||||
| n-6/n-3 | 16.38 | 15.20 | 16.21 | 15.48 | 0.22 | 0.162 |
| PUFA/SFA | 0.94 | 0.95 | 0.87 | 0.92 | 0.01 | 0.204 |
| AI | 0.40 | 0.39 | 0.43 | 0.41 | 0.01 | 0.106 |
| TI | 0.91 | 0.91 | 0.95 | 0.90 | 0.01 | 0.338 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavaniello, S.; De Marzo, D.; Bednarczyk, M.; Palazzo, M.; Zejnelhoxha, S.; Wu, M.; Peng, M.; Stadnicka, K.; Maiorano, G. Influence of a Commercial Synbiotic Administered In Ovo and In-Water on Broiler Chicken Performance and Meat Quality. Foods 2023, 12, 2470. https://doi.org/10.3390/foods12132470
Tavaniello S, De Marzo D, Bednarczyk M, Palazzo M, Zejnelhoxha S, Wu M, Peng M, Stadnicka K, Maiorano G. Influence of a Commercial Synbiotic Administered In Ovo and In-Water on Broiler Chicken Performance and Meat Quality. Foods. 2023; 12(13):2470. https://doi.org/10.3390/foods12132470
Chicago/Turabian StyleTavaniello, Siria, Davide De Marzo, Marek Bednarczyk, Marisa Palazzo, Sanije Zejnelhoxha, Mengjun Wu, Meng Peng, Katarzyna Stadnicka, and Giuseppe Maiorano. 2023. "Influence of a Commercial Synbiotic Administered In Ovo and In-Water on Broiler Chicken Performance and Meat Quality" Foods 12, no. 13: 2470. https://doi.org/10.3390/foods12132470






