“Biqi” Bayberry Extract Promotes Skeletal Muscle Fiber Type Remodeling by Increasing Fast Myofiber Formation via the Akt/FoxO1 Pathway in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
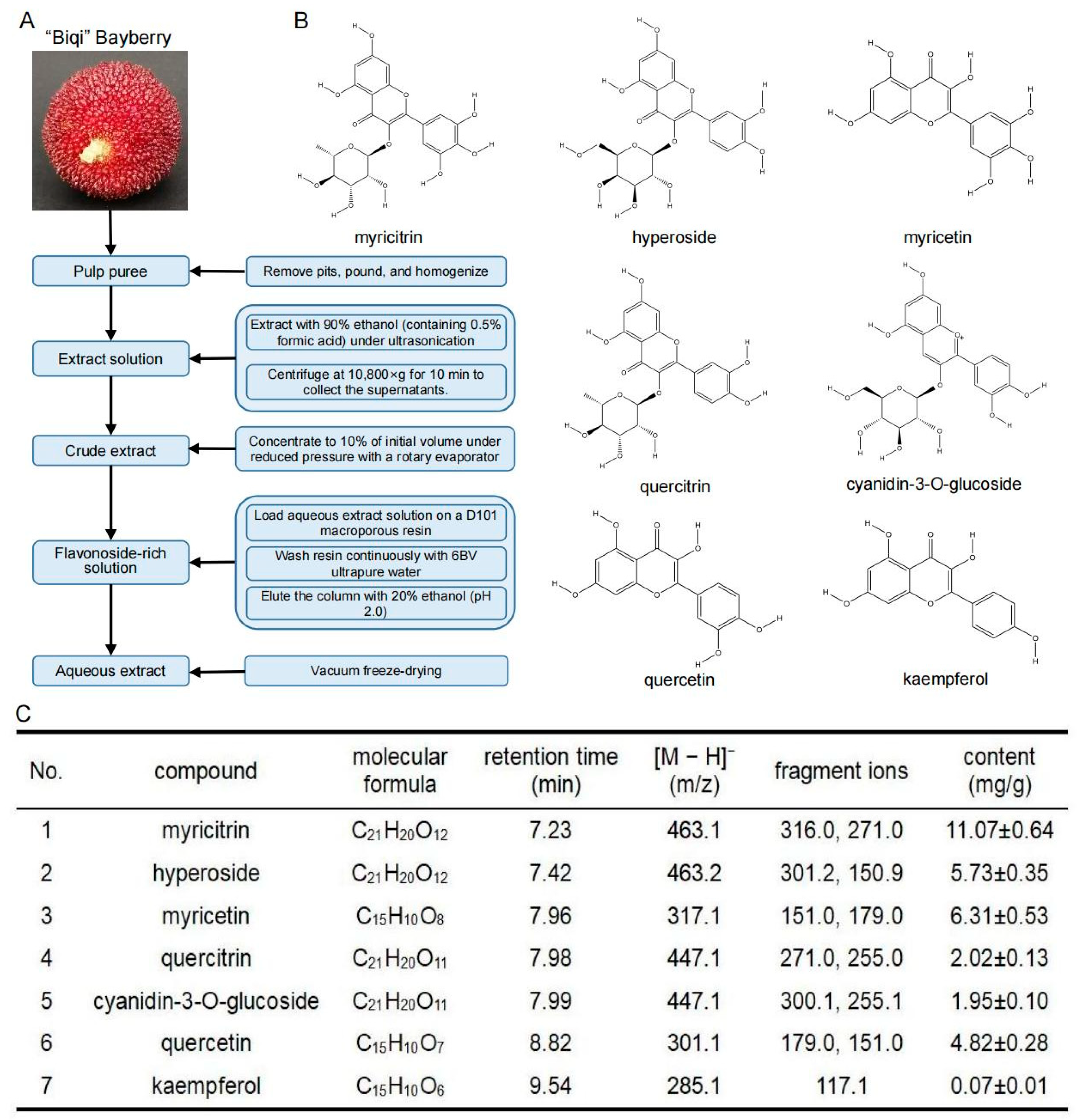
2.2. Preparation of the Bayberry Extracts
2.3. Quantification of Main Flavonoid Profiles by LC-MS/MS
2.4. Animals and BBE Treatment
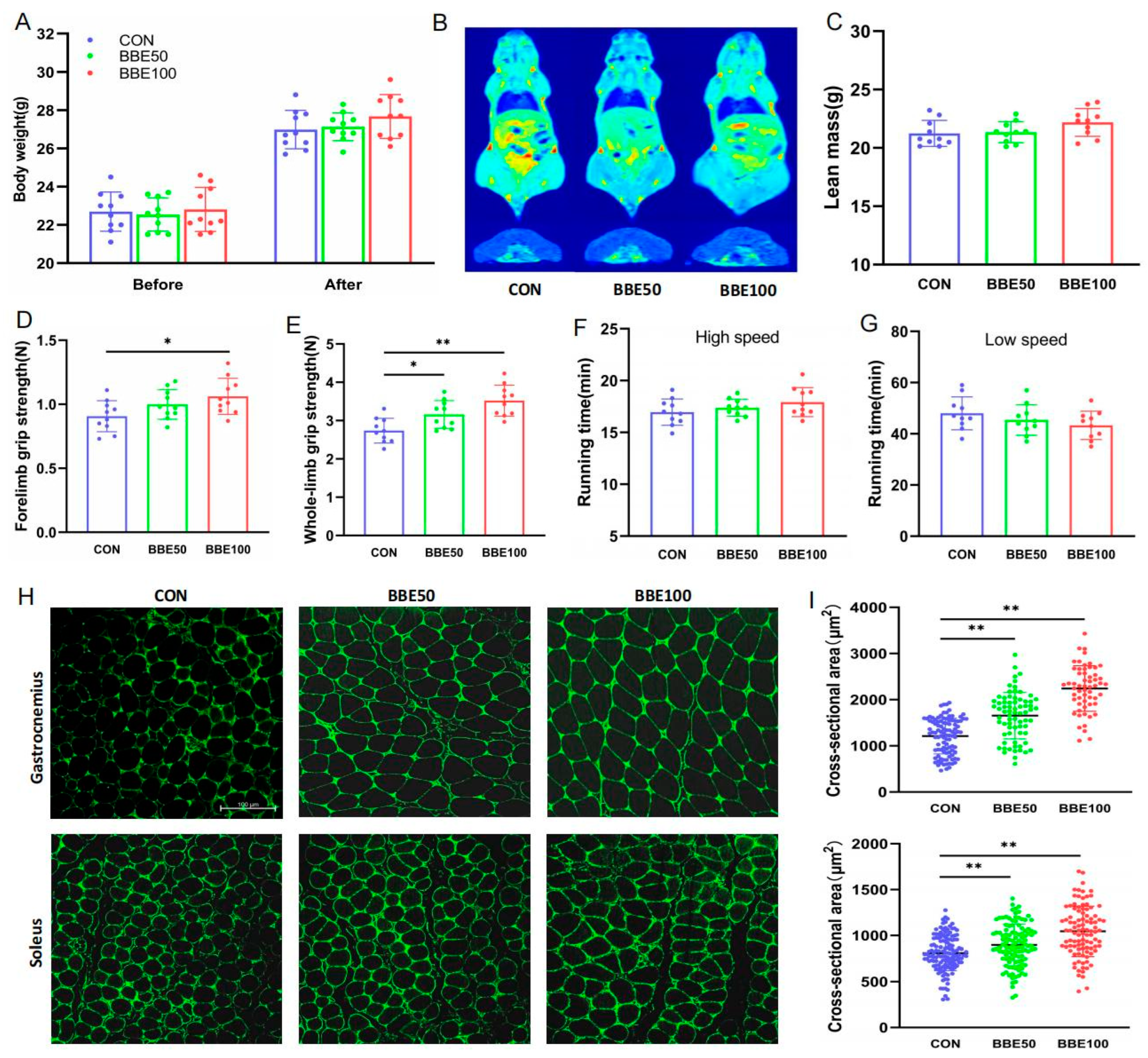
2.5. Grip Strength and Treadmill Running Tests
2.6. Body Composition and Magnetic Resonance Imaging (MRI) Analysis
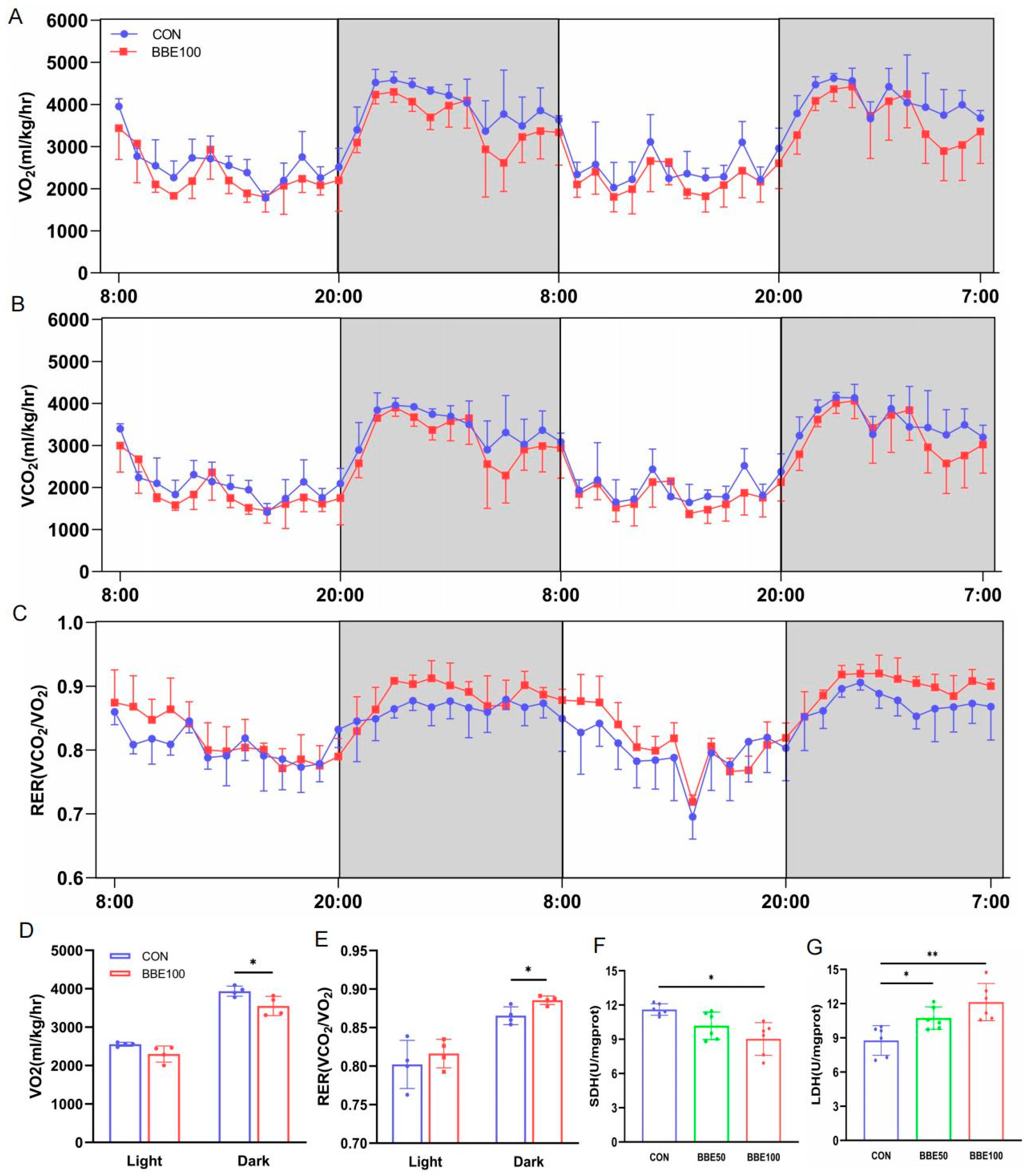
2.7. Metabolic Cage Monitoring
2.8. Enzyme Activities Assay
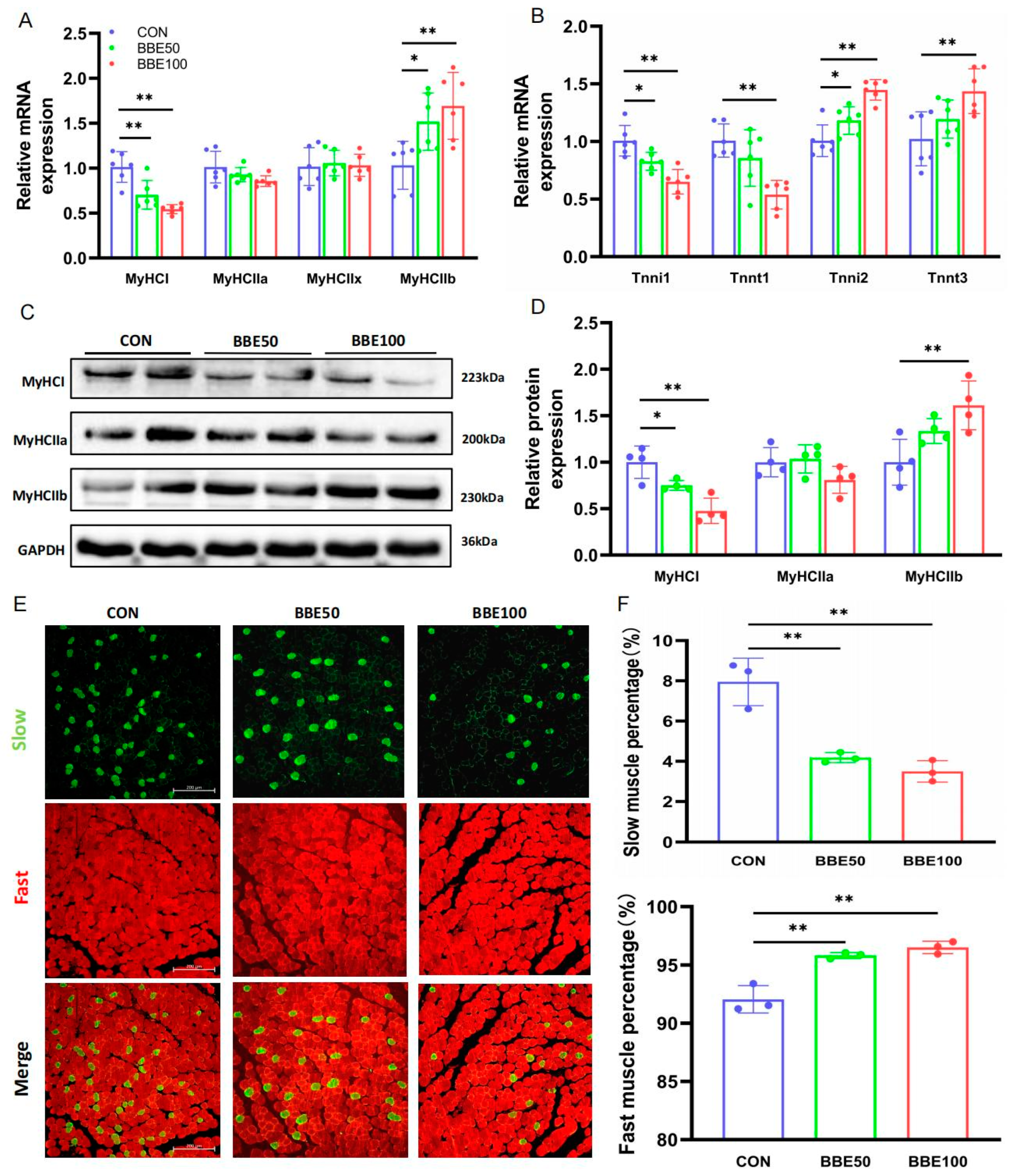
2.9. Quantitative Real-Time PCR
2.10. Western Blotting
2.11. Immunofluorescence Staining
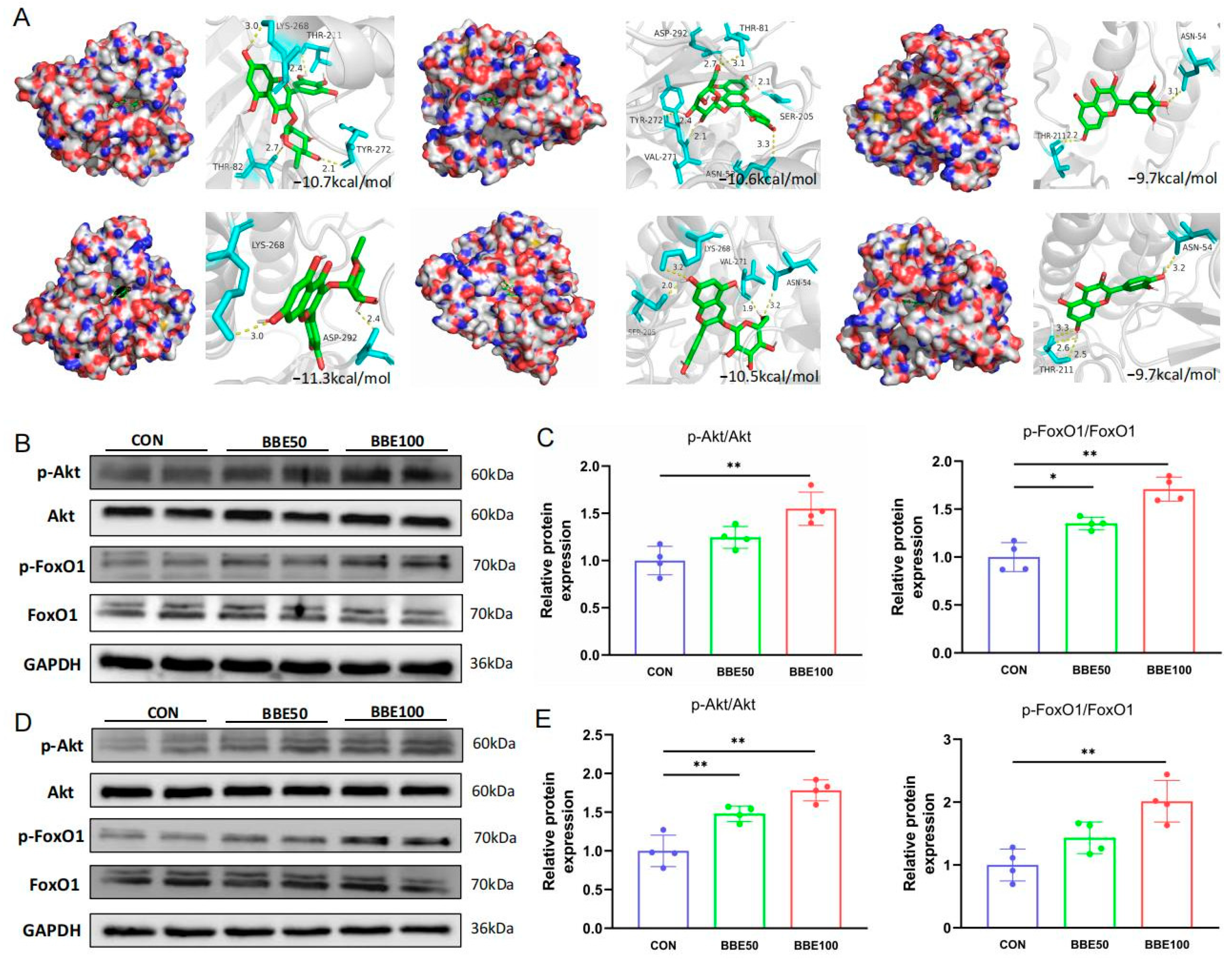
2.12. Molecular Docking
2.13. Statistical Analysis
3. Results
3.1. Quantitative Analysis of Flavonoids in “Biqi” Bayberry Extract
3.2. Effects of BBE on the Mass and Function of Skeletal Muscle in Mice
3.3. BBE Leads to the Transformation of Energy Metabolism Substrate in Mice
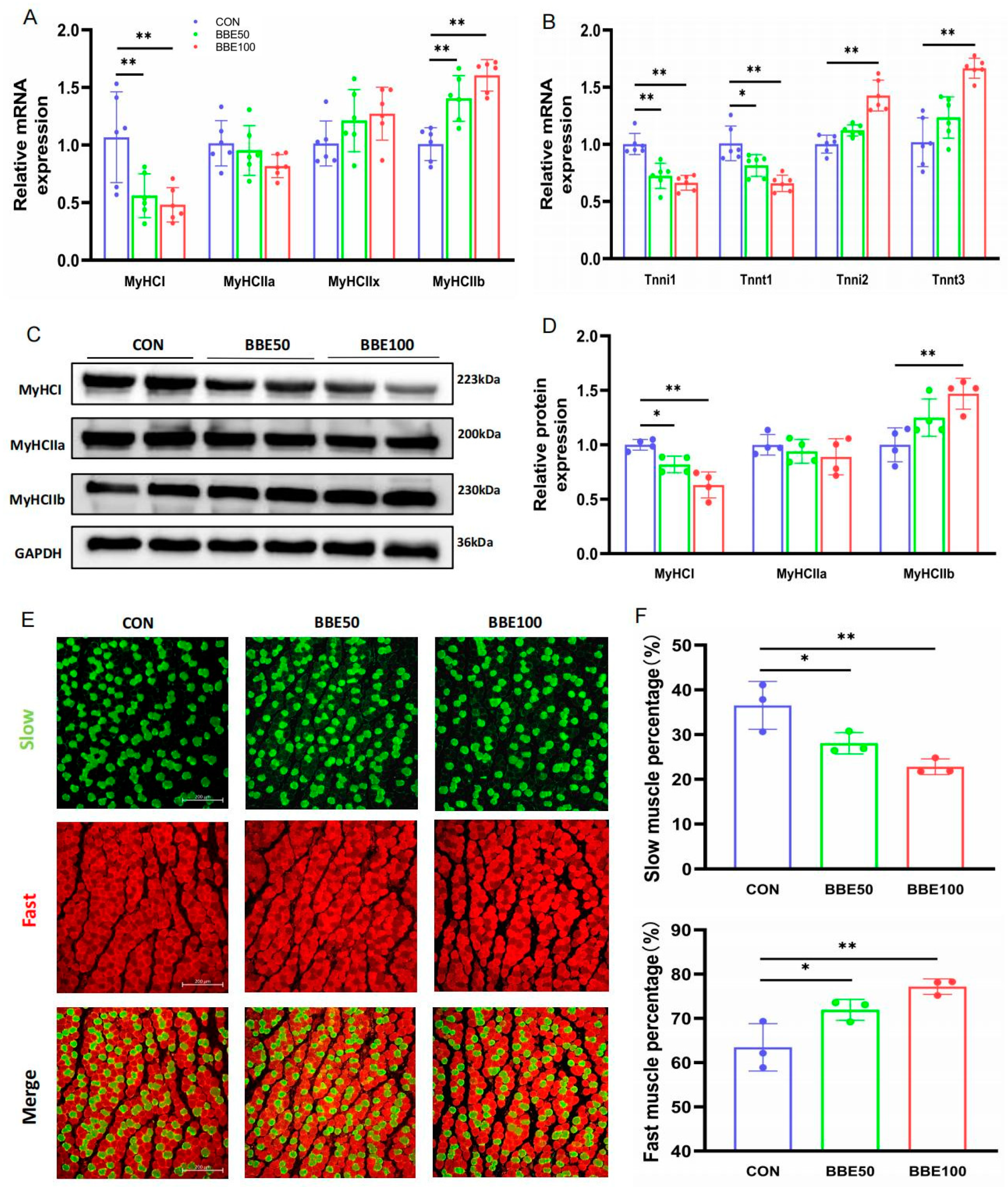
3.4. BBE Induces the Switch of Slow-to-Fast Fiber Types in the Gastrocnemius of Mice
3.5. BBE Regulates the Switch of Slow-to-Fast Fiber Types in the Soleus of Mice
3.6. BBE Activates the Akt-FoxO1 Pathway in the Gastrocnemius and Soleus of Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.H. Impact of Skeletal Muscle Mass on Metabolic Health. Endocrinol. Metab. 2020, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, H.; Zhang, X.; Wang, H.; Liu, S.; Zhang, H.; Qian, H.; Wang, L.; Ying, H. Geniposide Improves Glucose Homeostasis via Regulating FoxO1/PDK4 in Skeletal Muscle. J. Agric. Food Chem. 2019, 67, 4483–4492. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Huang, T.Y.; Linden, M.A.; Fuller, S.E.; Goldsmith, F.R.; Simon, J.; Batdorf, H.M.; Scott, M.C.; Essajee, N.M.; Brown, J.M.; Noland, R.C. Combined effects of a ketogenic diet and exercise training alter mitochondrial and peroxisomal substrate oxidative capacity in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1053–E1067. [Google Scholar] [CrossRef]
- Hall, E.C.R.; Semenova, E.A.; Bondareva, E.A.; Borisov, O.V.; Andryushchenko, O.N.; Andryushchenko, L.B.; Zmijewski, P.; Generozov, E.V.; Ahmetov, I. Association of muscle fiber composition with health and exercise-related traits in athletes and untrained subjects. Biol. Sport 2021, 38, 659–666. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Chen, H.; Luo, Y.; Zheng, P.; He, J.; Yu, J.; Yu, B. Procyanidin B2 Promotes Skeletal Slow-Twitch Myofiber Gene Expression through the AMPK Signaling Pathway in C2C12 Myotubes. J. Agric. Food Chem. 2020, 68, 1306–1314. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Yang, K.; Shu, G.; Wang, S.; Gao, P.; Zhu, X.; Xi, Q.; Zhang, Y.; Jiang, Q. Epigallocatechin Gallate Reduces Slow-Twitch Muscle Fiber Formation and Mitochondrial Biosynthesis in C2C12 Cells by Repressing AMPK Activity and PGC-1α Expression. J. Agric. Food Chem. 2016, 64, 6517–6523. [Google Scholar] [CrossRef]
- Lee, Y.G.; Woo, H.; Choi, C.; Ryoo, G.H.; Chung, Y.J.; Lee, J.H.; Jung, S.J.; Chae, S.W.; Bae, E.J.; Park, B.H. Supplementation with Vitis vinifera Jingzaojing Leaf and Shoot Extract Improves Exercise Endurance in Mice. Nutrients 2022, 14, 4033. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Luo, Y.; Zheng, P.; Yu, J.; He, J. Grape seed proanthocyanidin extract promotes skeletal muscle fiber type transformation via AMPK signaling pathway. J. Nutr. Biochem. 2020, 84, 108462. [Google Scholar] [CrossRef]
- Wang, L.; Luo, L.; Zhao, W.; Yang, K.; Shu, G.; Wang, S.; Gao, P.; Zhu, X.; Xi, Q.; Zhang, Y.; et al. Lauric Acid Accelerates Glycolytic Muscle Fiber Formation through TLR4 Signaling. J. Agric. Food Chem. 2018, 66, 6308–6316. [Google Scholar] [CrossRef]
- Huang, H.; Sun, Y.; Lou, S.; Li, H.; Ye, X. In vitro digestion combined with cellular assay to determine the antioxidant activity in Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruits: A comparison with traditional methods. Food Chem. 2014, 146, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Antibacterial activities of bayberry extract on foodborne pathogens and identification of its active components. Food Agric. Immunol. 2019, 30, 385–397. [Google Scholar] [CrossRef]
- Yao, W.R.; Wang, H.Y.; Wang, S.T.; Sun, S.L.; Zhou, J.; Luan, Y.Y. Assessment of the antibacterial activity and the antidiarrheal function of flavonoids from bayberry fruit. J. Agric. Food Chem. 2011, 59, 5312–5317. [Google Scholar] [CrossRef]
- Sun, C.D.; Zheng, Y.X.; Chen, Q.J.; Tang, X.L.; Jiang, M.; Zhang, J.K.; Li, X.; Chen, K.S. Purification and anti-tumour activity of cyanidin-3-O-glucoside from Chinese bayberry fruit. Food Chem. 2012, 131, 1287–1294. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhan, L.; Xu, C.; Sun, L.; Jiang, H.; Sun, C.; Li, X. LC-Q-TOF-MS Characterization of Polyphenols from White Bayberry Fruit and Its Antidiabetic Effect in KK-A(y) Mice. ACS Omega 2020, 5, 17839–17849. [Google Scholar] [CrossRef]
- Sun, C.D.; Zhang, B.; Zhang, J.K.; Xu, C.J.; Wu, Y.L.; Li, X.; Chen, K.S. Cyanidin-3-glucoside-rich extract from Chinese bayberry fruit protects pancreatic β cells and ameliorates hyperglycemia in streptozotocin-induced diabetic mice. J. Med. Food 2012, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health benefits of cyanidin-3-glucoside as a potent modulator of Nrf2-mediated oxidative stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef]
- Momtaz, S.; Salek-Maghsoudi, A.; Abdolghaffari, A.H.; Jasemi, E.; Rezazadeh, S.; Hassani, S.; Ziaee, M.; Abdollahi, M.; Behzad, S.; Nabavi, S.M. Polyphenols targeting diabetes via the AMP-activated protein kinase pathway; future approach to drug discovery. Crit. Rev. Clin. Lab. Sci. 2019, 56, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhang, Y.; Ma, J.; Du, M.; Xu, H.; Wang, J.; Zhang, L.; Li, W.; Hou, Y.; Liu, X.; et al. FHL3 promotes the formation of fast glycolytic muscle fibers by interacting with YY1 and muscle glycolytic metabolism. Cell. Mol. Life Sci. 2023, 80, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Yao, X.; Wang, H.; Zhao, L.; Jiang, H.; Yao, X.; Zhang, S.; Ye, C.; Liu, W.; et al. miR-182 Regulates Metabolic Homeostasis by Modulating Glucose Utilization in Muscle. Cell Rep. 2016, 16, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Seko, D.; Ogawa, S.; Li, T.S.; Taimura, A.; Ono, Y. μ-Crystallin controls muscle function through thyroid hormone action. FASEB J. 2016, 30, 1733–1740. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, F.; Gong, Q.; Cui, A.; Zhuo, S.; Hu, Z.; Han, Y.; Gao, J.; Sun, Y.; Liu, Z.; et al. Hepatic ATF6 Increases Fatty Acid Oxidation to Attenuate Hepatic Steatosis in Mice Through Peroxisome Proliferator-Activated Receptor α. Diabetes 2016, 65, 1904–1915. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Wang, Y.; Wang, Y.; Fu, L.; Su, L. Chinese bayberry (Myrica rubra) phenolics mitigated protein glycoxidation and formation of advanced glycation end-products: A mechanistic investigation. Food Chem. 2021, 361, 130102. [Google Scholar] [CrossRef]
- Fang, Z.X.; Zhang, M.; Wang, L.X.; Sun, J.C. Identification of anthocyanin in bayberry (Myrica rubra Sieb. et Zucc.) by HPLC-DAD-ESIMS and GC. J. Food Drug Anal. 2006, 14, 368–372. [Google Scholar]
- Lin, Q.H.; Zhong, Q.Z.; Zhang, Z.H. Comparative transcriptome analysis of genes involved in anthocyanin biosynthesis in the pink-white and red fruits of Chinese bayberry (Morella rubra). Sci. Hortic. 2019, 250, 278–286. [Google Scholar] [CrossRef]
- Akasaki, Y.; Ouchi, N.; Izumiya, Y.; Bernardo, B.L.; Lebrasseur, N.K.; Walsh, K. Glycolytic fast-twitch muscle fiber restoration counters adverse age-related changes in body composition and metabolism. Aging Cell 2014, 13, 80–91. [Google Scholar] [CrossRef]
- Gordon, B.A.; Benson, A.C.; Bird, S.R.; Fraser, S.F. Resistance training improves metabolic health in type 2 diabetes: A systematic review. Diabetes Res. Clin. Pract. 2009, 83, 157–175. [Google Scholar] [CrossRef]
- Meng, Z.X.; Li, S.; Wang, L.; Ko, H.J.; Lee, Y.; Jung, D.Y.; Okutsu, M.; Yan, Z.; Kim, J.K.; Lin, J.D. Baf60c drives glycolytic metabolism in the muscle and improves systemic glucose homeostasis through Deptor-mediated Akt activation. Nat. Med. 2013, 19, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Kitamura, Y.I.; Funahashi, Y.; Shawber, C.J.; Castrillon, D.H.; Kollipara, R.; DePinho, R.A.; Kitajewski, J.; Accili, D. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J. Clin. Investig. 2007, 117, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, F.G.; Zhang, W.S.; Pan, A.; Yang, Y.L.; Liu, J.F.; Li, P.; Liu, B.L.; Qi, L.W. Ginsenoside Rg1 Inhibits Glucagon-Induced Hepatic Gluconeogenesis through Akt-FoxO1 Interaction. Theranostics 2017, 7, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, Y.; Shen, Q.; Liu, G.; Ye, J.; Sun, G.; Sun, X. Myricitrin Attenuates High Glucose-Induced Apoptosis through Activating Akt-Nrf2 Signaling in H9c2 Cardiomyocytes. Molecules 2016, 21, 880. [Google Scholar] [CrossRef]
- Tzeng, T.F.; Liou, S.S.; Liu, I.M. Myricetin Ameliorates Defective Post-Receptor Insulin Signaling via β-Endorphin Signaling in the Skeletal Muscles of Fructose-Fed Rats. Evid. Based Complement. Alternat. Med. 2011, 2011, 150752. [Google Scholar] [CrossRef]
- Charachit, N.; Sukhamwang, A.; Dejkriengkraikul, P.; Yodkeeree, S. Hyperoside and Quercitrin in Houttuynia cordata Extract Attenuate UVB-Induced Human Keratinocyte Cell Damage and Oxidative Stress via Modulation of MAPKs and Akt Signaling Pathway. Antioxidants 2022, 11, 221. [Google Scholar] [CrossRef]
- Zeng, K.W.; Wang, X.M.; Ko, H.; Kwon, H.C.; Cha, J.W.; Yang, H.O. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid β-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2011, 672, 45–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, Y.; Suo, X.; Li, J.; Huang, D.; Kou, G. “Biqi” Bayberry Extract Promotes Skeletal Muscle Fiber Type Remodeling by Increasing Fast Myofiber Formation via the Akt/FoxO1 Pathway in Mice. Foods 2023, 12, 2471. https://doi.org/10.3390/foods12132471
Li J, Li Y, Suo X, Li J, Huang D, Kou G. “Biqi” Bayberry Extract Promotes Skeletal Muscle Fiber Type Remodeling by Increasing Fast Myofiber Formation via the Akt/FoxO1 Pathway in Mice. Foods. 2023; 12(13):2471. https://doi.org/10.3390/foods12132471
Chicago/Turabian StyleLi, Jinjie, Yi Li, Xiangying Suo, Jiangtao Li, Da Huang, and Guangning Kou. 2023. "“Biqi” Bayberry Extract Promotes Skeletal Muscle Fiber Type Remodeling by Increasing Fast Myofiber Formation via the Akt/FoxO1 Pathway in Mice" Foods 12, no. 13: 2471. https://doi.org/10.3390/foods12132471
APA StyleLi, J., Li, Y., Suo, X., Li, J., Huang, D., & Kou, G. (2023). “Biqi” Bayberry Extract Promotes Skeletal Muscle Fiber Type Remodeling by Increasing Fast Myofiber Formation via the Akt/FoxO1 Pathway in Mice. Foods, 12(13), 2471. https://doi.org/10.3390/foods12132471




