Abstract
Egg yolk forms have several health and industrial applications, but their storage characteristics and gel mechanisms have not been thoroughly studied. In order to investigate the relationship between the changes in structure and properties of egg yolk gel and egg yolk powder during storage, in this paper, egg yolk powder was stored at 37 °C for 0, 1, 3, and 6 months in an accelerated storage experiment, and the influence of storage time on the gel properties of egg yolk powder was analyzed. The results showed that the contents of protein carbonylation and sulfhydryl in the yolk decreased gradually with the extension of storage time. Circular dichroism and fluorescence spectra showed that the ordered structure and structural stability of egg yolk proteins decreased gradually. Oxidation led to the formation of intermolecular crosslinking in the egg yolk proteins and oxidized aggregates, resulting in a decrease in surface hydrophobicity, which affected the gel properties of the egg yolk powder after rehydration, resulting in the phenomenon of lipid migration and gel degradation. The results provide a theoretical basis for improving egg yolk powder’s overall quality and storage stability.
1. Introduction
Eggs are inexpensive, are a major source of high-quality protein and lipids, and provide many minerals and vitamins [1]. However, fresh eggs are easily spoiled and broken; they are unsuitable for long-distance transportation. In order to meet the food industry’s demand for eggs and egg products, spray-drying technology is often used to remove the moisture in egg liquid and process it into egg powder [2]. Compared with fresh eggs, egg powder can significantly reduce the weight of eggs, has higher biological safety and a shelf life of 1–2 years, and has been widely used in bakery products, mayonnaise, pasta, biscuits, and other food production domains [3]. During the storage process, egg yolk powder (EYP) is inevitably affected by various external environments, such as time, temperature, humidity, oxygen content, and light, which change the quality of the egg yolk powder. From pudding, egg tart, and other processing enterprises, problems are fed back concerning fat precipitation when egg yolk powder is stored for some time after its rehydration; heating to form egg yolk gel also entails a lot of oil precipitation, which affects the taste and flavor of the egg yolk powder itself, and then affects the application prospects of the egg yolk powder.
Egg yolk is a natural multiscale protein–lipid supramolecular assembly [4]. Its microstructure shows many spheroid particles with diameters between 0.8 and 10 μm embedded in the continuous phase [5]. The yolk liquid can be separated into precipitated yolk granules and yolk serous liquid with a centrifuge. The yolk granules are mainly composed of 70% high-density lipoprotein (HDL) micromicelles, 16% vitellophin, and 12% low-density lipoprotein (LDL) [6], while the yolk serous liquid is mainly composed of 85% low-density lipoprotein and 15% vitelloin [7]. LDL is structured as a spherical nanoparticle (17–60 nm) with a phospholipid membrane embedded with proteins surrounding a lipid core composed of triglycerides and cholesterol in a fluid state [8]. HDL is a dimer spherical molecule (7–22 nm) formed by two monomer molecules and presents a spherical micellar structure. At the same time, HDL is coupled with egg yolk hyper phosphoprotein and embedded LDL vesicles to form the main structure of egg yolk granules [9,10].
The gel mechanism of egg yolk liquid follows Flory theory. The formation process is mainly that egg yolk protein changes from a natural to denatured state, then forms soluble aggregates with high relative molecular weight through β folding. Finally, under the action of a disulfide bond, the aggregates gradually thicken to form a gel [11]. However, the difference with egg yolk gel is that the egg yolk liquid is a stable emulsion system. After heating, an emulsion particle gel will be formed, in which egg yolk protein acts as both an emulsifier and a gel matrix [4]. Therefore, the fat precipitation and lipid migration of the gel after the rehydration of yolk powder after storage are bound to be related to the property changes in yolk protein during storage.
Therefore, by accelerating the storage of yolk powder, this paper investigates the physicochemical property changes in yolk protein in yolk powder during storage and attempts to analyze the relationship between these physicochemical property changes and the precipitation of egg yolk gel lipids to provide a theoretical basis for solving the problem of the reduction in processing characteristics of yolk powder during storage.
2. Materials and Methods
2.1. Samples and Materials
The egg yolk powder used in this study was sourced from Shendi Agricultural Branch Trade Co., Ltd.,Wuhan, China. The protein content of the egg yolk powder was determined to be 31.34 ± 0.58% (w/v) using the BCA method. The lipid content was 61.22 ± 2.58% (w/v) as determined by the AOAC-recommended method, and the moisture content was 2.85 ± 0.27% (w/v). Dithiothreitol (DTT) (CAS: 3483-12-3) and 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) (CAS:69-78-3) were obtained from Sigma (St. Louis, MO, USA), while all other reagents were of analytical grade and purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China).
2.2. Sample Preparation
Accelerated shelf-life testing: The egg yolk powder was packed every 200 g in aluminum foil bags sealed using a vacuum and placed at 37 °C to avoid light for the accelerated test. After 0, 1, 3, and 6 months of storage, the egg yolk powder samples were taken for follow-up analysis.
Egg yolk gel preparation methods: EYP was mixed with 10 mM sodium phosphate buffers (pH 7.4) at a 1:1 ratio to restore the state of the egg yolk liquid. An amount of 20 mL well-stirred egg yolk was stored in a 25 mL beaker, which was sealed with plastic wrap to prevent water evaporation. Then, each beaker was boiled at 100 °C for 5 min to prepare egg yolk gel to explore lipid migration.
2.3. Protein Carbonyl Measurements
The protein carbonyl content was determined using a modified version of the method described by Bao et al. [12] EYP samples were dissolved in deionized water at a concentration of 3 mg/mL. Next, 1 mL of the EYP solution was mixed with 3 mL of a 10 mmol/mL solution of 2,4-dinitrophenylhydrazine (DNPH) and incubated at 25 °C for 2 h. Unreacted DNPH was removed using trichloroacetic acid and an ethanol/ethyl acetate solution. The results were expressed as nmol of carbonyl groups per milligram of soluble protein, with a molar extinction coefficient of 22,000 M−1 cm−1.
2.4. Free Sulphydryl Group Measurements
The oxidative changes in proteins during EYP storage were assessed by monitoring free sulphydryl groups (−SH) in proteins using the dithiothreitol colorimetric method, as reported by Chorna et al. [13]. The absorbance was measured at 412 nm. The results were expressed as nmol of −SH per milligram of soluble protein with a molar extinction coefficient of 13,600 M−1 cm−1.
2.5. Low-Field Magnetic Resonance Imaging Analyses
MRI analysis was measured according to Cheng et al. [14] with a little modification. Proton density images were acquired using a multi-spin-echo (MSE) imaging sequence. The specific parameter conditions were as follows: the field of view (FOV) was 70 mm × 70 mm, the slice width was 3.0 mm, read size = 256, phase size = 192, echo time (TE) = 20 ms, and the repeat sampling time (TR) was 2000 ms. Weighted imaging of the samples was obtained through the above parameters, and the images were processed using the software Osirix (Osirix life version 7.0.4, Geneva, Switzerland) for pseudocolor and quantitative processing.
2.6. Scanning Electron Microscope Analyses
A small sample piece was obtained from the top part and bottom part of the heated yolk gel after being freeze-dried in the vacuum. Then, the samples were coated with gold on a bronze stub. The microcosmic morphology of the egg yolk gel’s top part and bottom was obtained using an SU8010 (Hitachi, Ltd., Tokyo, Japan) scanning electron microscope in low-vacuum mode with an accelerating voltage of 10 kV, 10 μA and a working distance of 8 mm.
2.7. Determination of Spin–Spin Relaxation Time (T2)
According to the method described by Aursand et al. [15], the spin relaxation time (T2) of the sample was measured using a low-field pulsed NMR analyzer (Suzhou Niumag Co., Ltd., Suzhou, China). Test conditions: the instrument was set to CPMG sequence mode to collect attenuation signals from the sample; 90° pulse time was set to 20 μs and 180° pulse time was set to 200 μs; the cumulative number of repeats was 4 times; the waiting time was 3000 Ms; and the number of echoes was 8000. Using the SRIT algorithm of Multiple-Exp Inv Analysis software to invert data, a multiexponential fitting curve was obtained with 1,000,000 iterations.
2.8. Differential Scanning Calorimetry (DSC) Analyses
The DSC analysis of EYP was performed using a differential scanning calorimeter (DSC-250, TA Instruments, New Castle, DE, USA) calibrated with indium. Samples of EYP weighing 20.0 mg were placed in disposable aluminum hermetic cells and subjected to heating scans from 20 °C to 220 °C at a rate of 5 °C/min. The melting profiles were analyzed using TA-TRIOS software (TA Instruments, Version 4.4.0, New Castle, DE, USA), and the enthalpy (ΔH) and denaturation temperature (Td) were extracted.
2.9. Particle Size Distributions Analyses
EYP was mixed with 10 mM sodium phosphate buffers (pH 7.4) at a 1:1 ratio to restore the state of the egg yolk liquid. Particle size distribution was detected with an acoustic spectrometer (DT 1200, Dispersion Technology Inc., Bedford Hills, NY, USA). For high-viscosity and high-concentration samples, acoustic methods are more reliable and accurate than conventional light-scattering methods (Dukhin) [16]. The instrument manufacturer provided a calibration program to calculate the particle size distribution (PSD) from the attenuation spectra. Details of the instrument can be found elsewhere (Dukhin) [17].
2.10. Raman Spectrum Analyses
Raman spectra were measured using a Horiba LabRAM HR Evolution spectrometer (Horiba Jobin Yvon, Palaiseau, France). The collection parameters were as follows: excitation wavelength, 532 nm; laser power, 300 mW; range, 300–1800 cm−1; acquisition time, 60 s; and accumulation, 20 times. Background noise was removed and the baseline was adjusted using Labspec Spectrum 6 software (Horiba Jobin Yvon, France).
2.11. Secondary Structure Analyses
The Jasco J-1500 spectropolarimeter (Jasco Co., Tokyo, Japan) was used to determine the protein secondary structure. The measurement parameters included a scanning range of 190–260 nm, scanning speed of 50 nm/min, bandwidth of 1.0 nm, CD scale of 200 mdeg/1.0 d OD, and data interval of 0.1 nm. Prior to testing, the oxidized EYHDL samples (1 mg/mL) were dissolved in NaOH solution (pH 8.0).
2.12. Tertiary Structure Analysis
The EYP samples were analyzed for intrinsic fluorescence spectroscopy using Hitachi F-2700 fluorescence spectrometers (Hitachi, Japan). EYP solutions were prepared by dissolving 5 mg/mL in deionized water (pH 8.0) and adjusting the pH with 0.5 M NaOH solution. The measurement parameters included an excitation wavelength of 295 nm, a scanning range of 280–460 nm, and a slit width of 5 nm for both excitation and emission.
2.13. Statistics Analyses
The statistical package SPSS 20.0 (SPSS Inc., Chicago, IL, USA) was used to subject all data to statistics through one-way ANOVA. Mean values among treatments were compared and significant differences (p < 0.05) among treatments were identified using the least-square difference. All data were presented as means ± standard deviations of triplicate determinations.
3. Results
3.1. Changes in the Functional Group of Stored Egg Yolk Powder
Protein carbonyl content is currently the most commonly used index to characterize protein oxidation. Table 1 shows the changes in the carbonyl content of egg yolk powder during storage. With the extension of storage time, the carbonyl content of the egg yolk powder increased significantly, from 1.66 nmol/mg to 3.22 nmol/mg, indicating that egg yolk protein was oxidized during storage. The degree of protein oxidation deepened with the extension of storage time. Egg yolk powder has a high content of lipids. After spray drying, the chain oxidation reaction of lipids is caused by high temperature, and a large number of lipid free radicals and lipid active oxidation products are produced. These lipid oxidation products have strong biological activity and often cause the oxidative modification of protein structure and the carbonylation of egg yolk protein. Matumoto-Pintro, P. T. [18] found that lipid oxidation occurred in egg yolk powder during storage, and the TBARS value gradually increased with the extension of storage time. When Raitio, R [19] studied the quality of cauliflower soup powder, it was found that the protein in cauliflower soup powder was oxidized during storage, and the carbonyl content gradually increased. The conversion of some amino acid residues to carbonyl derivatives by reactive oxygen species increases carbonyl content [20]. Alternatively, lipid oxidation produces secondary dicarbonyl products, such as malondialdehyde, which combine with myosin to generate protein-bound carbonyls [21].

Table 1.
Carbonyl group, free sulfhydryl, and total disulfide of stored egg yolk powder samples.
The content of free sulfhydryl groups plays an important role in the gel structure of egg yolk powder. Sulfhydryl groups are the precursors for forming disulfide bonds, which play an important role in maintaining the tertiary structure of proteins. Therefore, sulfhydryl analysis is an indispensable analytical method to explore the structural and functional changes in proteins during storage [22]. Also shown in Table 1 are the changes in the free and total sulfhydryl contents of egg yolk powder with different storage times. With the extension of storage time, the content of free sulfhydryl and total sulfhydryl decreased (p < 0.05). Generally, reducing free sulfhydryl group content generally means protein denaturation or forming aggregates of intermolecular disulfide bonds or oxidation. Oxidation can change the REDOX state of cysteine in the protein and the equilibrium constant of sulfhydryl/disulfide bond interaction, thereby changing the amount and distribution of the sulfhydryl group and disulfide bond in the protein [23]. The content of disulfide bonds in egg yolk powder decreases, and sulfur-containing compounds with non-disulfide bonds are formed, which is not conducive to gel formation [24]. Sulfhydryl groups on the surface of egg yolk powder can be reversibly oxidized to a disulfide bond and sulfenic acid state and irreversibly oxidized to sulfenic acid and a sulfonic acid state. Generally, the decrease in free sulfhydryl group content indicates the oxidative denaturation of egg yolk powder. Studies have shown that reactive oxygen radicals can quickly react with the sulfhydryl group and convert to sulfonic radicals, thus reducing its content [25]. With the extended storage time, the exposed SH group and free SH group in egg yolk powder will rapidly undergo SH oxidation and SH-SS exchange reaction, resulting in decreased total sulfhydryl content. By analyzing the changes in the total sulfhydryl group and free sulfhydryl group content, it was concluded that oxidation would change the structural characteristics of egg yolk protein to a certain extent.
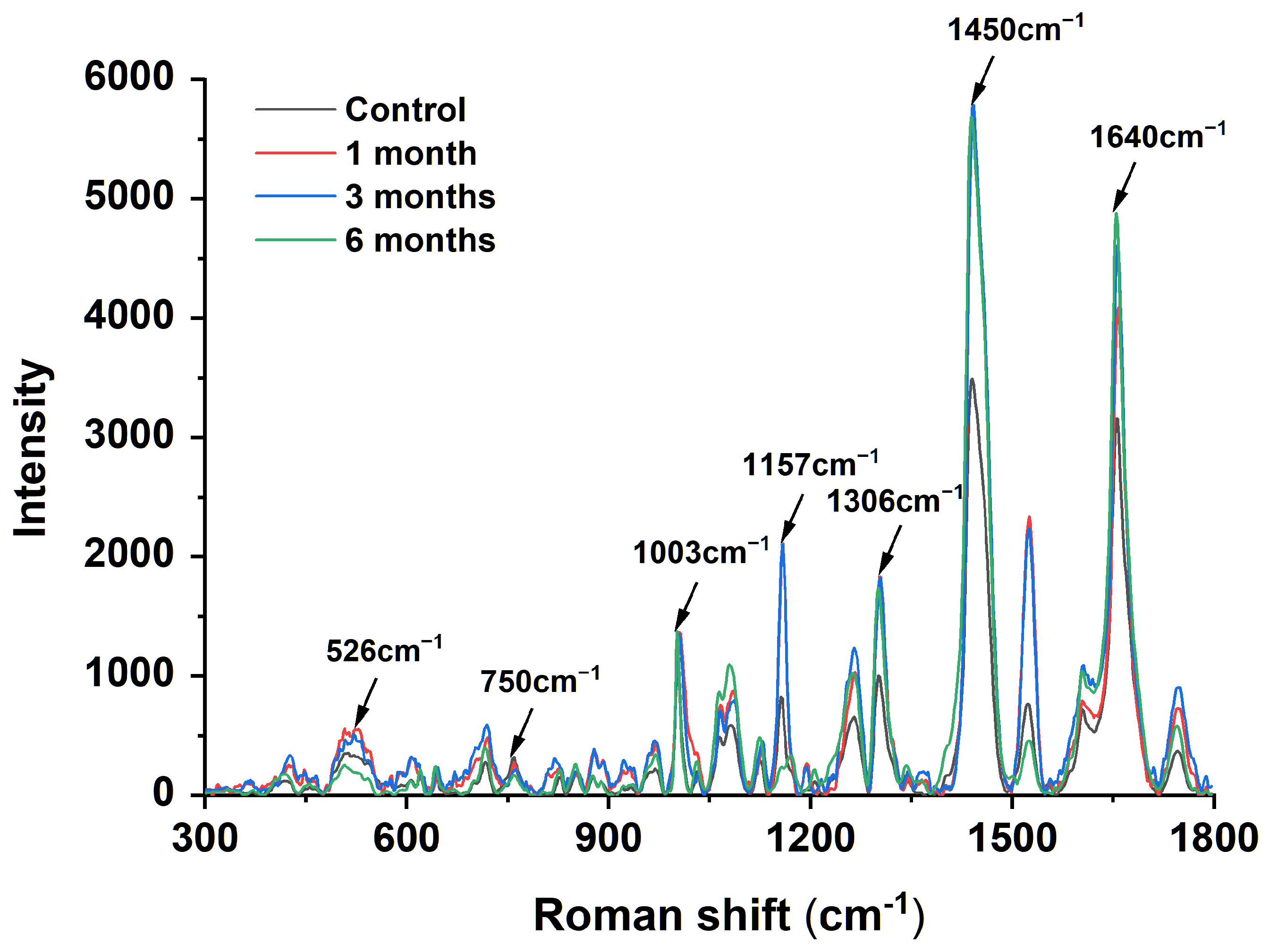
In recent years, Raman spectroscopy has been increasingly used to determine chemical structures [26]. The Raman spectra shown in Figure 1 were obtained after the accelerated storage of egg yolk powder at different times. In the protein Raman spectra, the spectral peak at 1640 cm−1 represents the stretching vibration of C=O, reflecting the carbonyl content. As shown in Figure 1, the spectral peak intensity gradually increased with the storage time, representing an increase in protein carbonyl content. The 500–530 cm−1 peak is usually associated with the S-S stretching vibration mode of the disulfide bond C-C-S-S-C-C structure [27]. It was found that the spectral peak intensity in this region decreased gradually with the extension of storage time, and the peak intensity of the egg yolk powder stored for 6 months was the lowest, indicating a reduction in disulfide bond content. The 750 cm−1 region represents the C-S vibration peak of cysteine residues, and it was found that the intensity of the spectral peak decreased gradually with the extension of storage time, which indicates that the content of cysteine (free sulfhydryl group) decreased gradually [28]. The above results are consistent with the results of the contents of each substance determined in Table 1. The peak intensity of 1157 cm−1 represents the tyrosine content, consistent with a mechanism by which lipid free radicals cause protein damage [29]. Hydrophobic interactions involve hydrophobic groups such as aliphatic amino acids. Compared with the control group, the peak strength of the egg yolk powder samples stored for 1–6 months was significantly increased near 1306 cm−1 and 1450 cm −1, and the peak strength of the egg yolk powder samples stored for 6 months was more obvious. Among them, the peak near 1306 cm−1 represents the bending vibration of C-H, while the peak intensity of 1450 cm−1 is related to the bending vibration of CH2 and CH3 [30]. These indicate that prolonged storage promotes the interaction between aromatic and aliphatic amino acid residues in egg yolk powder and facilitates protein aggregation through hydrophobic interactions.
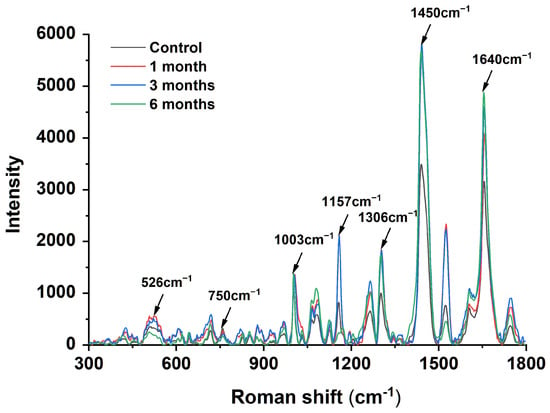
Figure 1.
Raman spectroscopy analysis of stored egg yolk powder samples.
3.2. Denaturation Degree Analysis of Stored Egg Yolk Powder
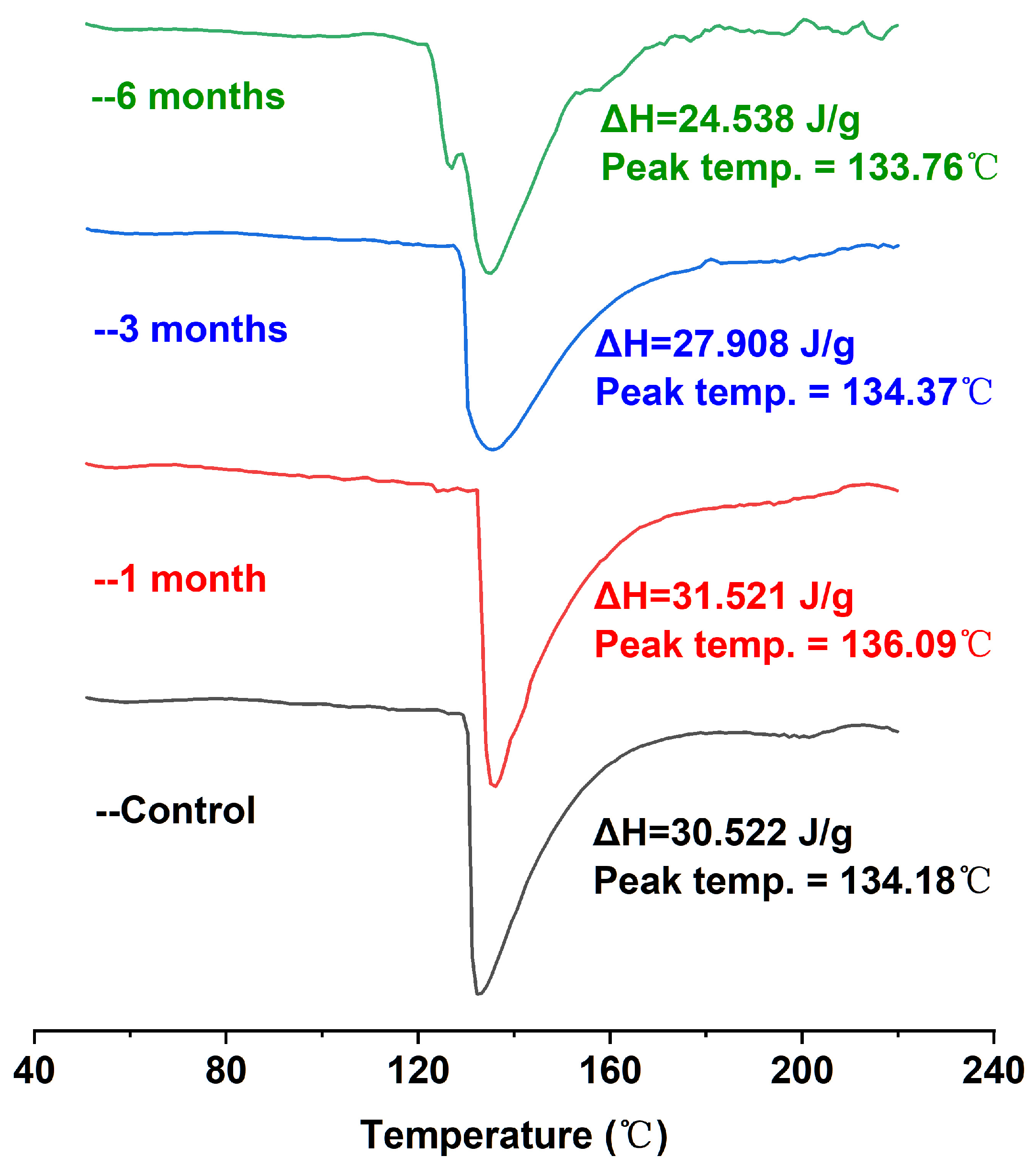
DSC can provide the temperature and enthalpic changes in the process of protein denaturation, which can effectively reflect the conformational changes and thermal stability of proteins. Proteins initially in the folded (native) conformation have higher enthalpic values; the protein undergoes conformational transformation (unfolding), and the ΔH value will gradually decrease [31]. As shown in Figure 2, the DSC scanning analysis of egg yolk powder samples under different storage periods showed a major endothermic peak of around 134 °C in the map. Because there were a large number of lipoproteins in the egg yolk powder samples, including low-density lipoprotein and high-density lipoprotein, they could not be distinguished well with calorimetry, so there was only a major endothermic peak on the image [32]. In addition, it was obvious that with the extension of storage time, the corresponding enthalpy (ΔH) of egg yolk powder gradually decreased. Combined with the results shown in Table 1, the increase in storage time led to the oxidation of proteins in egg yolk powder, indicating that conformational changes occurred in the egg yolk protein during storage, which also indicated that storage led to the oxidation and aggregation of most proteins in the egg yolk powder. As a result, the existing natural protein content was reduced, and the thermal stability was reduced.
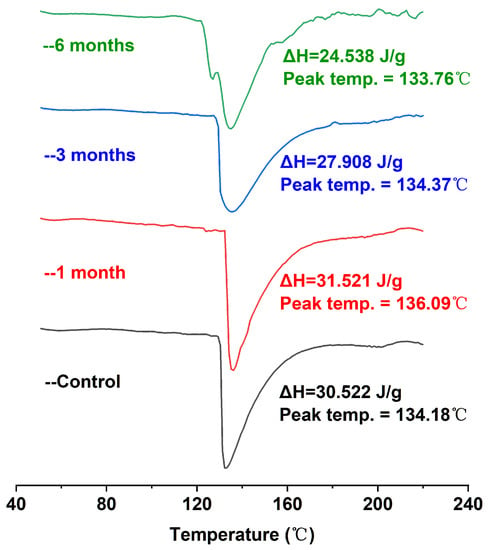
Figure 2.
DSC analysis of stored egg yolk powder samples.
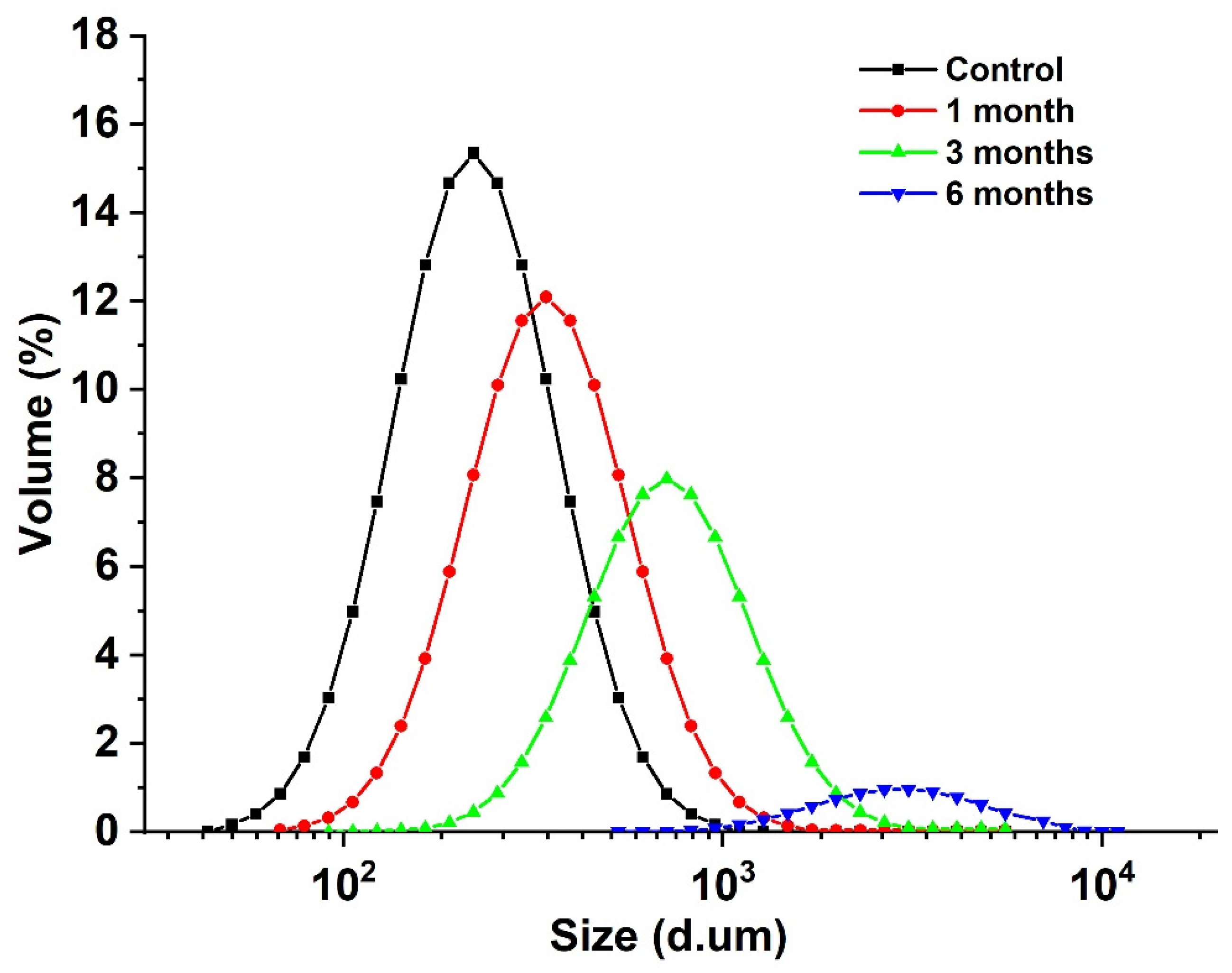
The particle size of the egg yolk liquid sample is a micron, and the particle size change of the egg yolk sample particle aggregate can be measured using a ultrasonic particle size meter, reflecting the effect of storage time on the degree of lipoprotein aggregation in egg yolk [33]. As shown in Figure 3, with the extension of storage time, the particle size of egg yolk liquid gradually increased from 116.27 μm to 3562.62 μm. Combined with the changes in carbonyl and sulfhydryl content, it was found that oxidation aggregation occurred in the egg yolk protein during storage, and with the extension of storage time, the degree of protein oxidation also increased. Covalent crosslinking occurs between protein molecules or subunits [34], and during storage, proteins fold and assemble under van der Waals forces, hydrogen bonds, hydrophobicity, and electrostatic forces, followed by the formation of a larger protein group, increasing in average particle size [35].
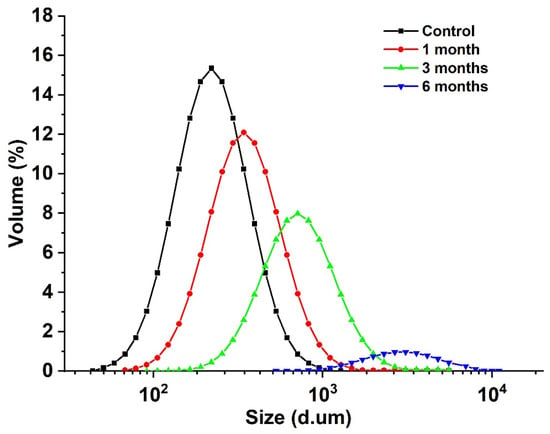
Figure 3.
Particle size distribution of stored egg yolk powder samples.
3.3. Changes in the Secondary/Tertiary Structure of Stored Egg Yolk Powder
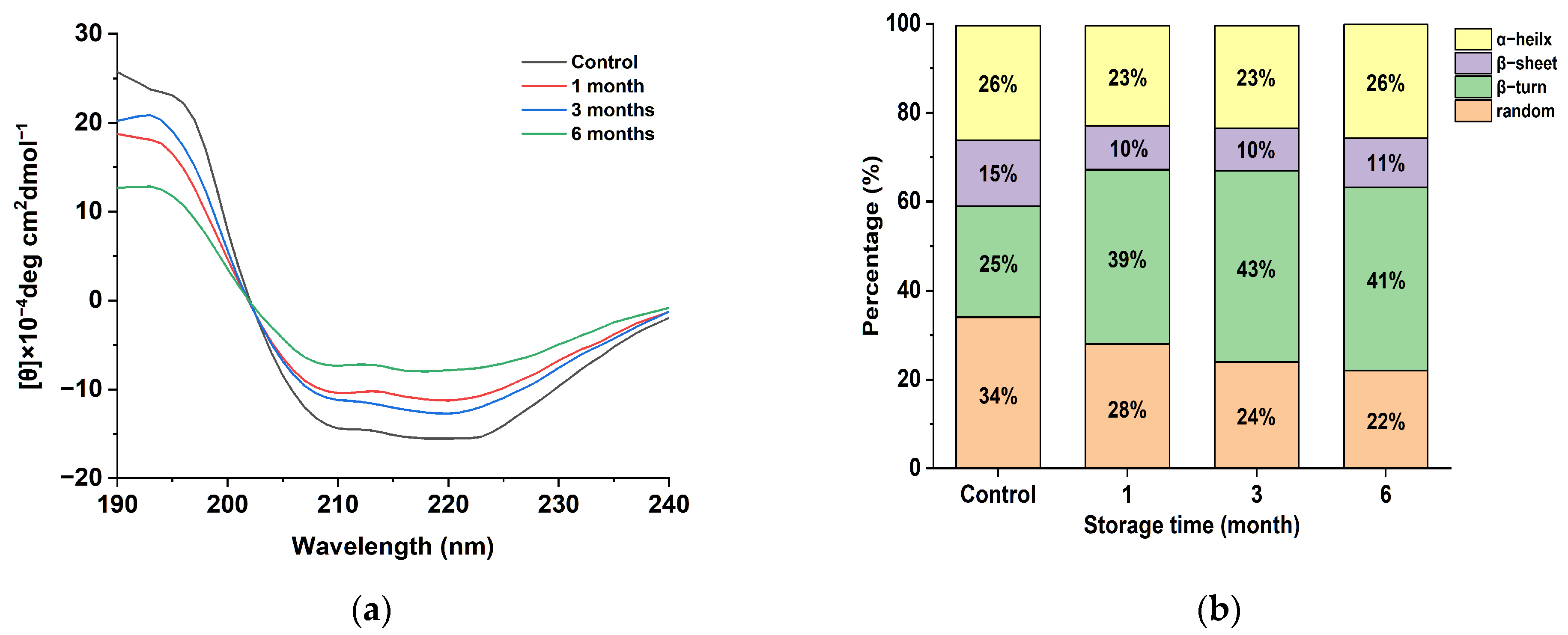
In order to investigate the effect of the spatial conformation change in the egg yolk powder on oil precipitation during storage, the secondary structure changes in the egg yolk powder in different storage periods were analyzed. As can be seen in Figure 4a, the yolk powder had negative peaks at 210 and 225 nm, which is consistent with the two negative characteristic shoulder peaks corresponding to the α-helical structure in this region due to the π-π and n-π transitions of the peptide bond [36]. A positive peak at 195 nm indicates that the yolk powder contains a β-folded structure. The intensity of the positive peak at 195 nm and the negative peak at 210 and 225 nm decreased with the storage time.
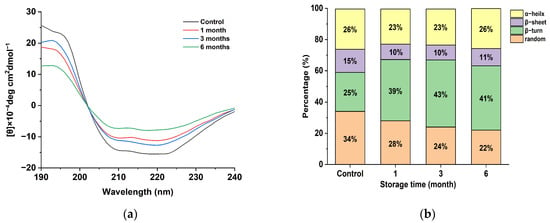
Figure 4.
Secondary structure analysis of stored egg yolk powder samples. (a) Circular dichroism of oxidized EYHDL samples. (b) Secondary structure content of oxidized EYHDL samples.
The secondary structure of proteins can be characterized by the presence of α-helices, β-folds, β-turns, and random coils. The α-helix structure represents a more ordered arrangement of protein molecules, while β-folds, β-turns, and random coils indicate a looser arrangement [37]. The secondary structure content of yolk protein, as shown in Figure 4b, was determined through software analysis. Egg yolk protein contains four types of secondary structures, with β-turn being the most abundant. The content of random coiling decreased gradually with the increasing storage time of the egg yolk powder, indicating that accelerated storage affected the secondary structure of proteins in the egg yolk powder. At the same time, it was also found that the β-Sheet in the secondary structure was reduced compared with the control group, and these phenomena indicate that protein oxidation occurred during the storage period of the egg yolk powder, leading to reduced stability [38].
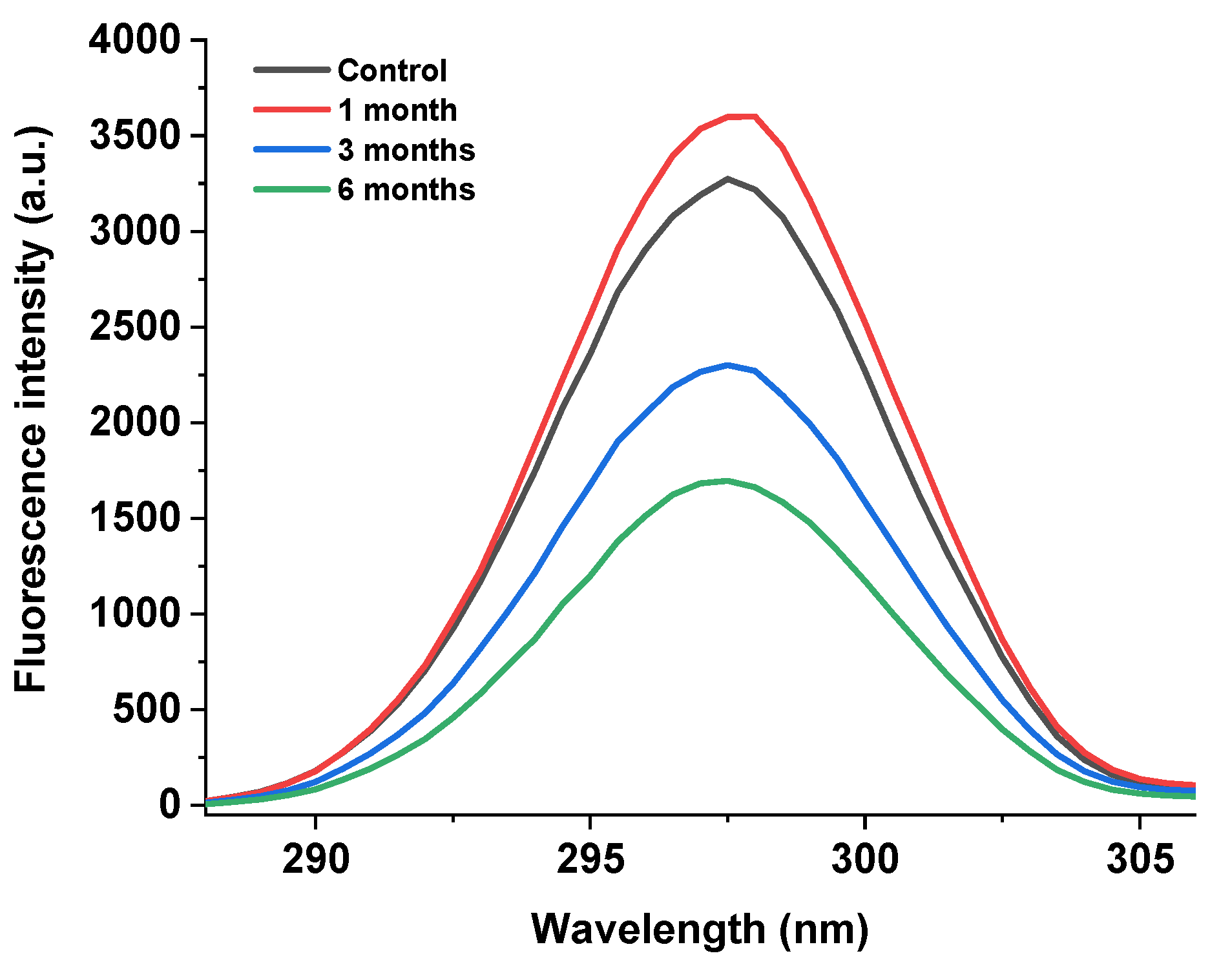
As shown in Figure 5, the endogenous fluorescence method was used to determine the fluorescence intensity of the egg yolk powder at different storage periods. It can be seen that the endogenous fluorescence intensity of the egg yolk powder showed a trend of first increasing and then decreasing with the extension of storage time compared with the control group. Among them, the yolk powder samples stored for 6 months showed the lowest fluorescence intensity.
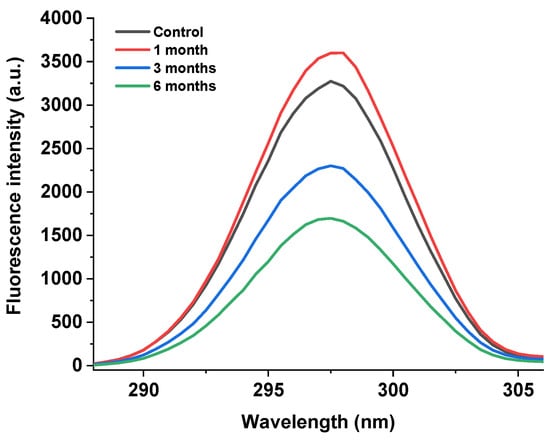
Figure 5.
Fluorescence intensity of stored egg yolk powder samples.
The fluorescence spectrum of the protein is mainly associated with tryptophan [39]. Tryptophan, a common amino acid in food proteins, is sensitive to its environment and ideal for molecular studies to determine structure and protein-folding dynamics and protein–protein interactions [40]. Changes in protein fluorescence intensity reflect the degree of oxidation of tryptophan residues and changes in their microenvironment [41]. However, in egg yolk powder, protein oxidation and lipid oxidation will occur during storage, so the analysis of the results in Figure 5 shows that the decrease in peak intensity may be due to the gradual oxidation of tryptophan by the environment with the increase in storage time, which promotes protein–protein or protein–other compound interactions. Thus, tryptophan is buried in the inner core of the newly formed protein aggregates, a conclusion consistent with the results of Guo et al. [42].
3.4. Changes in the Water Migration of Egg Yolk Gelation
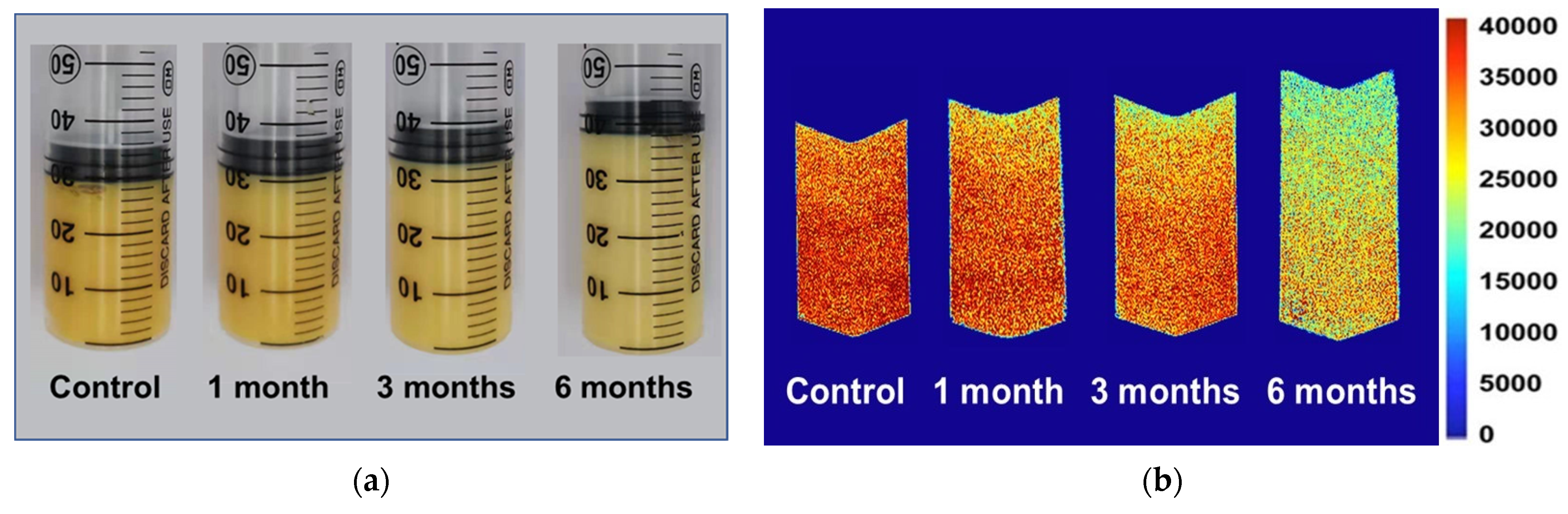
In order to better characterize the distribution and migration of hydrogen protons in different phases in the food system, the egg yolk powder was gelated at different storage periods, and its magnetic resonance imaging (MRI) was measured. In this sample, a 50 mL syringe was used to prepare the gel, so the longitudinal section of the gel was selected in the MRI detection (Figure 6a), and the pseudocolor image was obtained through later software processing. It can be intuitively seen from Figure 6b that the red color in the middle and lower part of the sample is darker, and the signal intensity in the upper part is weaker. Compared with the control group, the heated egg yolk gel volume increased with the extended storage time, and the proton signal gradually decreased in the MRI images. The proton signal of the egg yolk powder stored for 6 months significantly differed from that of the control group. In magnetic resonance imaging (MRI), protons with lower signal intensity tend to appear blue, while protons with higher signal intensity tend to appear red [43]. As shown in Figure 6b, the closer the sample is to the upper part, the lower the proton signal may be due to the large amount of lipids in the egg yolk, the oxidation of proteins during storage, and the change in lipoprotein structure, which reduces the lipid-binding ability of proteins and makes lipid molecules separate from lipoproteins. This results in a gradual movement of free lipid molecules to the top region of the yolk gel [44]. However, with the extension of storage time, the structural stability of lipoproteins in egg yolk powder decreased, and a large number of free lipid molecules were produced, so the MRI images of egg yolk powder stored for 6 months were significantly different from those of other groups.
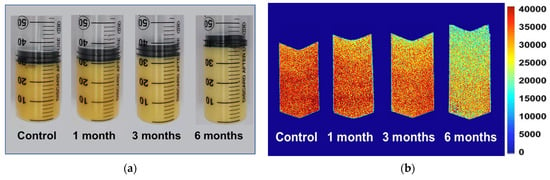
Figure 6.
MRI and morphological changes in egg yolk gelation at different stored times. (a) MRI changes in egg yolk gelation. (b) Images of egg yolk gelation.
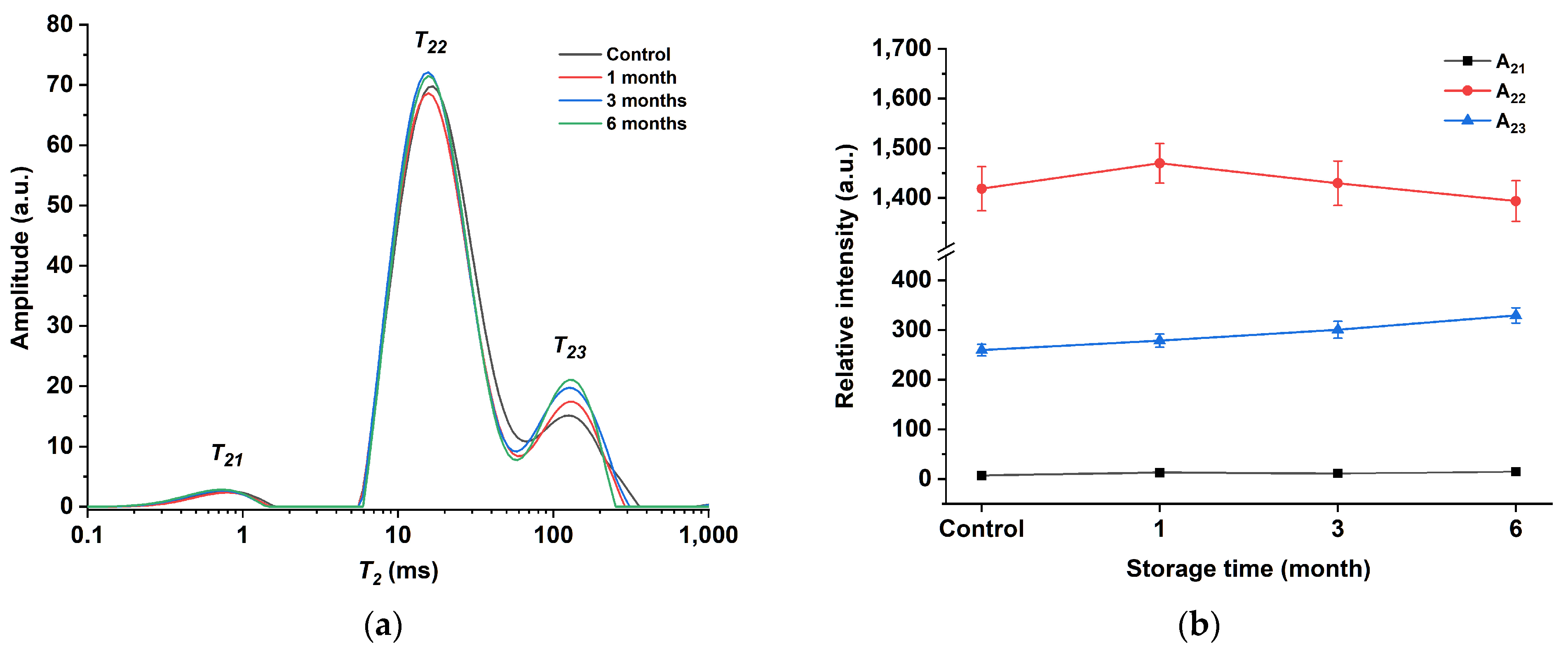
Additionally, LF-NMR was utilized to assess the alterations in water and lipid mobility of egg yolk powder during various storage periods. In LF-NMR, two critical parameters that are associated with the physicochemical structure of the sample are relaxation time and peak area. Relaxation times provide insight into the mobility of different water and fat components, while peak areas reflect the content of each water and fat component [45]. Figure 7a shows the T2 multiexponential fitted relaxation curves of the egg yolk powder at different storage periods. Three peaks (T21, T22, and T23) can be observed from this curve. The T21 peak is around 1 ms, which is the fastest relaxation component and belongs to water or lipids closely related to macromolecules [46]. The T22 peak is around 10 ms and reflects water and lipid molecules in the macromolecular network [47]. Peak T23 is around 100 ms and represents the hydrogen proton of lipids in the yolk [48]. By observing the curve in the figure, it can be found that T22 and T23 are continuous in the egg yolk gel. This phenomenon is consistent with the results obtained from the oil studied by Li et al., which contained two continuous characteristic peaks in the 10–100 ms range [49].
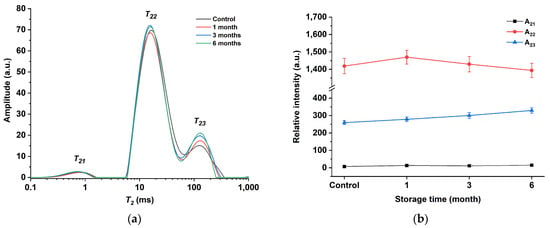
Figure 7.
Low-field NMR spectroscopy of egg yolk gelation at different stored times. (a) T2 curves of egg yolk gelation. (b) T2 peak area of egg yolk gelation.
Figure 7b shows the peak area plots of the T2 relaxation curves of gel samples prepared from egg yolk powder at different storage periods. As can be seen from the figure, with the extension of storage time, the change degree of A21 is not obvious, A22 shows a trend of first increasing and then decreasing, while A23 shows a trend of gradually increasing. This may be due to the conversion of part of the T22 fraction to T23 as protein molecules interact more closely with water and lipid molecules with prolonged storage time. However, in egg yolk powder with longer storage time, after the gelation of egg yolk induced by heating, the structure of lipoproteins is unstable and easier to be destroyed, thus reducing the lipid-binding ability of proteins, making lipid molecules separate from protein molecules and form protein–water–lipid complexes, making some changes in the structural and functional properties of proteins [44].
3.5. Changes in Microstructure of Egg Yolk Gelation
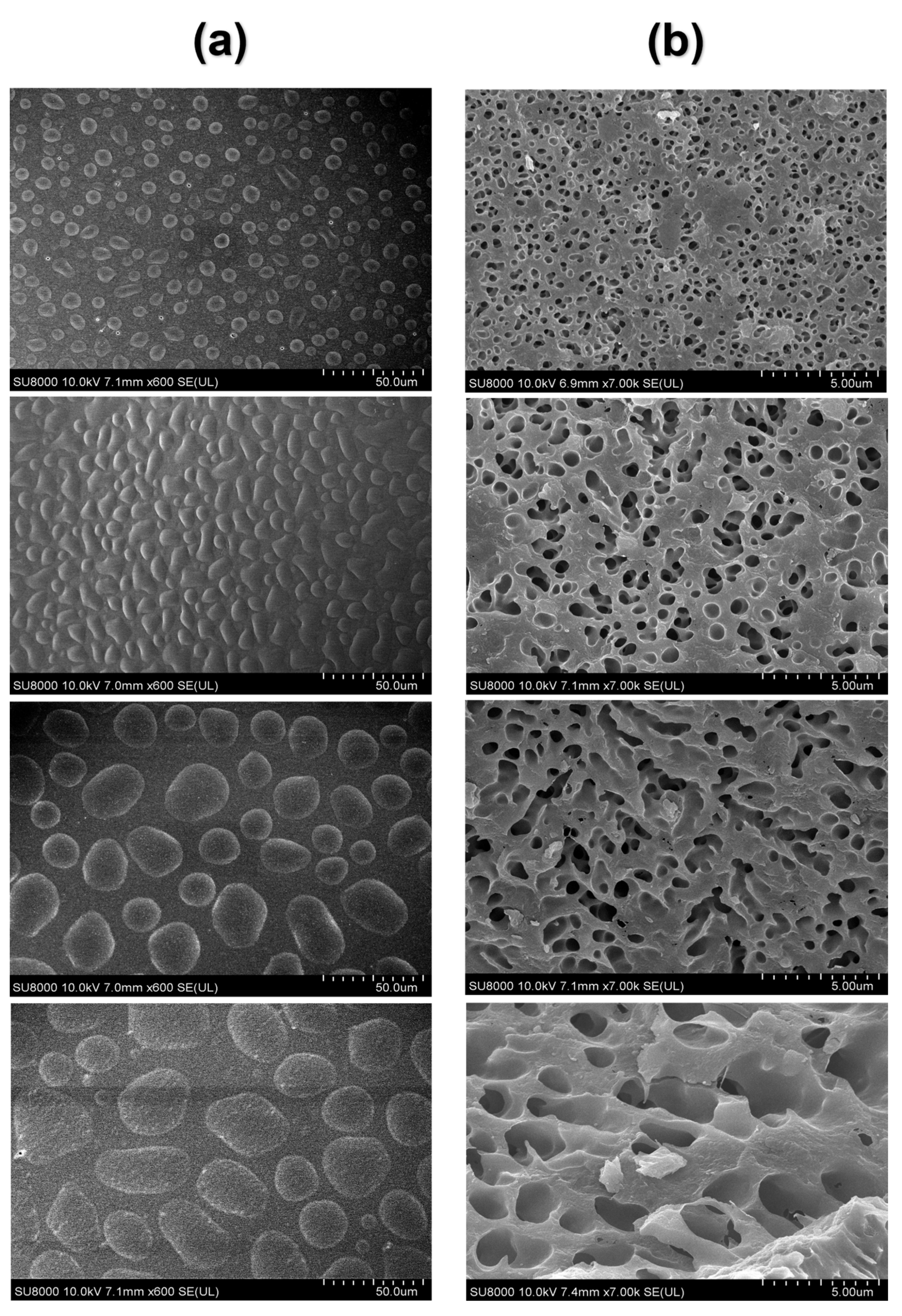
As shown in the figure, the top part and the inner region of the heated egg yolk gel were, respectively, intercepted, and the microstructure of the egg yolk gel sample was characterized with freezing scanning electron microscopy (SEM). It can be seen from Figure 8a that the microstructure of the upper part of the heated egg yolk gel showed an irregular spherical structure. The volume of the irregular spherical structure gradually increased with the storage time. However, the internal structure of the heated egg yolk gel showed a continuous void structure (Figure 8b), and with the extension of the storage time of egg yolk powder, the cavity increased, and the structure became more loose compared with the control group. The spherical structures in Figure 8a are oil droplets and lipoproteins, and the appearance of Figure 8 may be attributed to the heat-induced denaturation of lipoproteins in the egg yolk, releasing free lipids and migrating up and down [50]. It can be inferred that when the egg yolk is heat-treated, the denatured lipoproteins release more lipids, and then the released lipids migrate up and down. They also serve as solvents to extract more lipids [44]. With the increase in storage time, more free lipid molecules are produced in the egg yolk powder, and the increase in free lipids will make the heated egg yolk gel unfold more thoroughly inside, the structure collapse, and the cavity increase. The upper part of the yolk is bound to more lipids and water by hydrogen or hydrophobic interactions to maintain the spherical structure [8]. These results were consistent with the structural results measured using low-field NMR and MRI and confirmed the lipid precipitation problem in egg yolk powder after rehydration for a period of time.
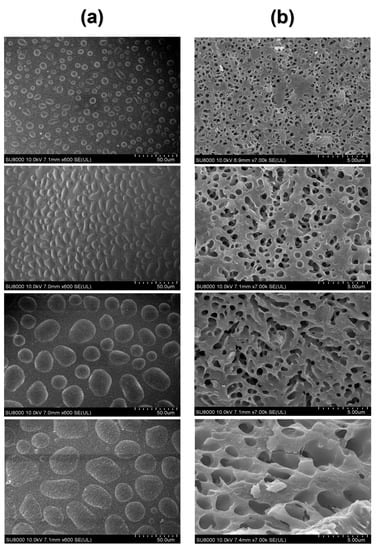
Figure 8.
SEM images of egg yolk gelation at different stored times. (a) surface SEM images of egg yolk gelation. (b) inside SEM images of egg yolk gelation.
4. Discussion
Protein is an important food component and plays various roles in food quality and stability. However, the interaction between lipid oxidation products and proteins can cause structural changes in proteins, affecting their functional properties [51]. Previous studies have reported that egg yolk powder contains many lipids, and spontaneous chain oxidation reactions will occur during storage. The production of lipid oxidation products in egg yolk powder increased with longer storage time [52]. The change in processing and storage conditions of egg yolk powder will lead to a change in the physical and functional characteristics of egg yolk powder [53], which will affect the storage stability of egg yolk powder and its nutritional characteristics [54,55]. This paper focuses on the processing characteristics of egg yolk powder and mainly studies and analyzes the changes in gel characteristics during storage. The essence of gel properties is a change in the protein system that allows the aggregation of partially denatured protein molecules and the binding of aggregates [56]. The results show that during storage, protein oxidation occurs in egg yolk powder, and covalent crosslinking and aggregation occur, which reduce the stability of the protein structure and leads to changes in the spatial conformation of lipoprotein. The protein gel has a loose three-dimensional network structure, and the activity of the lipid molecules embedded in the structure increases. The lipid molecules aggregate together with the driving force generated by heating and the effect of Oswald aging. Due to their low density, they gradually float up, and the final gel state shows the appearance of lipid precipitation and a loose gel structure.
There are many reasons for protein oxidation. As far as the yolk powder itself is concerned, the interaction between lipid oxidation products and protein oxidation is closely related. First, the protein’s secondary structure is mainly maintained by hydrogen bonds formed by carbonyl and amide groups on the backbone. When oxidation occurs, hydrogen bonds and electrostatic interactions that change the ordered structure of the protein occur. At the same time, disulfide bonds and non-covalent bonds generated during oxidation provide the force for protein polymerization. As a result, the protein particle size increases, the ordered structure is transformed into the disordered structure, and the random coil rises [57]. Secondly, the primary oxidation products produced in the process of lipid oxidation will be cleaved to generate free radicals. As potential initiating factors, free radicals cause the protein reaction system to generate free radicals, thus initiating the chain polymerization reaction and leading to protein aggregation [58]. At the same time, secondary oxidation products generated during lipid oxidation can react with the amino groups of protein molecules (Michael addition reaction), resulting in intrachain and interchain crosslinks of polypeptide chains. This interaction mainly acts on nucleophilic amino acid residues, forming disulfide and double tyrosine bonds, which lead to protein crosslinking [59].
The functions of proteins are diverse, and changes in protein structure will inevitably lead to changes in their functional properties, including food texture, water-holding capacity, emulsification, and dispersion. In order to more accurately study the effects of lipid oxidation on protein structure and function, scholars have established different oxidation models to study the effects of lipid oxidation products on proteins. SHEN et al. [60], when studying the effect of oxidation on the gel properties of pork myofibrillar protein and its binding ability with flavor compounds, found that mild oxidation could induce the partial unfolding of pork myofibrillar protein, thereby increasing the salt solubility of myofibrillar protein and improving the hardness of its gel. Bao [12,61] also studied the effects of malondialdehyde and AAPH on the structure and emulsification of high-density lipoprotein in egg yolk, and found that with the increase in oxidation degree, the emulsification characteristics of high-density lipoprotein changed approximately the same as the emulsification characteristics of egg yolk powder after storage. Combined with related studies, Bao also speculated that the unsynchronization might be the complex composition of egg yolk powder. For example, phospholipids are often used as antioxidants [62], and when the imidazole group of His is degraded, it can effectively quench the -OH radical [63]. The Glu-Leu sequence contributes to the O2− quenching capacity [64]. Therefore, lipid oxidation in fat-containing foods may be delayed due to the presence of certain proteins and amino acids, making the oxidation model out of sync with the changes in the processing characteristics of actual stored products.
5. Conclusions
The gel properties of egg yolk powder during storage were studied and analyzed through an accelerated storage experiment at 37 °C. The results showed that lipid migration of the rehydrated yolk gel appeared after storage. With the extension of storage time, the content of protein carbonyl increased, the content of free sulfhydryl and total sulfhydryl decreased, the secondary structure of the yolk protein was oxidized, the stability of the yolk protein changed from ordered to disordered, and the structural stability became worse. After storage, the oxidized aggregation of lipoproteins in the egg yolk powder resulted in insoluble aggregates, affecting the egg yolk protein’s gel properties. The experimental results provide a powerful scientific basis for further optimizing the processing parameters of yolk powder, reducing the degree of spontaneous reactions during storage, and improving the processing properties of yolk powder.
Author Contributions
Y.T.: Writing—original draft, validation verification, formal analysis. S.L.: Conceptualization, supervision, project administration. Z.B.: Writing—review and editing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32001725.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to them containing information that could compromise research participants’ consent.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Palacios, L.E.; Wang, T. Egg yolk lecithin fractionation and characterization. J. Am. Oil. Chem. Soc. 2005, 82, 571–578. [Google Scholar] [CrossRef]
- Rao, Q.C.; Rocca-Smith, J.R.; Labuza, T.P. Storage stability of hen egg white powders in three protein/water dough model systems. Food Chem. 2013, 138, 1087–1094. [Google Scholar] [CrossRef]
- Erçelebi, E.A.; Ibanoğlu, E. Effects of pectin and guar gum on creaming stability, microstructure and rheology of egg yolk plasma-stabilized emulsions. Eur. Food Res. Technol. Eur. 2010, 231, 297–302. [Google Scholar] [CrossRef]
- Anton, M.; Denmat, M.L.; Beaumal, V.; Pilet, P. Filler effects of oil droplets on the rheology of heat-set emulsion gels prepared with egg yolk and egg yolk fractions. Colloid Surface B 2001, 21, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.H.; Zhao, Y.; Yao, Y.; Wu, W.; Xu, M.S.; Du, H.Y.; Tu, Y.G. Biological activities of egg yolk lipids: A review. J. Agric. Food Chem. 2020, 68, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Monica, P.; Nuria, A.; Tong, W. Use of reconstituted yolk systems to study the gelation mechanism of frozen-thawed hen egg yolk. J. Agric. Food Chem. 2018, 66, 512–520. [Google Scholar]
- Monica, P.; Tao, F.; Nuria, A.; Tong, W. Effect of food additives on egg yolk gelation induced by freezing. Food Chem. 2018, 263, 142–150. [Google Scholar]
- Blume, K.; Dietrich, K.; Lilienthal, S.; Ternes, W.; Drotleff, A.M. Exploring the relationship between protein secondary structures, temperature-dependent viscosities, and technological treatments in egg yolk and LDL by FTIR and rheology. Food Chem. 2015, 173, 584–593. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Liu, F.; Luo, Y.C. Recent development of egg protein fractions and individual proteins as encapsulant materials for delivery of bioactive. Food Chem. 2023, 134353. [Google Scholar] [CrossRef]
- Razi, S.M.; Fahim, H.; Amirabadi, S.; Rashidinejad, A. An overview of the functional properties of egg white proteins and their application in the food industry. Food Hydrocolloid 2022, 135, 108183. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, F.; Yang, Y.; Xiong, C.; Tu, Y. Gelation behavior of egg yolk under physical and chemical induction: A review. Food Chem. 2021, 355, 129569. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.J.; Kang, D.; Xu, X.M.; Sun, N.; Lin, S.Y. Variation in the structure and emulsification of egg yolk high-density lipoprotein by lipid peroxide. J. Food Biochem. 2019, 43, e13019. [Google Scholar] [CrossRef] [PubMed]
- Chorna, I.V.; Dronik, G.B.; Lukashiv, T.O.; Yuzkova, V.D. Oxidatively modified proteins in kidneys of rats fed with glyphosate-resistant genetically modified soybean and the herbicide Roundup. Regul. Mech. Biosyst. 2019, 10, 319–325. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, T.; Wang, X.; Song, Y.; Wang, H.; Wang, H.; Tan, M. Influence of salting processes on water and lipid dynamics, physicochemical and microstructure of duck egg. LWT 2018, 95, 143–149. [Google Scholar] [CrossRef]
- Aursand, I.G.; Gallart, J.U.; Erikson, D.E.; Axelson; Rustad, T. Water distribution in brine salted cod (Gadus morhua) and salmon (Salmo salar): A low field 1H NMR study. J. Agric. Food Chem. 2008, 56, 6252. [Google Scholar] [CrossRef]
- Dukhin, A.S.; Goetz, P.J. Applications for emulsions and other soft particles. Stud. Interface Sci. 2010, 24, 369–400. [Google Scholar]
- Dukhin, A.S.; Goetz, P.J.; Truesdail, S. Titration of concentrated dispersion using electroacoustic ξ-potential probe. Langmuir 2001, 17, 964–968. [Google Scholar] [CrossRef]
- Matumoto-Pintro, P.T.; Murakami, A.E.; Pelaes Vital, A.C.; Croge, C.; Silva, D.; Ospina-Roja, I.C. Effects of storage time and temperature on lipid oxidation of egg powders enriched with natural antioxidants. Food Chem. 2017, 228, 463–468. [Google Scholar] [CrossRef]
- Raitio, R.; Orlien, V.; Skibsted, L.H. Storage stability of cauliflower soup powder: The effect of lipid oxidation and protein degradation reactions. Food Chem. 2011, 128, 371–379. [Google Scholar] [CrossRef]
- Donkeun, P.; Youling, X.; Amy, L.A.; Tooru, O. Biochemical Changes in Myofibrillar Protein Isolates Exposed to Three Oxidizing Systems. J. Agric. Food Chem. 2006, 54, 4445–4451. [Google Scholar]
- Delles, R.M.; Xiong, Y.L. The effect of protein oxidation on hydration and water-binding in pork packaged in an oxygen-enriched atmosphere. Meat Sci. 2014, 97, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Mallis, R.J. Aging and oxidation of reactive protein sulfhydryls. Exp. Gerontol. 2001, 36, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [CrossRef]
- Xia, X.F.; Kong, B.H.; Xiong, Y.L. Decreased gelling and emulsifying properties of myofibrillar protein from repeatedly frozen—thawed porcine longissimus muscle are due to protein denaturation and susceptibility to aggregation. Meat Sci. 2010, 85, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Vander, P.I.; Van, L.A.; Hendrickx, M.E. Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment. J. Agric. Food Chem. 2005, 53, 5726–5733. [Google Scholar]
- Nicolaï, A.; Delarue, P.; Senet, P. Low-frequency, functional, modes of proteins: All-atom and coarse-grained normal mode analysis. Springer 2014, 1, 4233. [Google Scholar]
- Bao, P.; Liu, X.; Huang, T.; Wu, T. Comparative study of the structure of TvNPV polyhedrin and TvNPV virion protein by Raman spectroscopy. J. Raman Spectrosc. 1991, 22, 445–447. [Google Scholar] [CrossRef]
- Lawson, E.E.; Barry, B.W.; Williams, A.C.; Edwards, H.G.M. Biomedical applications of Raman spectroscopy. J. Raman Spectrosc. 1997, 28, 111–117. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, Y.L. Comparative time-course of lipid and myofibrillar protein oxidation in different biphasic systems under hydroxyl radical stress. Food Chem. 2018, 243, 231–238. [Google Scholar] [CrossRef]
- Mizutani, Y.; Kamogawa, K.; Nakanishi, K. Effect of urea on hydrophobic interaction: Raman difference spectroscopy on the carbon-hydrogen stretching vibration of acetone and the carbon-ni-trogen stretching vibration of urea. J. Phys. Chem. 1989, 93, 5650–5654. [Google Scholar] [CrossRef]
- Ibarra, M.B.; Naganathan, A.N.; Sanchez-Ruiz, J.M.; Munoz, V. Modern Analysis of Protein Folding by Differential Scanning Calorimetry. Methods Enzymol. 2016, 567, 281–318. [Google Scholar]
- Arntfield, S.D.; Murray, E.D.; Ismond, M.A.H. Effect of salt on the thermal stability of storage proteins from fababean (Vicia faba). Food Sci. 1986, 51, 371–377. [Google Scholar] [CrossRef]
- Wang, R.; Ma, Y.; Ma, Z. Changes in gelation, aggregation and intermolecular forces in frozen-thawed egg yolks during freezing. Food Hydrocolloid. 2020, 108, 105947. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.T.; Ding, W. Effect of dose rate on lipid and protein oxidation and the properties of beef proteins. Mod. Food Sci. Technol. 2015, 31, 122–128. [Google Scholar]
- Amin, S.; Barnett, G.V.; Pathak, J.A.; Roberts, C.J.; Sarangapani, P.S. Protein aggregation, particle formation, characterization & rheology. Cur. Opin. Col. Interf. Sci. 2014, 19, 438–449. [Google Scholar]
- Terabayashi, T.; Tsuda, M.; Kawanishi, Y. The characteristic negative Cotton effect of ganglioside lactones observed by circular dichroism spectrometry. Anal. Biochem. 1992, 204, 15–21. [Google Scholar] [CrossRef]
- Shen, X.C.; Liang, H.; He, X.W.; Wang, X.S. Recent Trends and Spectroscopic Methods for Analysis of the Protein Conformation with Circular Dichroism. Chin. J. Anal. Chem. 2004, 13, 235–258. [Google Scholar]
- Nicolini, C.; Erokhin, V.; Antolini, F.; Catasti, P.; Facci, P. Thermal stability of protein secondary structure in Langmuir-Blodgett films. B BA-Biomembranes. 1993, 1158, 273–278. [Google Scholar] [CrossRef]
- Bao, Z.J.; Wu, J.P.; Cheng, Y. Effects of lipid peroxide on the structure and gel properties of ovalbumin. Process. Biochem. 2017, 57, 124–130. [Google Scholar] [CrossRef]
- Ji, J.A.; Zhang, B.; Cheng, W.; Wang, Y.J. Methionine, tryptophan, and histidine oxidation in a model protein, pth: Mechanisms and stabilization. J. Pharm. Sci. 2009, 98, 4485–4500. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Kinsella, J.E. Changes induced in beta-lactoglobulin B following interactions with linoleic acid 13-hydroperoxide. J. Agric. Food Chem. 2002, 37, 860–866. [Google Scholar] [CrossRef]
- Guo, M. Storage Stability of a Commercial Spray Dried Hen Egg Yolk Powder. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2016. [Google Scholar]
- Bao, Z.; Kang, D.; Li, C.; Zhang, F.; Lin, S. Effect of salting on the water migration, physicochemical and textural characteristics, and microstructure of quail eggs. LWT 2020, 132, 109847. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, Y.; Wang, X.Y.; Jin, Y.G. Changes in structure and flavor of egg yolk gel induced by lipid migration under heating. Food Hydrocolloid. 2019, 98, 105257. [Google Scholar]
- Liu, Y.Y.; Cai, Z.X.; Sheng, L.; Ma, M.H.; Xu, Q. Influence of nanosilica on inner structure and performance of chitosan-based films. Carbohydr. Polym. 2019, 212, 421–429. [Google Scholar] [CrossRef]
- Shao, J.H.; Deng, Y.M.; Song, L.; Batur, A.; Jia, N.; Liu, D.Y. Investigation the effects of protein hydration states on the mobility water and fat in meat batters by LF-NMR technique. LWT 2016, 66, 1–6. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Li, T.; Zheng, H.; Khan, M.A.; Xu, X. Effect of protein structure on water and fat distribution during meat gelling. Food Chem. 2016, 204, 239–245. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.M.; Shen, Q. Discrimination of edible vegetable oil adulteration with used frying oil by low field nuclear magnetic resonance. Food Bioprocess Tech. 2013, 6, 2562–2570. [Google Scholar] [CrossRef]
- Li, T.; Rui, X.; Tu, C.; Li, W.; Dong, M. NMR relaxometry and imaging to study water dynamics during soaking and blanching of soybean. Int. J. Food Eng. 2015, 12, 181–188. [Google Scholar] [CrossRef]
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W. Effects of salting processes and time on the chemical composition, textural properties, and microstructure of cooked duck egg. J. Food Sci. 2011, 76, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Boeren, S.; Ertbjerg, P. Myofibrillar protein oxidation affects filament charges, aggregation and water-holding. Meat Sci. 2018, 135, 102–108. [Google Scholar] [CrossRef]
- Li, S.X.; Cherian, G.; Sim, J.S. Cholesterol Oxidation in Egg Yolk Powder During Storage and Heating as Affected by Dietary Oils and Tocopherol. J. Food Sci. 1996, 61, 721–725. [Google Scholar] [CrossRef]
- Rannou, C.; Queveau, D.; Beaumal, V.; David-Briand, E.; Le Borgne, C.; Meynier, A.; Anton, M.; Prost, C.P.; Schuck, C. Loisel Effect of spray-drying and storage conditions on the physical and functional properties of standard and n−3 enriched egg yolk powders. J. Food Eng. 2015, 154, 58–68. [Google Scholar] [CrossRef]
- Rao, Q.C.; Fisher, M.C.; Guo, M.F.; Labuza, T.P. Storage Stability of a Commercial Hen Egg Yolk Powder in Dry and Intermediate-Moisture Food Matrices. J. Agric. Food Chem. 2013, 61, 8676–8686. [Google Scholar] [CrossRef]
- Wenzel, M.; Ingrid, S.B.; Elmar, S. Influences of storage time and temperature on the xanthophyll content of freeze-dried egg yolk. Food Chem. 2011, 124, 1343–1348. [Google Scholar] [CrossRef]
- Cordobés, F.; Partal, P.; Guerrero, A. Rheology and microstructure of heat-induced egg yolk gels. Rheol. Acta. 2004, 43, 184–195. [Google Scholar] [CrossRef]
- Wu, W.; Wu, X.; Hu, Y. Structural modification of soy protein by the lipid peroxidation product acrolein. LWT 2010, 43, 133–140. [Google Scholar] [CrossRef]
- Rakotondramavo, A.; Rabesona, H.; Brou, C.; de Lamballerie, M.; Pottier, L. Ham processing: Effects of tumbling, cooking and high pressure on proteins. Eur. Food Res. Technol. 2019, 245, 273–284. [Google Scholar] [CrossRef]
- Alavi, F.; Emam, D.Z.; Momen, S. Effect of free radical-induced aggregation on physicochemical and interface-related functionality of egg white protein. Food Hydrocolloid. 2019, 87, 734–746. [Google Scholar] [CrossRef]
- Shen, H.; Elmore, J.S.; Zhao, M. Effect of oxidation on the gel properties of porcine myofibrillar proteins and their binding abilities with selected flavour compounds. Food Chem. 2020, 329, 127032. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, S.Y.; Jiang, P.F.; Jiang, G.S.; Bao, Z.J. Oxidative modification of malondialdehyde influences the structure and emulsification properties of egg yolk high-density lipoprotein. Food Biosci. 2023, 52, 102444. [Google Scholar] [CrossRef]
- Chen, D.W.; Wan, P.; Yao, J.Y.; Yang, X.Y.; Liu, J. Egg yolk phospholipids as an ideal precursor of fatty note odorants for chicken meat and fried foods: A review. Food Chem. 2023, 407, 135177. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.M.; Knoerzer, K.; Arcot, J. Effect of low moisture extrusion on a pea protein isolate′s expansion, solubility, molecular weight distribution and secondary structure as determined by fourier transform infrared spectroscopy (FTIR). J. Food Eng. 2017, 214, 166–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).