A Turn-On Fluorescence Sensor Based on Nitrogen-Doped Carbon Dots and Cu2+ for Sensitively and Selectively Sensing Glyphosate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of NCDs
2.3. Characterization and Photophysical Investigation
2.4. Sample Preparation for Glyphosate Analysis
3. Results and Discussion
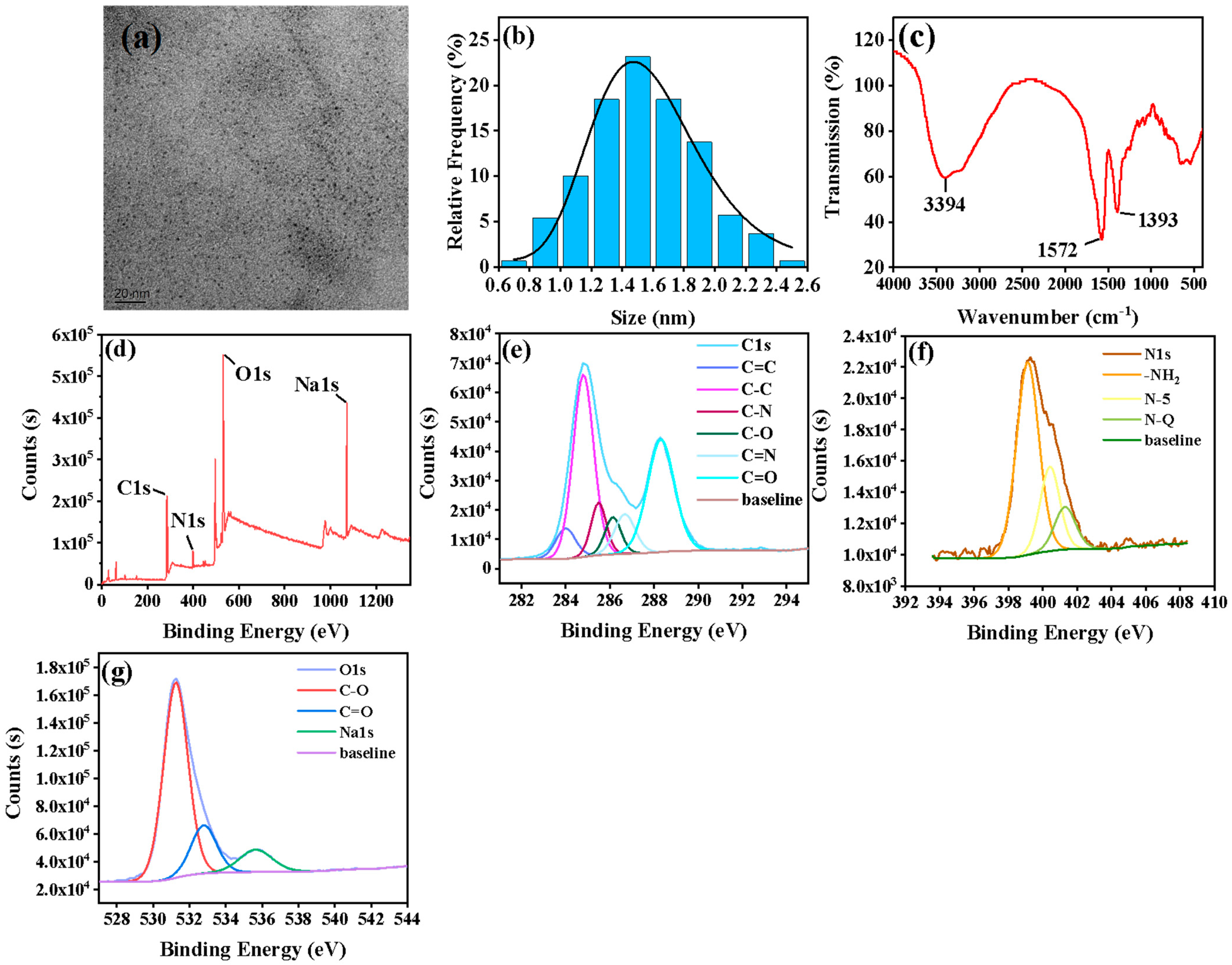
3.1. Characterization of NCDs
3.2. Optical Properties of NCDs
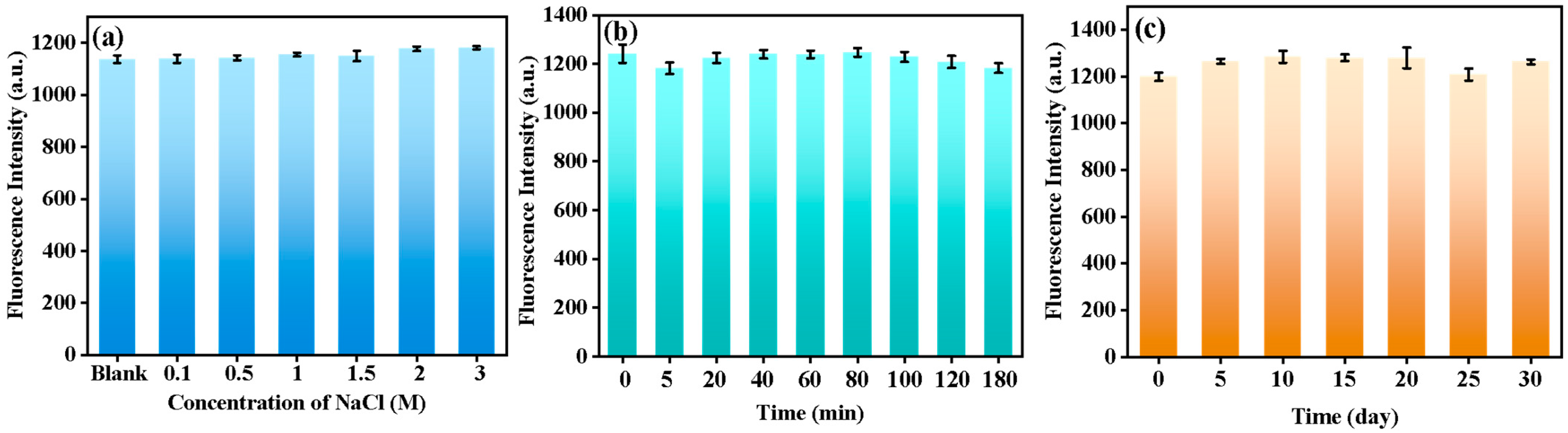
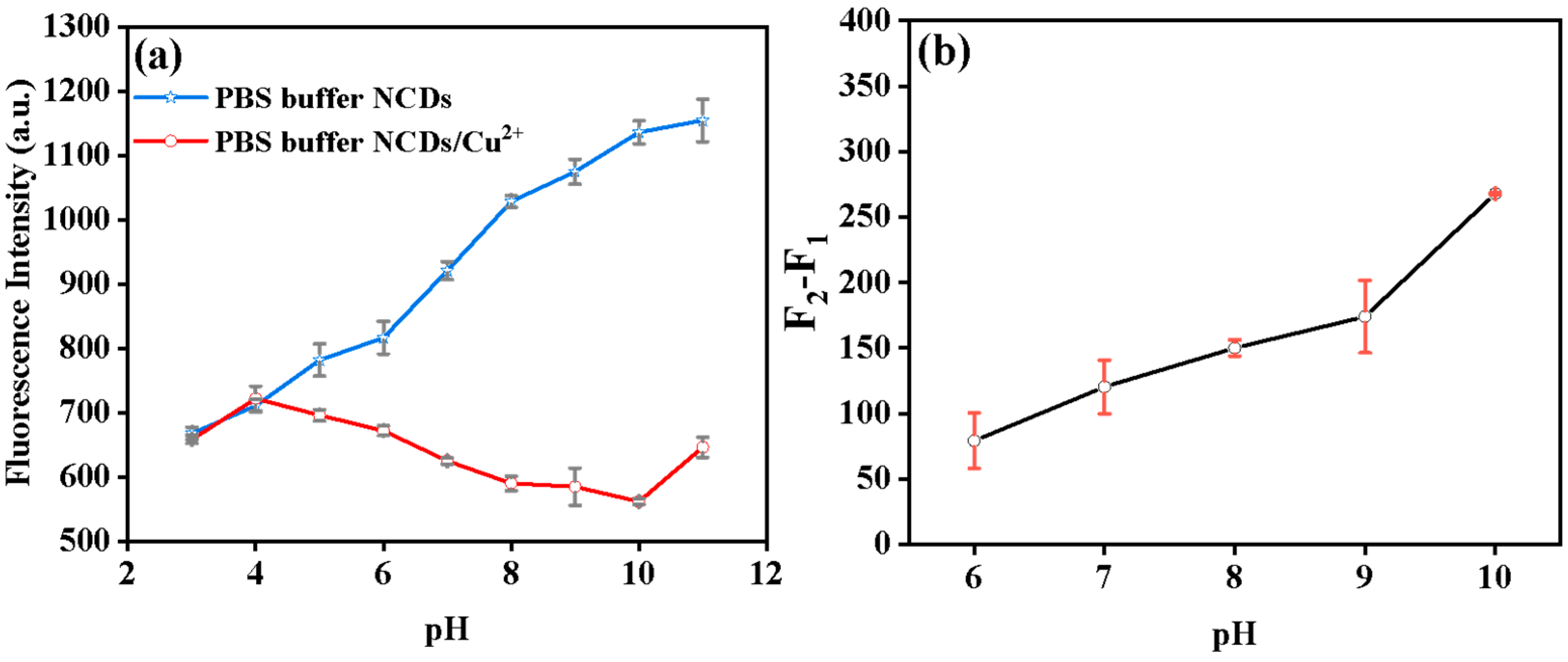
3.3. Optimizing Parameters for Glyphosate Analysis
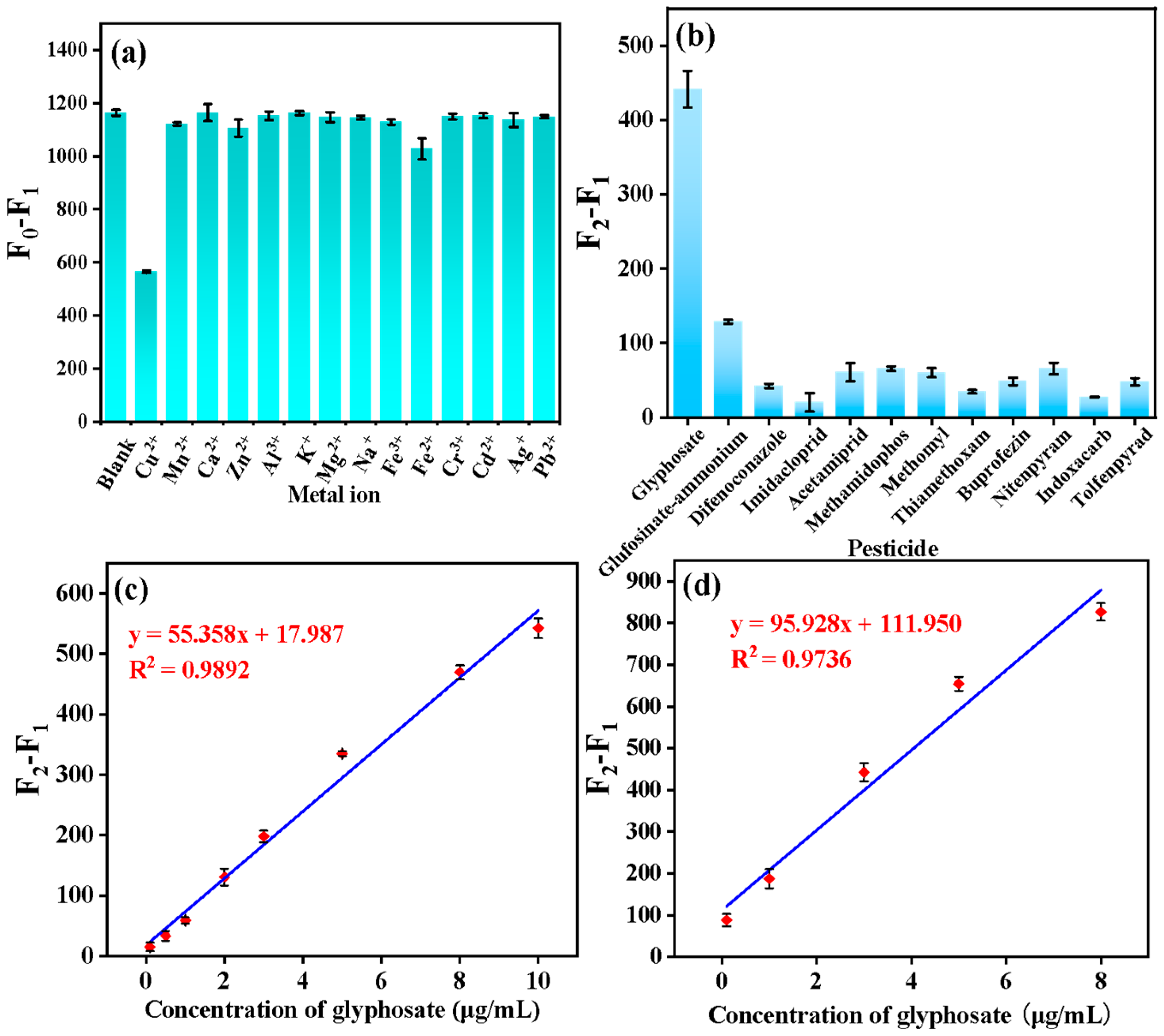
3.4. Selectivity and Sensitivity of Glyphosate Detection
3.5. Fluorescent Sensing Principle of Glyphosate
3.6. Sample Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Li, G.D.; Liu, H.; Leung, C.H.; Ma, D.L. G-quadruplex-based detection of glyphosate in complex biological systems by a time-resolved luminescent assay. Sens. Actuator B-Chem. 2020, 320, 128393. [Google Scholar] [CrossRef]
- Wan, C.Q.; Pang, Y.H.; Feng, Y.W.; Shen, X.F. A ratio fluorescence sensor based on rhodamine B embedded metal-organic framework for glyphosate detection in agri-food products. Food Chem. 2022, 394, 133446. [Google Scholar] [CrossRef] [PubMed]
- Van Stempvoort, D.R.; Roy, J.W.; Brown, S.J.; Bickerton, G. Residues of the herbicide glyphosate in riparian groundwater in urban catchments. Chemosphere 2014, 95, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pedersen, T.; Fischer, M.; White, R.; Young, T. Herbicide runoff along highways. 1. field observations. Environ. Sci. Technol. 2004, 38, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Z.; Keleş, M. A reaction-based system for the colorimetric detection of glyphosate in real samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120501. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.; Llorent-Martinez, E.; Ortega-Barrales, P.; Ruiz-Medina, A. Graphene quantum dots-silver nanoparticles as a novel sensitive and selective luminescence probe for the detection of glyphosate in food samples. Talanta 2020, 207, 120344. [Google Scholar] [CrossRef]
- Yuan, Y.N.; Liu, W.; Liu, Q.; He, Q.; Hu, Y.Y.; Jiang, X.Y.; Chen, X.Q. A dual-channel probe based on copper ion-mediated metal organic framework composite for colorimetric and ratiometric fluorescence monitoring of glyphosate degradation in soil and water. Microchim. Acta 2022, 189, 372. [Google Scholar] [CrossRef]
- Fang, H.J.; Zhang, X.P.; Gao, D.X.; Xiao, Y.; Ma, L.Y.; Yang, H.L.; Zhou, Y. Fluorescence determination of glyphosate based on a DNA-templated copper nanoparticle biosensor. Microchim. Acta 2022, 189, 158. [Google Scholar] [CrossRef]
- European Commission. EU Pesticides Database. 2022. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 15 March 2023).
- You, J.; Koropchak, J.A. Condensation nucleation light scattering detection with ion chromatography for direct determination of glyphosate and its metabolite in water. J. Chromatogr. A 2003, 989, 231–238. [Google Scholar] [CrossRef]
- Tseng, S.H.; Lo, Y.W.; Chang, P.C.; Chou, S.S.; Chang, H.M. Simultaneous quantification of glyphosate, glufosinate, and their major metabolites in rice and soybean sprouts by gas chromatography with pulsed flame photometric detector. J. Agric. Food Chem. 2004, 52, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Demonte, L.D.; Michlig, N.; Gaggiotti, M.; Adam, C.G.; Beldomenico, H.R.; Repetti, M.R. Determination of glyphosate, AMPA and glufosinate in dairy farm water from Argentina using a simplified UHPLC-MS/MS method. Sci. Total Environ. 2018, 645, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Gotti, R.; Fiori, J.; Furlanetto, S.; Orlandini, S.; Candela, M.; Franzellitti, S. Assessment of bioaccumulation of glyphosate and aminomethylphosphonic acid in marine mussels using capillary electrophoresis with light-emitting diode-induced fluorescence detection. J. Chromatogr. A 2022, 1681, 463452. [Google Scholar] [CrossRef] [PubMed]
- Geerdink, R.B.; Hassing, M.; Ayarza, N.; Bruggink, C.; Wielheesen, M.; Claassen, J.; Epema, O.J. Analysis of glyphosate, AMPA, glufosinate and MPPA with ion chromatography tandem mass spectrometry using a membrane suppressor in the ammonium form application to surface water of low to moderate salinity. Anal. Chim. Acta 2020, 1133, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Gao, Y.; Li, Y.L.; Li, X.L.; Zhang, H.J.; Han, X.X.; Zhao, B.; Su, L. Indirect glyphosate detection based on ninhydrin reaction and surface-enhanced raman scattering spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Mortl, M.; Nemeth, G.; Juracsek, J.; Darvas, B.; Kamp, L.; Rubio, F.; Székács, A. Determination of glyphosate residues in Hungarian water samples by immunoassay. Microchem. J. 2013, 107, 143–151. [Google Scholar] [CrossRef]
- Vaghela, C.; Kulkarni, M.; Haram, S.; Aiyer, R.; Karve, M. A novel inhibition based biosensor using urease nanoconjugate entrapped biocomposite membrane for potentiometric glyphosate detection. Int. J. Biol. Macromol. 2018, 108, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, Y.; Hou, J.; Li, H.; Xu, Y.; Wang, B.; Ding, H.; Ding, L. Facile, green and clean one-step synthesis of carbon dots from wool: Application as a sensor for glyphosate detection based on the inner filter effect. Talanta 2016, 160, 268–275. [Google Scholar] [CrossRef]
- Hou, J.Z.; Wang, X.F.; Lan, S.Y.; Zhang, C.; Hou, C.J.; He, Q.; Huo, D. A turn-on fluorescent sensor based on carbon dots from Sophora japonica leaves for the detection of glyphosate. Anal. Methods 2020, 12, 4130–4138. [Google Scholar] [CrossRef]
- Munoz, R.; Guevara-Lara, A.; Santos, J.L.M.; Miranda, J.M.; Rodriguez, J.A. Determination of glyphosate in soil samples using CdTe/CdS quantum dots in capillary electrophoresis. Microchem. J. 2019, 146, 582–587. [Google Scholar] [CrossRef]
- Shahdost-fard, F.; Fahimi-Kashani, N.; Hormozi-nezhad, M. A ratiometric fluorescence nanoprobe using CdTe QDs for fast detection of carbaryl insecticide in apple. Talanta 2021, 221, 121467. [Google Scholar] [CrossRef]
- Bala, R.; Swami, A.; Tabujew, I.; Peneva, K.; Wangoo, N.; Sharma, R.K. Ultra-sensitive detection of malathion using quantum dots-polymer based fluorescence aptasensor. Biosens. Bioelectron. 2018, 104, 45–49. [Google Scholar] [CrossRef]
- Wang, X.F.; Yang, Y.X.; Huo, D.Q.; Ji, Z.; Ma, Y.; Yang, M.; Luo, H.B.; Hou, C.J.; Lv, J.Y. A turn-on fluorescent nanoprobe based on N-doped silicon quantum dots for rapid determination of glyphosate. Microchim. Acta 2020, 187, 341. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Bano, D.; Sahoo, K.; Kumar, D.; Kumar, V.; Yadav, P.K.; Hasan, S.H. Synthesis of fluorescent carbon quantum dots from Jatropha fruits and their application in fluorometric sensor for the detection of chlorpyrifos. Microchem. J. 2022, 172, 106953. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, K.; Zhao, T.T.; Liu, B.H.; Wang, Z.Y.; Zhang, Z.P. Selective phosphorescence sensing of pesticide based on the inhibition of silver(I) quenched ZnS:Mn2+ quantum dots. Sens. Actuator B-Chem. 2017, 252, 1083–1088. [Google Scholar] [CrossRef]
- Wang, Y.Y.; He, J.; Zheng, M.D.; Qin, M.D.; Wei, W. Dual-emission of Eu based metal-organic frameworks hybrids with carbon dots for ratiometric fluorescent detection of Cr(VI). Talanta 2019, 191, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Chaghaghazardi, M.; Kashanian, S.; Nazari, M.; Omidfar, B.; Joseph, Y.; Rahimi, P. Nitrogen and sulfur co-doped carbon quantum dots fluorescence quenching assay for detection of mercury (II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 293, 122448. [Google Scholar] [CrossRef]
- Wang, T.S.; Wang, A.L.; Wang, R.X.; Liu, Z.Y.; Sun, Y.; Shan, G.Y.; Chen, Y.W.; Liu, Y.C. Carbon dots with molecular fluorescence and their application as a “turn-off” fluorescent probe for ferricyanide detection. Sci. Rep. 2019, 9, 10723. [Google Scholar] [CrossRef] [Green Version]
- Dang, V.D.; Ganganboina, A.B.; Doong, R.A. Bipyridine- and copper-dunctionalized n-doped carbon dots for fluorescence turn off-on detection of ciprofloxacin. ACS Appl. Mater. Interfaces 2020, 12, 32247–32258. [Google Scholar] [CrossRef]
- Lv, P.P.; Xie, D.D.; Zhang, Z.H. Magnetic carbon dots based molecularly imprinted polymers for fluorescent detection of bovine hemoglobin. Talanta 2018, 188, 145–151. [Google Scholar] [CrossRef]
- Kou, X.; Jiang, S.; Park, S.J.; Meng, L.Y. A review: Recent advances in preparations and applications of heteroatom-doped carbon quantum dots. Dalton Trans. 2020, 49, 6915–6938. [Google Scholar] [CrossRef]
- Mu, Y.X.; Zhuang, Q.F.; Huang, S.P.; Hu, M.Y.; Wang, Y.; Ni, Y.N. Adenine-stabilized carbon dots for highly sensitive and selective sensing of copper(II) ions and cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118531. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Bera, M.K.; Das, R.K. Rapid “turn-on” detection of atrazine using highly luminescent N-doped carbon quantum dot. Sens. Actuator B-Chem. 2018, 263, 459–468. [Google Scholar] [CrossRef]
- Jiang, X.H.; Qin, D.M.; Mo, G.C.; Feng, J.S.; Yu, C.H.; Mo, W.M.; Deng, B.Y. Ginkgo leaf-based synthesis of nitrogen-doped carbon quantum dots for highly sensitive detection of salazosulfapyridine in mouse plasma. J. Pharm. Biomed. Anal. 2019, 164, 514–519. [Google Scholar] [CrossRef]
- Wang, K.J.; Ji, Q.J.; Xu, J.L.; Li, H.X.; Zhang, D.Y.; Liu, X.Y.; Wu, Y.J.; Fan, H.Y. Highly sensitive and selective detection of amoxicillin using carbon quantum dots derived from beet. J. Fluoresc. 2018, 28, 759–765. [Google Scholar] [CrossRef]
- Yu, C.H.; Qin, D.M.; Jiang, X.H.; Zheng, X.F.; Deng, B.Y. Facile synthesis of bright yellow fluorescent nitrogen-doped carbon quantum dots and their applications to an off-on probe for highly sensitive detection of methimazole. Microchem. J. 2021, 168, 106480. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Fang, X.; Zhao, H.; Li, Z.X. A highly sensitive and selective detection of Cr(VI) and ascorbic acid based on nitrogen-doped carbon dots. Talanta 2018, 181, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.U.; Wang, D.; Zhang, W.; Tang, Z.; Ma, X.; Ding, X.; Du, S.S.; Wang, Y.H. High quantum yield green-emitting carbon dots for Fe (ІІІ) detection, biocompatible fluorescent ink and cellular imaging. Sci. Rep. 2017, 7, 14866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Chen, Y.; Mo, W.Q.; Li, X.B.; Ma, H.W.; Zhao, X.H. An “on-off-on” fluorescent system based on the microwave-assisted preparation of copper-functionalized carbon quantum dots for sensitive detection of ascorbic acid. Opt. Mater. 2021, 115, 111041. [Google Scholar] [CrossRef]
- Wang, L.; Bi, Y.D.; Gao, J.; Li, Y.J.; Ding, H.; Ding, L. Carbon dots based turn-on fluorescent probes for the sensitive determination of glyphosate in environmental water samples. RSC Adv. 2016, 6, 85820–85828. [Google Scholar] [CrossRef]
- Sun, F.; Ye, X.L.; Wang, Y.B.; Yue, M.L.; Li, P.; Yang, L.; Liu, Y.L.; Fu, Y. NPA-Cu2+ complex as a fluorescent sensing platform for the selective and sensitive detection of glyphosate. Int. J. Mol. Sci. 2021, 22, 9816. [Google Scholar] [CrossRef]
- Wang, X.F.; Sakinati, M.; Yang, Y.X.; Ma, Y.; Yang, M.; Luo, H.B.; Hou, C.J.; Huo, D.Q. The construction of a CND/Cu2+ fluorescence sensing system for the ultrasensitive detection of glyphosate. Anal. Methods 2020, 12, 520–527. [Google Scholar] [CrossRef]
- Yang, Y.X.; Li, L.; Lin, L.Y.; Wang, X.F.; Li, J.W.; Liu, H.; Liu, X.F.; Huo, D.Q.; Hou, C.J. A dual-signal sensing strategy based on ratiometric fluorescence and colorimetry for determination of Cu2+ and glyphosate. Anal. Bioanal. Chem. 2022, 414, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Huang, G.L.; Li, Y.C.; Guo, J.; Chen, X.K.; Tan, Y.W.; Tang, W.Z.; Li, Z.H. Dual-modes of ratiometric fluorescent and smartphone-integrated colorimetric detection of glyphosate by carbon dots encapsulated porphyrin metal-organic frameworks. Appl. Surf. Sci. 2022, 602, 154368. [Google Scholar] [CrossRef]



| Matrix | Linear Equation | R2 | LOD | Linear Range |
|---|---|---|---|---|
| Water | F2 − F1 = 55.358 [Cgly] + 17.987 | 0.9892 | 0.021 μg/mL | 0.10–10 μg/mL |
| Rice | F2 − F1 = 95.928 [Cgly] + 111.950 | 0.9736 | 0.049 μg/mL | 0.10–8.0 μg/mL |
| Sensor | Linear Range (μg/mL) | LOD (μg/mL) | Reference |
|---|---|---|---|
| CND/Cu2+ | 0.020–1.5 | 0.0183 | [42] |
| N-SiQDs/Cu2+ | 0.10–1.0 | 0.0078 | [23] |
| CDs/Cu2+ | 0.030–10 | 0.016 | [40] |
| CDs/AgNPs | 0.025–2.5 | 0.012 | [18] |
| SiNP/OPD/Cu2+ | 0.15–10 | 0.070 | [43] |
| N-CDs@PCN-222 | 0.010–6.6 | 0.0090 | [44] |
| ZnMOF-74@Al-MOF | 0.10–1.4 | 0.070 | [7] |
| NH2-MIL-88(Fe)@RhB/Cu2+ | 0.10–2.5 | 0.030 | [2] |
| NCDs/Cu2+ | 0.10–10 | 0.021–0.049 | This work |
| Sample Type | Spiked (μg/mL) | Found (μg/mL) | Recovery (%) | RSD (%, n = 5) |
|---|---|---|---|---|
| Tap water | 0 | ND * | — | — |
| 1 | 0.838 | 83.8 | 14.3 | |
| 3 | 3.080 | 102.7 | 2.5 | |
| 5 | 5.296 | 105.9 | 7.9 | |
| River water | 0 | ND * | — | — |
| 1 | 0.854 | 85.4 | 12.7 | |
| 3 | 3.176 | 105.9 | 6.0 | |
| 5 | 5.650 | 113.0 | 2.6 | |
| Rice | 0 | ND * | — | — |
| 1 | 0.821 | 82.1 | 13.6 | |
| 3 | 3.178 | 105.9 | 6.7 | |
| 5 | 5.554 | 111.1 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liang, S.; Zhou, L.; Luo, F.; Lou, Z.; Chen, Z.; Zhang, X.; Yang, M. A Turn-On Fluorescence Sensor Based on Nitrogen-Doped Carbon Dots and Cu2+ for Sensitively and Selectively Sensing Glyphosate. Foods 2023, 12, 2487. https://doi.org/10.3390/foods12132487
Li Z, Liang S, Zhou L, Luo F, Lou Z, Chen Z, Zhang X, Yang M. A Turn-On Fluorescence Sensor Based on Nitrogen-Doped Carbon Dots and Cu2+ for Sensitively and Selectively Sensing Glyphosate. Foods. 2023; 12(13):2487. https://doi.org/10.3390/foods12132487
Chicago/Turabian StyleLi, Ziqiang, Shuang Liang, Li Zhou, Fengjian Luo, Zhengyun Lou, Zongmao Chen, Xinzhong Zhang, and Mei Yang. 2023. "A Turn-On Fluorescence Sensor Based on Nitrogen-Doped Carbon Dots and Cu2+ for Sensitively and Selectively Sensing Glyphosate" Foods 12, no. 13: 2487. https://doi.org/10.3390/foods12132487





