Rapid and Automated Method for Detecting and Quantifying Adulterations in High-Quality Honey Using Vis-NIRs in Combination with Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Adulteration
2.3. Visible near Infrared Spectroscopy (Vis-NIRs)
2.4. Data Analysis
3. Results
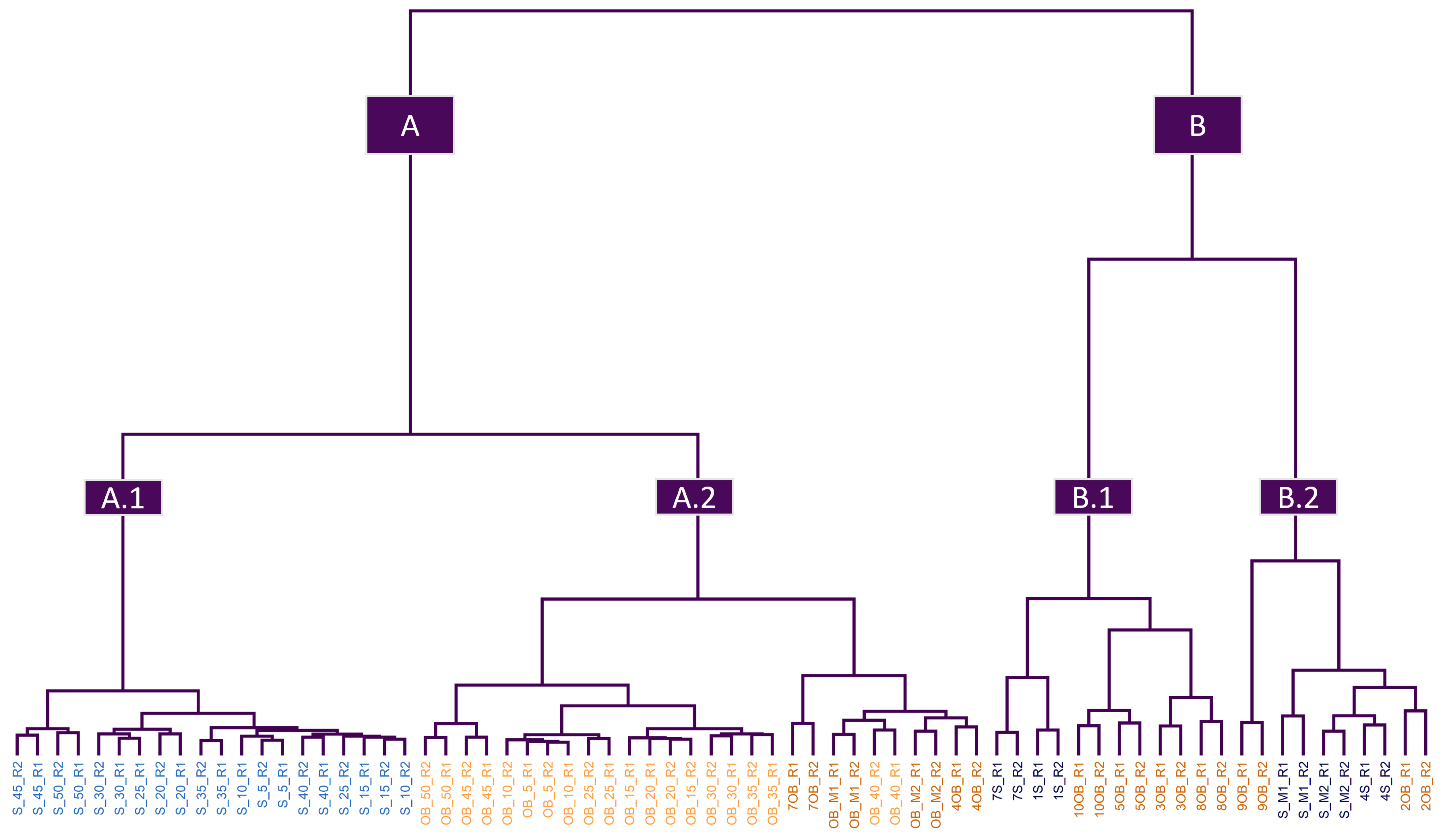
3.1. Exploratory Analysis
3.2. Classification Models for Adulterant Detection
3.2.1. Support Vector Machines (SVM) with Radial Basis Function (RBF)
3.2.2. Lineal Support Vector Machines (SVM-Lineal)
3.2.3. Lineal Discriminant Analysis (LDA)
3.2.4. Random Forest (RF)
3.3. Regression Models for Adulterant Quantification
3.3.1. Partial Least Squares (PLS)
3.3.2. Shrinkage Methods
Lasso
Ridge
Elastic Net
3.3.3. Support Vector Regression (SVR) with Gaussian Kernel
3.3.4. Random Forest Regression (RF)
3.4. Application Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilia, G.; Simulescu, V.; Merghes, P.; Varan, N. The Health Benefits of Honey as an Energy Source with Antioxidant, Antibacterial and Antiseptic Effects. Sci. Sport. 2021, 36, 272.e1–272.e10. [Google Scholar] [CrossRef]
- European Parliament Committee on the Environment, Public Health and Food Safety. REPORT on the Food Crisis, Fraud in the Food Chain and the Control Thereof; European Parliament: Strasbourg, France, 2013. [Google Scholar]
- European Parliament. Directive 2014/63/EU of the European Parliament and of the Council Amending Council Directive 2001/110/EC Relating to Honey; European Parliament: Strasbourg, France, 2014. [Google Scholar]
- Se, K.W.; Wahab, R.A.; Syed Yaacob, S.N.; Ghoshal, S.K. Detection Techniques for Adulterants in Honey: Challenges and Recent Trends. J. Food Compos. Anal. 2019, 80, 16–32. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Kosarev, M.N.; Neal, A.; Yumaguzhin, F.G. Burzyan Wild-Hive Honeybee A.M. Mellifera in South Ural. Bee World 2015, 92, 7–11. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Beatriz, M.; Oliveira, P.P.; Mafra, I. A Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Zhang, G.; Abdulla, W. On Honey Authentication and Adulterant Detection Techniques. Food Control 2022, 138, 108992. [Google Scholar] [CrossRef]
- Cotte, J.F.; Casabianca, H.; Giroud, B.; Albert, M.; Lheritier, J.; Grenier-Loustalot, M.F. Characterization of Honey Amino Acid Profiles Using High-Pressure Liquid Chromatography to Control Authenticity. Anal. Bioanal. Chem. 2004, 378, 1342–1350. [Google Scholar] [CrossRef]
- García-Seval, V.; Martínez-Alfaro, C.; Saurina, J.; Núñez, O.; Sentellas, S. Characterization, Classification and Authentication of Spanish Blossom and Honeydew Honeys by Non-Targeted HPLC-UV and Off-Line SPE HPLC-UV Polyphenolic Fingerprinting Strategies. Foods 2022, 11, 2345. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Q.; Wang, L.; Lin, L.; Shi, H.; Cao, H.; Cao, B. Detection of Honey Adulteration with Starch Syrup by High Performance Liquid Chromatography. Food Chem. 2015, 172, 669–674. [Google Scholar] [CrossRef]
- Xue, X.; Wang, Q.; Li, Y.; Wu, L.; Chen, L.; Zhao, J.; Liu, F. 2-Acetylfuran-3-Glucopyranoside as a Novel Marker for the Detection of Honey Adulterated with Rice Syrup. J. Agric. Food Chem. 2013, 61, 7488–7493. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Tatlisu, N.B.; Toker, O.S.; Karaman, S.; Dertli, E.; Sagdic, O.; Arici, M. Steady, Dynamic and Creep Rheological Analysis as a Novel Approach to Detect Honey Adulteration by Fructose and Saccharose Syrups: Correlations with HPLC-RID Results. Food Res. Int. 2014, 64, 634–646. [Google Scholar] [CrossRef]
- Sotiropoulou, N.S.; Xagoraris, M.; Revelou, P.K.; Kaparakou, E.; Kanakis, C.; Pappas, C.; Tarantilis, P. The Use of SPME-GC-MS IR and Raman Techniques for Botanical and Geographical Authentication and Detection of Adulteration of Honey. Foods 2021, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Sun, J.; Guo, J.; Li, X.; Zhang, X.; Xiao, F. Authentication of Chaste Honey Adulterated with High Fructose Corn Syrup by HS-SPME-GC-MS Coupled with Chemometrics. LWT 2023, 176, 114509. [Google Scholar] [CrossRef]
- Kružík, V.; Grégrová, A.; Rajchl, A.; Čížková, H. Study on Honey Quality Evaluation and Detection of Adulteration by Analysis of Volatile Compounds. J. Apic. Sci. 2017, 61, 17–27. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Barbero, G.F.; Palma, M. Novel Method Based on Ion Mobility Spectroscopy for the Quantification of Adulterants in Honeys. Food Control 2020, 114, 107236. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Castell, A.; Campillo, N.; Viñas, P.; López-García, I.; Hernández-Córdoba, M. Untargeted Headspace Gas Chromatography—Ion Mobility Spectrometry Analysis for Detection of Adulterated Honey. Talanta 2019, 205, 120123. [Google Scholar] [CrossRef] [PubMed]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Palma, M.; Barbero, G.F. A Screening Method Based on Headspace-Ion Mobility Spectrometry to Identify Adulterated Honey. Sensors 2019, 19, 1621. [Google Scholar] [CrossRef]
- Cagliani, L.R.; Maestri, G.; Consonni, R. Detection and Evaluation of Saccharide Adulteration in Italian Honey by NMR Spectroscopy. Food Control 2022, 133, 108574. [Google Scholar] [CrossRef]
- Rachineni, K.; Rao Kakita, V.M.; Awasthi, N.P.; Shirke, V.S.; Hosur, R.V.; Chandra Shukla, S. Identifying Type of Sugar Adulterants in Honey: Combined Application of NMR Spectroscopy and Supervised Machine Learning Classification. Curr. Res. Food Sci. 2022, 5, 272–277. [Google Scholar] [CrossRef]
- Mohamadzade Namin, S.; Yeasmin, F.; Choi, H.W.; Jung, C. DNA-Based Method for Traceability and Authentication of Apis cerana and A. Dorsata Honey (Hymenoptera: Apidae), Using the NADH Dehydrogenase 2 Gene. Foods 2022, 11, 928. [Google Scholar] [CrossRef]
- Truong, A.; Kim, S.; Yoon, B. Determination of Honey Adulterated with Corn Syrup by Quantitative Amplification of Maize Residual DNA Using Ultra-rapid Real-time PCR. J. Sci. Food Agric. 2022, 102, 774–781. [Google Scholar] [CrossRef]
- Machuca, G.; Staforelli, J.; Rondanelli-Reyes, M.; Garces, R.; Contreras-Trigo, B.; Tapia, J.; Sanhueza, I.; Jara, A.; Lamas, I.; Troncoso, J.M.; et al. Hyperspectral Microscopy Technology to Detect Syrups Adulteration of Endemic Guindo Santo and Quillay Honey Using Machine-Learning Tools. Foods 2022, 11, 3868. [Google Scholar] [CrossRef] [PubMed]
- Pirmoradi, M.; Mostafaei, M.; Naderloo, L.; Javadikia, H. Modeling Honey Adulteration by Processing Microscopic Images Using Artificial Intelligence Methods. J. Agric. Sci. Technol. 2022, 24, 365–378. [Google Scholar]
- Devi, A.; Jangir, J.; Appaiah, K.A. Chemical Characterization Complemented with Chemometrics for the Botanical Origin Identification of Unifloral and Multifloral Honeys from India. Food Res. Int. 2018, 107, 216–226. [Google Scholar] [CrossRef]
- Berghian-Grosan, C.; Hategan, A.R.; David, M.; Magdas, D.A. Untargeted Metabolomic Analysis of Honey Mixtures: Discrimination Opportunities Based on ATR-FTIR Data and Machine Learning Algorithms. Microchem. J. 2023, 188, 108458. [Google Scholar] [CrossRef]
- Rios-Corripio, M.A.; Rojas-López, M.; Delgado-Macuil, R. Analysis of Adulteration in Honey with Standard Sugar Solutions and Syrups Using Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy and Multivariate Methods. CyTA—J. Food 2012, 10, 119–122. [Google Scholar] [CrossRef]
- Riswahyuli, Y.; Rohman, A.; Setyabudi, F.M.C.S.; Raharjo, S. Indonesian Wild Honey Authenticity Analysis Using Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy Combined with Multivariate Statistical Techniques. Heliyon 2020, 6, e03662. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, J.; Lin, L.; Zhang, J.; Zhu, J.; Yao, L.; Wang, S.; Yao, Z.; Wu, Z. Low Risk of Category Misdiagnosis of Rice Syrup Adulteration in Three Botanical Origin Honey by ATR-FTIR and General Model. Food Chem. 2020, 332, 127356. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H. Honey Discrimination Using Visible and Near-Infrared Spectroscopy. ISRN Spectrosc. 2012, 2012, 487040. [Google Scholar] [CrossRef]
- Gok, S.; Severcan, M.; Goormaghtigh, E.; Kandemir, I.; Severcan, F. Differentiation of Anatolian Honey Samples from Different Botanical Origins by ATR-FTIR Spectroscopy Using Multivariate Analysis. Food Chem. 2015, 170, 234–240. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Ye, Z.; Zhao, J.; Xue, X.; Vander Heyden, Y.; Sun, Q. Classification of Chinese Honeys According to Their Floral Origin by near Infrared Spectroscopy. Food Chem. 2012, 135, 338–342. [Google Scholar] [CrossRef]
- Valinger, D.; Longin, L.; Grbeš, F.; Benković, M.; Jurina, T.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Detection of Honey Adulteration—The Potential of UV-VIS and NIR Spectroscopy Coupled with Multivariate Analysis. LWT 2021, 145, 111316. [Google Scholar] [CrossRef]
- Yang, X.; Guang, P.; Xu, G.; Zhu, S.; Chen, Z.; Huang, F. Manuka Honey Adulteration Detection Based on Near-Infrared Spectroscopy Combined with Aquaphotomics. LWT 2020, 132, 109837. [Google Scholar] [CrossRef]
- Huang, F.; Song, H.; Guo, L.; Guang, P.; Yang, X.; Li, L.; Zhao, H.; Yang, M. Detection of Adulteration in Chinese Honey Using NIR and ATR-FTIR Spectral Data Fusion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 235, 118297. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Palma, M.; Barbero, G.F. A Screening Method Based on Visible-NIR Spectroscopy for the Identification and Quantification of Different Adulterants in High-Quality Honey. Talanta 2019, 203, 235–241. [Google Scholar] [CrossRef]
- Ferreiro-González, M.; Espada-Bellido, E.; Guillén-Cueto, L.; Palma, M.; Barroso, C.G.; Barbero, G.F. Rapid Quantification of Honey Adulteration by Visible-near Infrared Spectroscopy Combined with Chemometrics. Talanta 2018, 188, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, X.; Shan, Y.; Su, D.; Ma, Q.; Wen, R.; Li, J. Qualitative and Quantitative Detection of Honey Adulterated with High-Fructose Corn Syrup and Maltose Syrup by Using near-Infrared Spectroscopy. Food Chem. 2017, 218, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Bázár, G.; Romvári, R.; Szabó, A.; Somogyi, T.; Éles, V.; Tsenkova, R. NIR Detection of Honey Adulteration Reveals Differences in Water Spectral Pattern. Food Chem. 2016, 194, 873–880. [Google Scholar] [CrossRef]
- Kumaravelu, C.; Gopal, A. Detection and Quantification of Adulteration in Honey through Near Infrared Spectroscopy. Int. J. Food Prop. 2015, 18, 1930–1935. [Google Scholar] [CrossRef]
- Mishra, P.; Biancolillo, A.; Roger, J.M.; Marini, F.; Rutledge, D.N. New Data Preprocessing Trends Based on Ensemble of Multiple Preprocessing Techniques. TrAC Trends Anal. Chem. 2020, 132, 116045. [Google Scholar] [CrossRef]
- Calle, J.L.P.; Barea-Sepúlveda, M.; Ruiz-Rodríguez, A.; Álvarez, J.Á.; Ferreiro-González, M.; Palma, M. Rapid Detection and Quantification of Adulterants in Fruit Juices Using Machine Learning Tools and Spectroscopy Data. Sensors 2022, 22, 3852. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Stevens, A.; Ramirez-Lopez, L. An Introduction to the Prospectr Package; R Package Vignette R Package Version 0.2.6; R Foundation for Statistical Computing: Indianapolis, IN, USA, 2020. [Google Scholar]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 20 June 2023).
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Chang, W.; Cheng, J.; Allaire, J.; Sievert, C.; Schloerke, B.; Xie, Y.; Allen, J.; McPherson, J.; Dipert, A.; Borges, B. _shiny: Web Application Framework for R. R Package Version 1.7.4. 2022. Available online: https://CRAN.R-project.org/package=shiny (accessed on 20 June 2023).
- Calle, J.L.P.; Ferreiro-González, M.; Ruiz-Rodríguez, A.; Fernández, D.; Palma, M. Detection of Adulterations in Fruit Juices Using Machine Learning Methods over FT-IR Spectroscopic Data. Agronomy 2022, 12, 683. [Google Scholar] [CrossRef]
- Calle, J.L.P.; Falatová, B.; Aliaño-González, M.J.; Ferreiro-González, M.; Palma, M. Machine Learning Approaches over Ion Mobility Spectra for the Discrimination of Ignitable Liquids Residues from Interfering Substrates. Talanta Open 2022, 6, 100125. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S.; Shan, Y.; Zhang, Z.; Li, G.; Su, D.; Liu, F. Detection of Adulterants Such as Sweeteners Materials in Honey Using Near-Infrared Spectroscopy and Chemometrics. J. Food Eng. 2010, 101, 92–97. [Google Scholar] [CrossRef]
- Heidari, A.; Navimipour, N.J.; Jamali, M.A.J.; Akbarpour, S. A Green, Secure, and Deep Intelligent Method for Dynamic IoT-Edge-Cloud Offloading Scenarios. Sustain. Comput. Inform. Syst. 2023, 38, 100859. [Google Scholar] [CrossRef]
- Heidari, A.; Javaheri, D.; Toumaj, S.; Navimipour, N.J.; Rezaei, M.; Unal, M. A New Lung Cancer Detection Method Based on the Chest CT Images Using Federated Learning and Blockchain Systems. Artif. Intell. Med. 2023, 141, 102572. [Google Scholar] [CrossRef]
- Elhamdaoui, O.; El Orche, A.; Cheikh, A.; Mojemmi, B.; Nejjari, R.; Bouatia, M. Development of Fast Analytical Method for the Detection and Quantification of Honey Adulteration Using Vibrational Spectroscopy and Chemometrics Tools. J. Anal. Methods Chem. 2020, 2020, 8816249. [Google Scholar] [CrossRef]
- Šnurkovič, P. Quality Assessment of Fruit Juices by NIR Spectroscopy. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 803–812. [Google Scholar] [CrossRef]
- Li, C.; He, M.; Cai, Z.; Qi, H.; Zhang, J.; Zhang, C. Hyperspectral Imaging with Machine Learning Approaches for Assessing Soluble Solids Content of Tribute Citru. Foods 2023, 12, 247. [Google Scholar] [CrossRef]

| Models | Hyperparameter | CV-5-Fold Accuracy | Training Set Accuracy | Test Set Accuracy |
|---|---|---|---|---|
| SVM GAUSSIAN | C = 1 Y = 1.38 × 10−3 | 100% | 100% | 100% |
| SVM LINEAL | C = 9.77 × 10−4 | 100% | 100% | 100% |
| LDA | - | - | 100% | 94.12% |
| RF | mtry = 64 ntree = 500 | - | 100% | 100 % |
| MODELS | Hyperparameters | LOOCV Performance | Training Set Performance | Test Set Performance |
|---|---|---|---|---|
| PLS | 8 principal components | RMSE = 3.961 R2 = 0.921 | RMSE = 2.102 R2 = 0.981 | RMSE = 2.784 R2 = 0.976 |
| SVR | C = 64 Y = 5.52 × 10−3 | RMSE = 2.075 R2 = 0.987 | RMSE = 1.432 R2 = 0.994 | RMSE = 1.894 R2 = 0.991 |
| RF | mtry = 5 | RMSE = 7.328 R2 = 0.851 | RMSE = 2.754 R2 = 0.985 | RMSE = 8.475 R2 = 0.813 |
| LASSO | λ = 1 | RMSE = 3.138 R2 =0.964 | RMSE = 1.983 R2 = 0.996 | RMSE = 2.081 R2 = 0.986 |
| RIDGE | λ = 4 | RMSE = 5.312 R2 = 0.871 | RMSE = 5.071 R2 = 0.885 | RMSE = 12.352 R2 = 0.723 |
| ELASTIC NET | λ = 0.22 α = 0.53 | RMSE = 3.501 R2 = 0.952 | RMSE = 3.031 R2 = 0.969 | RMSE = 3.586 R2 = 0.939 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calle, J.L.P.; Punta-Sánchez, I.; González-de-Peredo, A.V.; Ruiz-Rodríguez, A.; Ferreiro-González, M.; Palma, M. Rapid and Automated Method for Detecting and Quantifying Adulterations in High-Quality Honey Using Vis-NIRs in Combination with Machine Learning. Foods 2023, 12, 2491. https://doi.org/10.3390/foods12132491
Calle JLP, Punta-Sánchez I, González-de-Peredo AV, Ruiz-Rodríguez A, Ferreiro-González M, Palma M. Rapid and Automated Method for Detecting and Quantifying Adulterations in High-Quality Honey Using Vis-NIRs in Combination with Machine Learning. Foods. 2023; 12(13):2491. https://doi.org/10.3390/foods12132491
Chicago/Turabian StyleCalle, José Luis P., Irene Punta-Sánchez, Ana Velasco González-de-Peredo, Ana Ruiz-Rodríguez, Marta Ferreiro-González, and Miguel Palma. 2023. "Rapid and Automated Method for Detecting and Quantifying Adulterations in High-Quality Honey Using Vis-NIRs in Combination with Machine Learning" Foods 12, no. 13: 2491. https://doi.org/10.3390/foods12132491
APA StyleCalle, J. L. P., Punta-Sánchez, I., González-de-Peredo, A. V., Ruiz-Rodríguez, A., Ferreiro-González, M., & Palma, M. (2023). Rapid and Automated Method for Detecting and Quantifying Adulterations in High-Quality Honey Using Vis-NIRs in Combination with Machine Learning. Foods, 12(13), 2491. https://doi.org/10.3390/foods12132491










