Abstract
Wood Decay Fungi (WDF) are fungi specialized in degrading wood. An interesting perspective is their use as a source of Novel Foods or food ingredients. Here, for the first time, the metabolite profiling of hydroalcoholic and organic extracts from A. biennis, F. iberica, S. hirsutum mycelia was investigated by NMR methodology. Amino acids (alanine, arginine, asparagine, aspartate, betaine, GABA, glutamate, glutamine, histidine, isoleucine, leucine, lysine, phenylalanine, threonine, tryptophan, tyrosine, valine), sugars (galactose, glucose, maltose, trehalose, mannitol), organic acids (acetate, citrate, formate, fumarate, lactate, malate, succinate), adenosine, choline, uracil and uridine were identified and quantified in the hydroalcoholic extracts, whereas the 1H spectra of organic extracts showed the presence of saturated, mono-unsaturated and di-unsaturated fatty chains, ergosterol,1,2-diacyl-sn-glycero-3-phosphatidylethanolamine, and 1,2-diacyl-sasglycero-3-phosphatidylcholine. A. biennis extracts showed the highest amino acid concentration. Some compounds were detected only in specific species: betaine and mannitol in S. hirsutum, maltose in A. biennis, galactose in F. iberica, GABA in F. iberica and S. hirsutum, and acetate in A. biennis and S. hirsutum. S. hirsutum showed the highest saturated fatty chain concentration, whereas DUFA reached the highest concentration in A. biennis. A high amount of ergosterol was measured both in A. biennis and F. iberica. The reported results can be useful in the development of WDF-based products with a high nutritional and nutraceutical value.
Keywords:
Abortiporus biennis; Fomitopsis iberica; Stereum hirsutum; mycelia; NMR; metabolomic; Novel Food 1. Introduction
Wood Decay Fungi (WDF) are a group of fungi able to grow on different forms of wood substrates such as living plants, dying trees, fallen wood or dead wood [1]. Due to their rich enzymatic pools, WDF specifically degrade wood lignin, cellulose, hemicelluloses, and pectins, becoming useful in many applicative fields such as degradation of organic pollutants, bioremediation, bioadsorption, and bioaccumulation of metal ions in living or dead biomass [2]. An interesting perspective is the possible use of WDF as source of nutraceuticals or Novel Foods.
Recently, several mushroom ingredients or mycelia powders have been studied, tested and approved as Novel Foods. It is the case of the chitin–glucan from Aspergillus niger Tiegh. and Fomes fomentarius (L.) Fr. [3], the recently approved vitamin D2 from Agaricus bisporus (J.E. Lange) Imbach powder [4] or the dehydrated mycelia powder from different WDF species including Ganoderma lucidum (Curtis) P. Karst., Grifola frondosa (Dicks.) Gray, Hericium erinaceus (Bull.) Pers., Lentinula edodes (Berk.) Pegler, Pleurotus eryngii (DC.) Quèl., Pleurotus ostreatus (Jacq.) P. Kumm., and Polyporus umbellatus (Pers.) Fr. [5].
Regarding WDF, only the mycelia of a few species such as G. lucidum or G. frondosa have been extensively investigated because of their relevance in the medical field. In particular, G. lucidum has turned out to be rich in phenolics, polysaccharides, and triterpenoids, responsible for both immunomodulatory and probiotic activities [6], whereas G. frondosa, rich in amino acids, sugars, peptides, and polysaccharides has shown antimicrobial, antidiabetic, probiotic, antioxidant, lipid metabolism regulation, and anti-hypertensive activities [7].
Other WDF species of potential interest in the nutraceutical and Novel Food fields are Abortiporus biennis (Bull.) Singer, Fomitopsis iberica Melo, Ryvarden, and Stereum hirsutum (Willd.) Pers. Up to now, the chemical analysis of these three species has been focused mainly on the determination of polysaccharides content, namely alpha-glucans and beta-glucans in A. biennis and F. iberica, respectively, and chitin in S. hirsutum [2,8]. S. hirsutum mycelium has also been investigated through GC-TOF-MS [9] analysis, showing the presence of sugars (meso-erythritol, ribose, glucose, 6-deoxyglucose, trehalose), organic acids (2-hydroxyhexanoic acid, 2,3-dihydroxybutanoic acid, citramalic acid, 2-oxoglutaric acid), and amino acids (glycine, alanine, threonine, glutamate).
As widely reported [10,11], NMR untargeted approach is one of the main powerful methodologies useful to achieve the metabolite profiling of complex biological matrices and to carry out quantitative comparison among different vegetable varieties [12,13].
Although NMR spectroscopy is characterized by a lower sensitivity with respect to other methodologies such as Mass Spectroscopy (alone or coupled with separation systems), it provides a sure structural determination since it is confirmed by 1H spectrum features (chemical shift, J coupling constants, multiplicity) and two-dimensional experiments. In the context of first-time characterization of the metabolite profile of a certain matrix, this approach is very useful to offer concrete information.
In this paper, a detailed NMR investigation of the hydroalcoholic and organic A. biennis, F. iberica and S. hirsutum mycelia extracts was carried out to obtain their complete metabolites profile as the first step in the development of WDF-based Novel Foods or food ingredients. The identified metabolites were quantified to determine strengths and weaknesses of each investigated species, thus suggesting potential food applications for the analyzed species.
2. Materials and Methods
2.1. Fungal Strains
The Wild Type (WT) sporophores of A. biennis, F. iberica and S. hirsutum were collected in Italy and strains were isolated in pure culture. As reported in [14], all the strains were identified both by macro- and micro-morphological cultural characteristics and by molecular analysis of the Internal Transcribed Spacer (ITS) region. The three analyzed strains belong both to the Fungal Research Culture Collection (MicUNIPV) of the Mycology Laboratory at the Department of Earth and Environmental Sciences (DSTA) (University of Pavia, Italy) and MOGU’s fungal strain collection (MFSC). The strains, which, respectively, have the following codes: MicUNIPV A.b.6, MicUNIPV F.b.1, MicUNIPV S.h.1, and MFSC 064-18, MFSC 104-19, MFSC 073-18, were maintained through different cultural media and preserved at −80 °C in MicUNIPV.
2.2. Mycelia Samples Preparation
Fungal strains were first grown up to 15 days at 25 °C in Petri dishes with a 2%w/v Malt Extract Agar (MEA) to standardize the inoculum conditions and reactivity. Ten colonized portions of MEA (about 0.125 cm3 each) were sterilely inoculated into flasks (capacity of 1 L) containing a 2%w/v ME previously sterilized by autoclave (121 °C, 20 min) and corked by raw cotton to favor gaseous exchange. Incubation was carried out in dark and static condition at 25 °C. After 15 days, each mycelium was gently washed with deionized water and lyophilized for 24 h at −50 °C and 1 mbar. Mycelia were stored in a freezer at −20 °C.
2.3. Samples Extraction
Extraction of both hydroalcoholic and organic fractions was carried out using the Bligh–Dyer protocol by modifying a previously described procedure [15]. A 100 mg aliquot of dried and pulverized sample was added to 3 mL of a CH3OH/CHCl3 2:1 v/v mixture and 0.8 mL of distillated H2O, followed by sonication. Afterwards, 1 mL of CHCl3 and 1 mL of distillated H2O were added to the system that was finally centrifugated, allowing the separation of hydroalcoholic and organic phases. Extraction was repeated two more times on residual pellet and the reunited hydroalcoholic and organic phases were dried with an N2 flux.
2.4. NMR Analysis
NMR analyses were carried out on a 600 MHz spectrometer (Jeol JNM-ECZ 600R) equipped with a 5 mm FG/RO DIGITAL AUTOTUNE probe.
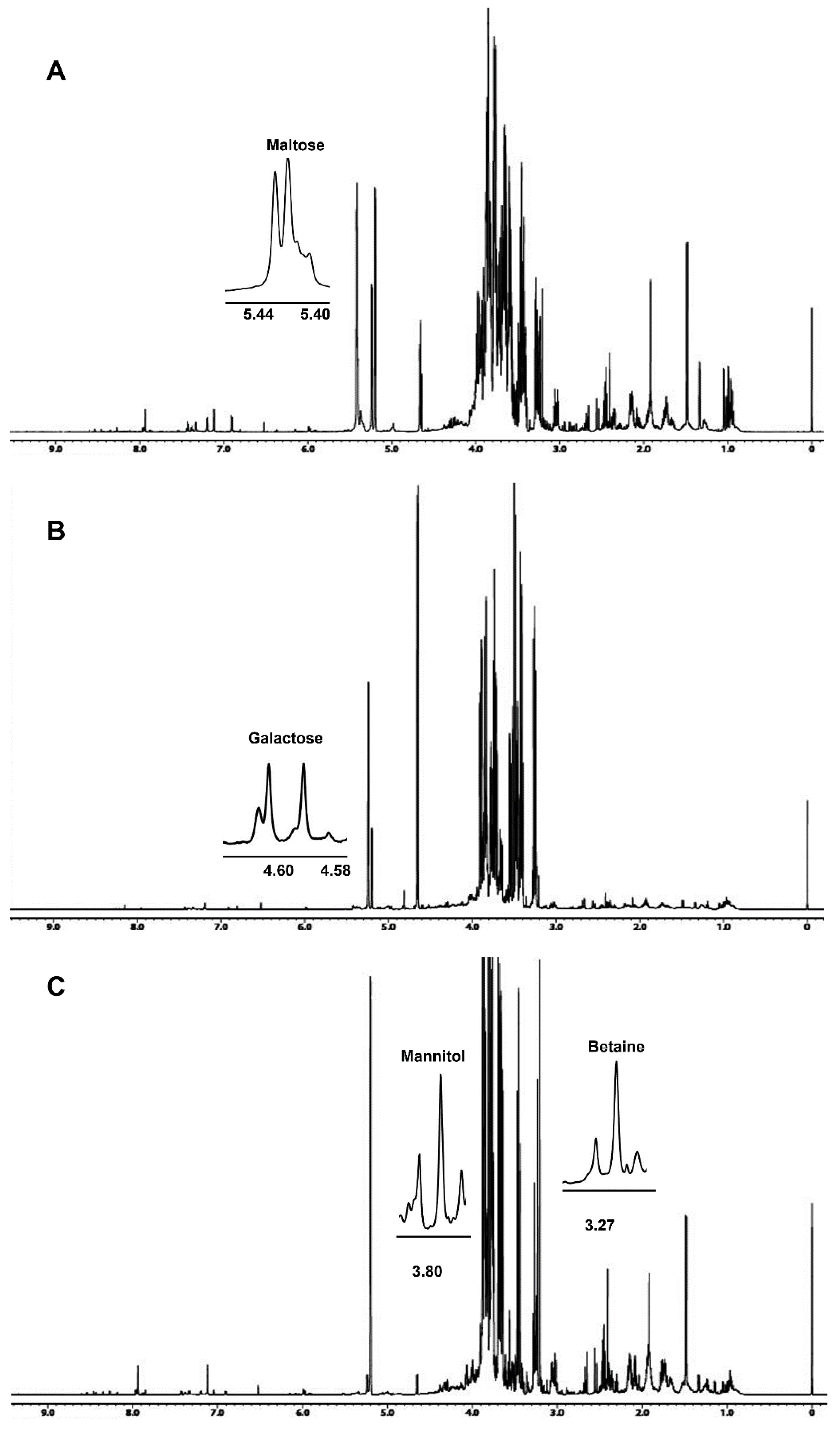
Dried hydroalcoholic phases were dissolved in 700 μL of a 100 mM phosphate buffer/D2O, containing a 0.5 mM TSP (3-(trimethylsilyl)propionic acid sodium salt) as internal standard. 1H spectra, Figure 1, were obtained at 298 K using the following parameters: 128 scans, residual HDO signal suppression with a pre-saturation pulse, a 7.7 s relaxation delay, a 90° pulse of 8.3 μs, 64 k data points, and a 9000 Hz spectral width. 1H spectra were referenced to a TSP methyl group signal in D2O (δH = 0.00 ppm).
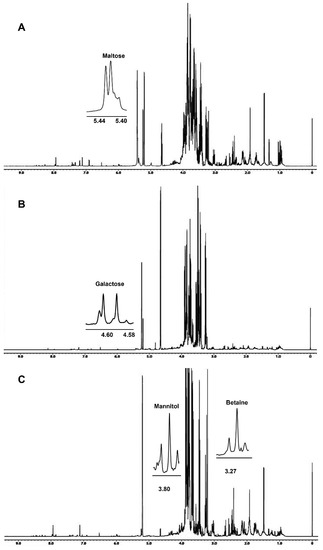
Figure 1.
The 600.13 MHz 1H NMR spectra of Bligh–Dyer hydroalcoholic extracts of (A) A. biennis, (B) F. iberica and (C) S. hirsutum mycelia. Signals characteristic of the metabolites detected only in the corresponding species are expanded in the spectra.
Dried organic phases were dissolved in 700 μL of a CDCl3/CD3OD 2:1 v/v mixture. 1H spectra were obtained at 298 K using the following parameters: 128 scans, a 7.7 s relaxation delay, a 90° pulse of 8.3 μs, 64 k data points, and a 9000 Hz spectral width. 1H spectra were referenced to a CHD2 residual signal of methanol (δH = 3.34 ppm).
Two-dimensional NMR experiments, namely 1H-1H TOCSY, 1H-13C HSQC, and 1H-13C HMBC, were carried out on hydroalcoholic extracts. In particular, 1H-1H TOCSY were acquired with 56 scans, 8 k data points in f2 and 128 in f1, a 50 ms mixing time, a 2 s relaxation delay, and a 9000 Hz spectral width in both dimensions. The 1H-13C HSQC experiments were acquired with 88 scans, 8 k data points in f2 and 256 in f1, a 3 s relaxation delay, and a spectral width of 9000 Hz and 33,000 Hz for f2 and f1, respectively. The 1H-13C HMBC experiments were acquired with 84 scans, 8 k data points in f2 and 165 in f1, a 2 s relaxation delay, and a spectral width of 9000 Hz and 37,500 Hz for f2 and f1, respectively Spectrum processing and signal integration were carried out with the JEOL Delta software (v5.3.1).
To quantify the identified metabolites in the hydroalcoholic extracts, the integrals of the corresponding selected 1H resonances were measured with respect to TSP. Three replicates were made, and the results were expressed as mg/100 g of sample ± SD.
To quantify the identified metabolites in the organic extracts, integrals of the corresponding selected 1H resonances were measured and expressed as molar % ± SD, on three replicates, by applying the following equations:
%ERG = 100 × (2IERG/Itot),
%DUFA = 100 × (IDUFA/Itot),
%MUFA = 100 × (ITOT UFA − 2IDUFA)/Itot,
%TOT FA = 100 × (ITOT FA/Itot),
%TOT UFA = %MUFA + %DUFA,
%TOT SFA = %TOT FA − %TOT UFA,
%PC = 100 × (4IPC/9Itot),
%PE = 100 × (2IPE/Itot).
IERG, IDUFA, ITOT UFA, ITOT FA, IPC, and IPE are the integral values of ergosterol, di-unsaturated fatty acids, mono-unsaturated fatty acids, total fatty acids, total unsaturated fatty acids, total saturated fatty acids, phosphatidylcholine, and phosphatidylethanolamine signals, respectively; see Table 1. In particular, to integrate TOT UFA, signals in the range of 5.33–5.35 ppm were considered, corresponding to double-bound protons. To integrate TOT FA, signals in the range of 2.28–2.30 were considered, corresponding to α-CH2 groups of all fatty acids.

Table 1.
Metabolites identified in the 600.17 MHz 1H NMR spectra of WDG Bligh–Dyer hydroalcoholic extracts dissolved in 100 mM phosphate buffer/D2O containing TSP 0.5 mM and Bligh–Dyer organic extracts dissolved in CDCl3/MeOD 2:1 v/v solution.
Itot is obtained by the following equation:
Itot = ITOT FA + 2IERG.
%ERG, %DUFA, %MUFA, %TOT FA, %TOT UFA, %TOT SFA, %PC, and %PE are the molar % of ergosterol, di-unsaturated fatty acids, mono-unsaturated fatty acids, total fatty acids, total unsaturated fatty acids, total saturated fatty acids, phosphatidylcholine, and phosphatidylethanolamine, respectively.
3. Results and Discussion
3.1. NMR Assignment of Bligh–Dyer Extracts
The 1H NMR spectra of A. biennis, F. iberica and S. hirsutum hydroalcoholic extracts are reported in Figure 1. Spectral assignments reported in Table 1 were obtained by means of 2D experiments and literature data relative to other vegetal matrices analyzed in the same experimental conditions [16,17].
The 1H-1H TOCSY was useful to confirm some assignments or to solve dubious cases. For instance, the 1H spectrum showed the presence of two doublets at 1.33 ppm and 1.34 ppm characterized by the same J coupling constant of 6.6 Hz, typically due to terminal CH3. The doublet at 1.33 ppm was assigned to CH3 of lactate, showing in the TOCSY map a typical spin correlation with the α-CH at 4.13, whereas the doublet at 1.34 ppm was assigned to threonine, showing a correlation with α-CH and β-CH at 3.58 and 4.27 ppm, respectively.
In some cases, due to strong signal overlapping, the addition of standard compounds was necessary to confirm metabolite assignment. It is the case of betaine and mannitol of S. hirsutum hydroalcoholic extracts, whose signals at 3.27 ppm (betaine) and 3.68, 3.77, 3.80, 3.87 ppm (mannitol) showed an increase in intensity after the standard addition.
The 1H spectra of organic extracts were assigned by literature data [18] showing the presence of saturated fatty acids, mono-unsaturated fatty acids, di-unsaturated fatty acids, ergosterol, 1,2-diacyl-sn-glycero-3-phosphatidylethanolamine, and 1,2-diacyl-sn-glycero-3-phosphatidylcholine.
3.2. Quantitative Metabolite Profile: Comparison between A. biennis, F. iberica and S. hirsutum Mycelia
The metabolites identified in the hydroalcoholic and organic extracts were quantified according to the procedure reported in Experimental section. Data are reported as histograms and discussed separately according to the class of compounds.
3.2.1. Amino Acids
It is noteworthy that no chemical or enzymatic protein hydrolysis was carried out before the NMR analysis, so the here-discussed amino acids (AAs)are referred to as the free ones, naturally present in the samples.
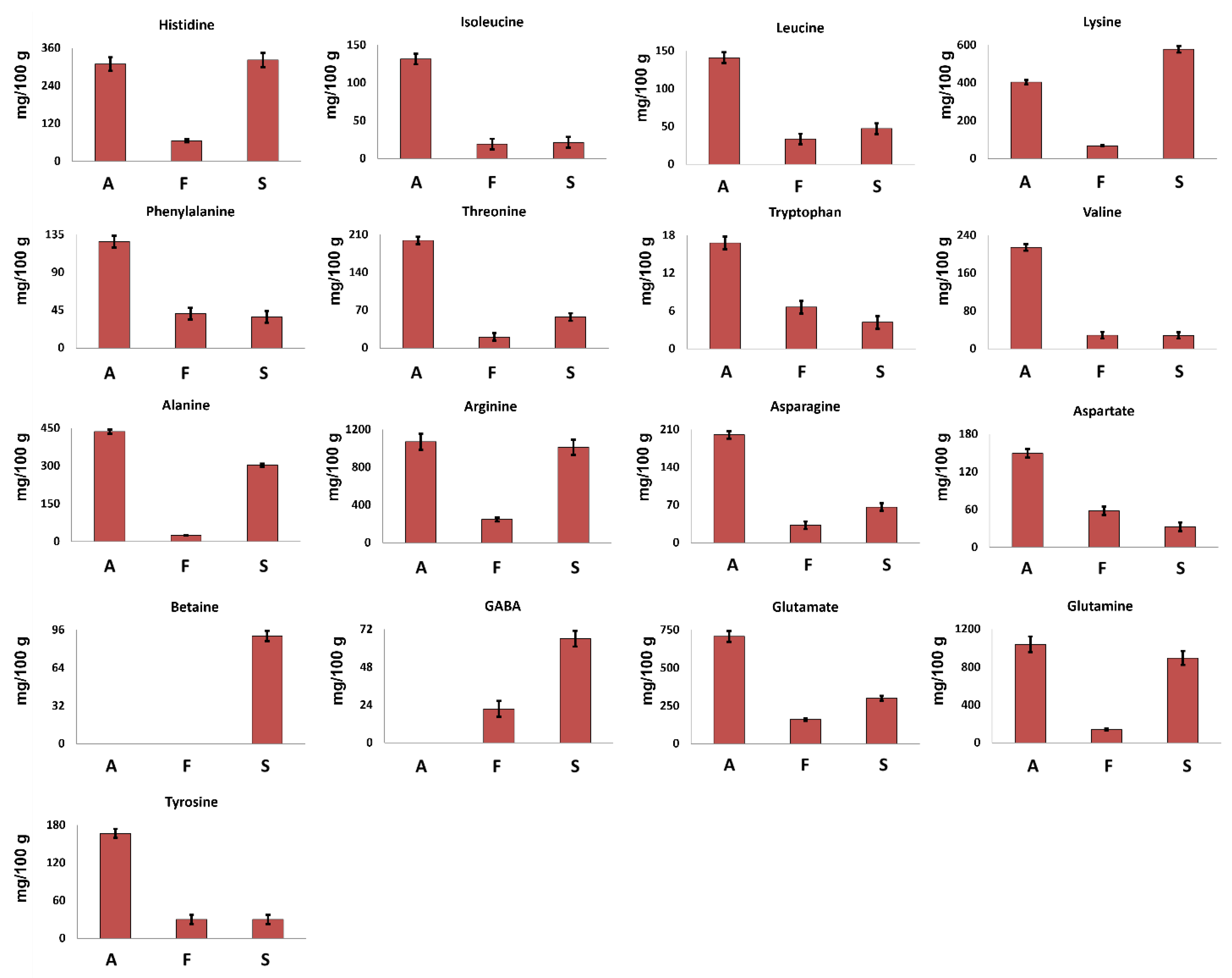
Seventeen amino acids were identified in the 1H spectra of hydroalcoholic extracts.
Betaine, a non-protein amino acid, was detected only in S. hirsutum mycelium (see Figure 1) at a concentration of 91 mg/100 g (Figure 2), whereas GABA was detected in S. hirsutum as well as F. iberica but not in A. biennis mycelium.
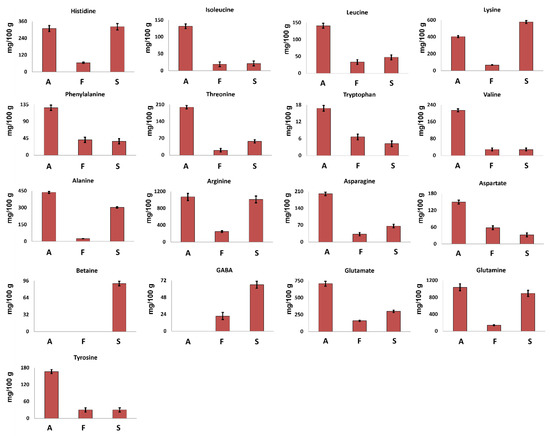
Figure 2.
Quantitative histograms relative to free amino acids measured in 1H NMR spectra of Bligh–Dyer hydroalcoholic extracts. A. biennis (A), F. iberica (F), S. hirsutum (S).
The other 15 amino acids were detected in all the investigated samples. Arginine was the most abundant amino acid, whereas tryptophan was present at the lowest concentration.
A. biennis was characterized by the highest concentration of all AAs, except for lysine, suggesting this species as a significative AAs source.
All the investigated samples showed the presence of all the essential amino acids (EAAs) except methionine, confirming their nutritional value. In A. biennis and S. hirsutum in particular, EAAs represent more than 35% of the total free AAs, whereas in F. iberica, EAAs represent less than 30%.
In all the investigated samples according to literature data [19], lysine turned out to be the most abundant EAA, reaching a concentration of 404 mg/100 g and 579 mg/100 g in A. biennis and S. hirsutum, respectively.
From a quantitative point of view, glutamine and glutamate were found to be the most abundant amino acids in A. biennis, followed by alanine and lysine. In particular, the high concentration of glutamate was expected, since it has been found to be the main non-essential amino acid in several mushrooms, playing a role as a precursor for the synthesis of other amino acids. Moreover, the umami taste that is common in certain kinds of foods, including mushrooms, can be linked to the presence of glutamic acid [20].
Regarding S. hirsutum, comparing the results obtained here with those of a previously reported study [9], an improvement of the amino acids profile was achieved. In the cited paper [9], just four amino acids have been identified (glycine, alanine, threonine, glutamate), whereas in this work, seventeen molecules of this class were identified. In any case, glycine reported by Peiris et al. [9] was not detected in this work.
In Table 2, AAs content of some species proposed/approved as Novel Foods (Agaricus blazei Murril, G. frondosa, L. edodes) [21,22,23] is reported together with AAs content of A. biennis species determined here. Due to the different extraction procedures and the different analytical methods, it is not possible to carry out a direct quantitative comparison. However, it is possible to observe interesting trends regarding the AAs profile of the different species.

Table 2.
AAs concentrations in A. biennis mycelium compared to the ones of other mushroom mycelia proposed/approved as Novel Foods.
The four species are characterized by different amino acid profiles. Among the essential amino acids, methionine was not detected in A. biennis. On the other hand, tryptophan was identified and quantified in A. biennis and not detected in the other three species. Regarding the non-essential amino acids, asparagine and glutamine were detected only in A. biennis, whereas arginine and serine were detected only in the other three species.
In order to understand whether the AAs content of A. biennis can be considered advantageous in comparison with other non-fungal food, a comparison between its AAs content and the ones of some vegetables is reported here; see Table 3. Considering the classification of cereals and grain products, starchy roots and tubers, dry legumes, nuts and seeds, vegetables and, finally, fruit offered by the Food and Agriculture Organization of United Nations (FAO) [24], in order to make comparison clearer, only one representative food from each category was taken into account.

Table 3.
AAs concentrations in A. biennis mycelium compared to other food sources.
Eggs, milk, meat and fish were excluded from the comparison because they were not in compliance with the purpose of the work.
Although the data reported in Table 3 cannot be considered representative of all the non-fungal foods listed in the FAO document, the comparison of the indicated values can be useful for making some considerations. In particular, A. biennis mycelium showed the highest concentration of asparagine and glutamate and was proven to be second after lentils in regard to histidine and lysine (both EAAs) concentrations.
3.2.2. Sugars
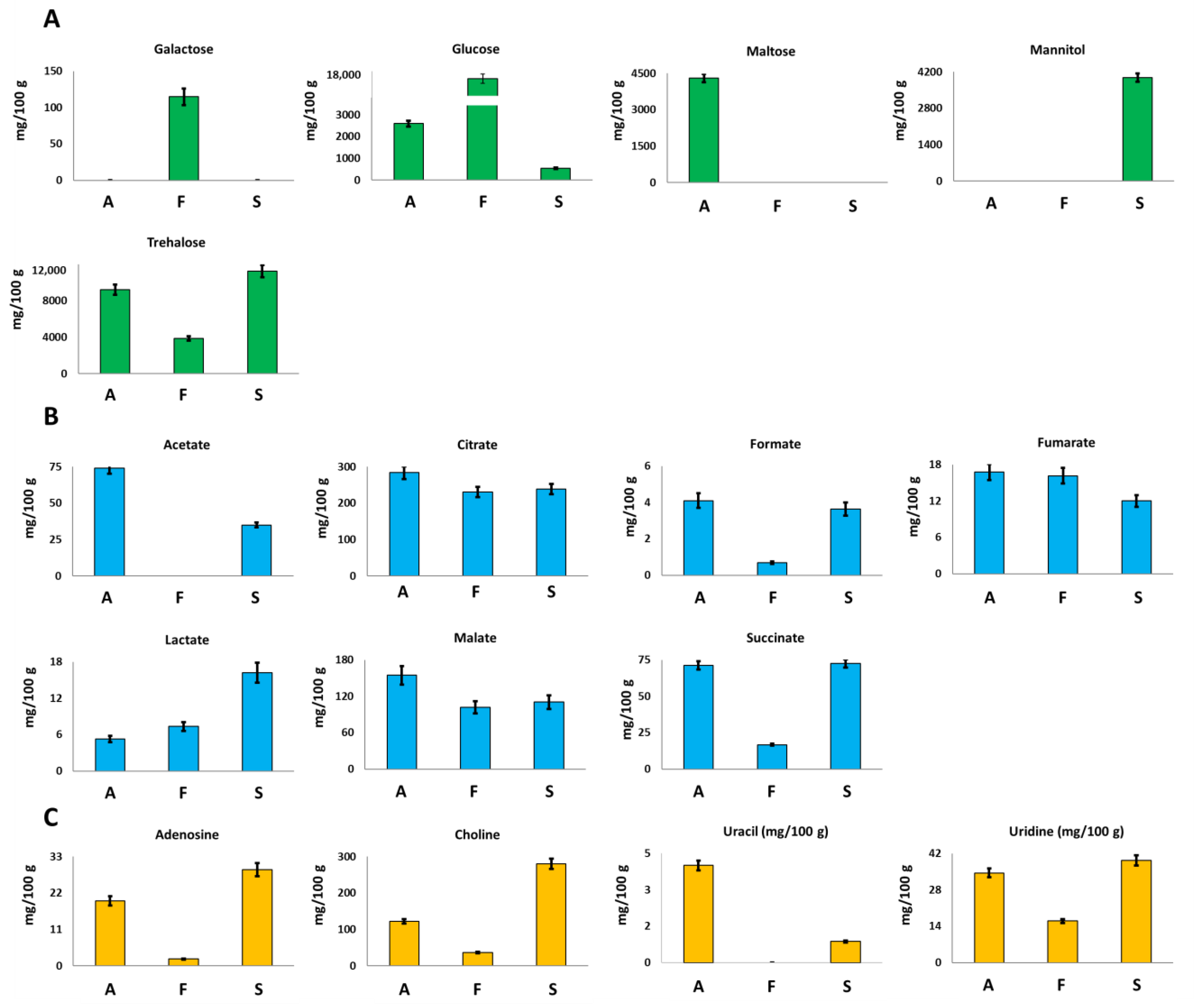
Glucose and trehalose were identified and quantified in all the investigated mycelia samples. Trehalose, a typical sugar of mushrooms, is a low glycemic disaccharide able to lower the postprandial glycaemia and to induce biogenesis of lysosomes and autophagosomes [25].
F. iberica showed the highest amount of glucose, 16,491 mg/100 g, about 6 times and 30 times higher than the amount detected in A. biennis and S. hirsutum, respectively; see Figure 3A.
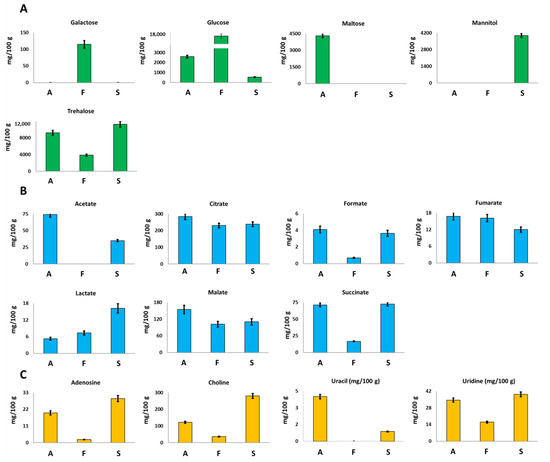
Figure 3.
Quantitative histograms relative to (A) free sugars, (B) organic acids, and (C) Adenosine, Choline, Uracil and Uridine quantified in NMR analysis of Bligh–Dyer hydroalcoholic extracts. A. biennis (A), F. iberica (F), S. hirsutum (S).
In A. biennis and S. hirsutum, trehalose turned out to be the most abundant sugar. In particular, among the three species, S. hirsutum showed the highest content, whereas F. iberica displayed the lowest one.
Interestingly, other sugars were detected only in the specific species: galactose was detected only in F. iberica, maltose was detected in A. biennis, and mannitol was detected in S. hirsutum, which offers the potential role of species markers to these metabolites.
Also in the case of sugars, the comparison between the results for S. hirsutum reported here and those of Peiris et al. [9] underlined the presence of qualitative differences. In particular, glucose and trehalose were detected in both studies, whereas meso-erythritol, ribose, and 6-deoxyglucose were not detected here. This difference could be due to the lower sensitivity of NMR spectroscopy in respect to GC-TOF-MS, or to the used strain, whereas a difference due to the growth medium is excluded since both studies were carried out using the same medium (Malt extract). On the contrary, mannitol was reported here.
3.2.3. Organic Acids
Mushrooms are well known and largely used for the production of organic acids in food, pharmaceutical, and technical sectors [26]. The samples investigated here showed the presence of seven organic acids, namely acetate, citrate, formate, fumarate, lactate, malate, and succinate. Among them, citrate turned out to be present in the highest concentration in the three species. In particular, A. biennis species showed the highest concentration of citrate (Figure 3B), malate, formate, fumarate, and acetate. Acetate was not detected in F. iberica. Lactate concentration turned out to be at least three times higher in S. hirsutum than in the other two species. The obtained qualitative data are in accordance with the literature data regarding the organic acids profile of edible mushrooms [27,28]. In particular, except for acetate, the metabolites detected here have been already identified in other fungal species. Moreover, it is noteworthy that it was not possible to verify the presence of oxalate, a dicarboxylic acid typical of fungal species, since it consists of two bounded carboxylic groups whose signals cannot be detected in the 1H NMR spectrum of aqueous samples.
In the case of organic acids identified in S. hirsutum, the comparison with Peiris et al. [9] underlined a completely different qualitative profile, with 2-hydroxyhexanoic acid, 2,3-dihydroxybutanoic acid, citramalic acid, and 2-oxoglutaric acid identified in the cited paper but not in the present work.
3.2.4. Other Compounds
Adenosine, choline, and uridine were present at the highest concentration in S. hirsutum, whereas the highest amount of uracil was measured in A. biennis, Figure 3C. This last metabolite was not detected in F. iberica.
Choline, or Vitamin J, is an amine only partially synthesized by the human body (due to the presence of cobalamin and folic acid); therefore, its supply should be ensured, above all, through food. This metabolite is important for the synthesis of phospholipids in cell membranes, methyl metabolism, acetylcholine synthesis, and cholinergic neurotransmission in humans [29].
According to the National Academy of Sciences, USA, foods with the highest choline concentrations are beef liver, chicken liver and eggs [30]. No data are reported in the literature about choline determination on the mushrooms considered here.
Considering that the recommended dietary daily intake of choline is 550 mg of total choline per day for men and 425 mg per day for women, S. hirsutum may represent an interesting dietary source for this nutrient. This is reinforced by the presence of betaine (not detected in A. biennis and F. iberica), that is a choline metabolite that cannot be converted to choline but can be used as a methyl donor, sparing some choline requirements [31].
3.2.5. Apolar Fraction
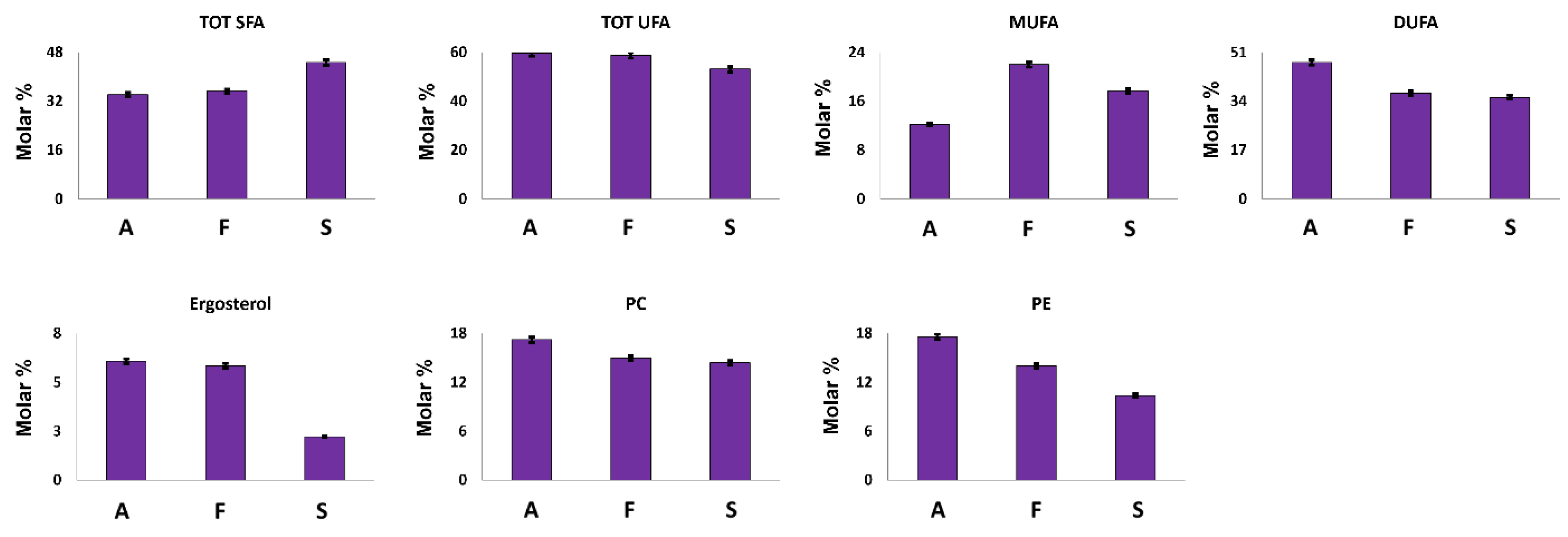
Ergosterol is a sterol present in the cell membrane of mushrooms where it exerts a similar function to those of cholesterol in animal cells. It can also be considered an important nutritional compound since it is the precursor of vitamin D [32]. As expected, ergosterol was identified in all the investigated samples; its highest concentration was measured in A. biennis and F. iberica extracts (Figure 4).
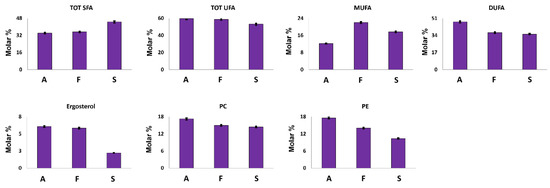
Figure 4.
Quantitative histograms relative to quantified fatty chains, ergosterol and polar lipid heads from the NMR analysis of Bligh–Dyer hydroalcoholic extracts. A. biennis (A), F. iberica (F), S. hirsutum (S).
Fatty chains represent the major compounds of Bligh–Dyer organic extracts. In A. biennis and F. iberica samples, unsaturated fatty chains (UFA) showed an average molar concentration of 59% (Figure 4). In particular, A. biennis was characterized by the highest DUFA and the lowest MUFA concentrations. Among them, DUFA were present in high concentration and can be attributed to ω-6 linoleic acid, an essential fatty acid strongly recommended in the human diet, and whose presence in mushrooms has been largely demonstrated [33,34,35].
It is noteworthy that tri-unsaturated fatty chains were not detected in the 1H NMR spectra of the investigated samples, thus suggesting the absence, or at least a non-NMR relievable concentration, of this essential fatty acid. These data are strongly supported by the literature data, where tri-unsaturated fatty acids in mushrooms have shown to be absent or present in very low concentrations [33,34,35,36].
4. Conclusions
In this study, the NMR-based chemical profile of three fungal mycelia, namely A. biennis, F. iberica and S. hirsutum, was reported, each species characterized by a peculiar chemical profile and thus by a potential nutritional value. The overall chemical profile can represent an important first step towards the potential use of WDF mycelia as food matrices or food ingredients. New products with nutritional and nutraceutical properties could be developed as a mixture of different species, opening new perspective in WDF sectors.
Author Contributions
Conceptualization, E.S., L.G., L.M., M.S. and P.R.; methodology, L.G., M.C., M.S. and R.M.B.; software, L.M. and M.S.; validation, E.S., L.M. and P.R.; writing—original draft preparation, E.S., L.G., L.M. and M.S.; writing—review and editing, E.S., L.G., L.M., M.C., M.S., P.R. and R.M.B.; supervision, E.S., L.M. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by Fondazione Cariplo and Regione Lombardia, grant number 2018-1765, project entitled ‘MYCO-ADVANCED LEATHER MATERIALS (MATER)’.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, T.; Cui, L.; Song, X.; Cui, X.; Wei, Y.; Tang, L.; Mu, Y.; Xu, Z. Wood decay fungi: An analysis of worldwide research. J. Soils Sediments 2022, 22, 1688–1702. [Google Scholar] [CrossRef]
- Girometta, C.; Dondi, D.; Baiguera, R.M.; Bracco, F.; Branciforti, D.S.; Buratti, S.; Lazzaroni, S.; Savino, E. Characterization of mycelia from wood-decay species by TGA and IR spectroscopy. Cellulose 2020, 27, 6133–6148. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the safety of ‘Chitin-glucan’ as a Novel Food ingredient. EFSA J. 2010, 8, 1687. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of vitamin D2 mushroom powder as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, e05948. [Google Scholar] [CrossRef]
- European Commision Consultation Process on Novel Food Status. Available online: https://food.ec.europa.eu/safety/novel-food/consultation-process-novel-food-status_en (accessed on 26 April 2023).
- Swallah, M.S.; Bondzie-Quaye, P.; Wu, Y.; Acheampong, A.; Sossah, F.L.; Elsherbiny, S.M.; Huang, Q. Therapeutic potential and nutritional significance of Ganoderma lucidum—A comprehensive review from 2010 to 2022. Food Funct. 2023, 14, 1812–1838. [Google Scholar] [CrossRef]
- Wu, J.Y.; Siu, K.C.; Geng, P. Bioactive ingredients and medicinal values of Grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Cartabia, M.; Girometta, C.E.; Milanese, C.; Baiguera, R.M.; Buratti, S.; Branciforti, D.S.; Vadivel, D.; Girella, A.; Babbini, S.; Savino, E.; et al. Collection and characterization of wood decay fungal strains for developing pure mycelium mats. J. Fungi 2021, 7, 1008. [Google Scholar] [CrossRef]
- Peiris, D.; Dunn, W.B.; Brown, M.; Kell, D.B.; Roy, I.; Hedger, J.N. Metabolite profiles of interacting mycelial fronts differ for pairings of the wood decay basidiomycete fungus, Stereum hirsutum with its competitors Coprinus micaceus and Coprinus disseminatus. Metabolomics 2008, 4, 52–62. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Viel, S. Liquid state 1H high field NMR in food analysis. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 66, 1–39. [Google Scholar] [CrossRef]
- Moco, S. Studying Metabolism by NMR-Based Metabolomics. Front. Mol. Biosci. 2022, 9, 372. [Google Scholar] [CrossRef]
- Marcone, M.F.; Wang, S.; Albabish, W.; Nie, S.; Somnarain, D.; Hill, A. Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Res. Int. 2013, 51, 729–747. [Google Scholar] [CrossRef]
- Parlak, Y.; Guzeler, N. Nuclear magnetic resonance spectroscopy applications in foods. Curr. Res. Nutr. Food Sci. 2016, 4, 161–168. [Google Scholar] [CrossRef]
- Cartabia, M.; Girometta, C.E.; Baiguera, R.M.; Buratti, S.; Babbini, S.; Bernicchia, A.; Savino, E. Lignicolous Fungi Collected in Northern Italy: Identification and Morphological Description of Isolates. Diversity 2022, 14, 413. [Google Scholar] [CrossRef]
- Di Matteo, G.; Spano, M.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L.; Ingallina, C.; Sobolev, A.P. Nmr characterization of ten apple cultivars from the piedmont region. Foods 2021, 10, 289. [Google Scholar] [CrossRef]
- Ingallina, C.; Spano, M.; Sobolev, A.P.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L. Characterization of local products for their industrial use: The case of Italian potato cultivars analyzed by untargeted and targeted methodologies. Foods 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Botta, B.; Quaglio, D.; Ghirga, F.; Balducci, S.; Cammarone, S.; Campiglia, E.; Giusti, A.M.; et al. A multimethodological characterization of cannabis sativa L. Inflorescences from seven dioecious cultivars grown in Italy: The effect of different harvesting stages. Molecules 2021, 26, 2912. [Google Scholar] [CrossRef]
- Spano, M.; Maccelli, A.; Di Matteo, G.; Ingallina, C.; Biava, M.; Crestoni, M.E.; Bardaud, J.X.; Giusti, A.M.; Mariano, A.; D’abusco, A.S.; et al. Metabolomic profiling of fresh goji (Lycium barbarum L.) berries from two cultivars grown in central italy: A multi-methodological approach. Molecules 2021, 26, 5412. [Google Scholar] [CrossRef] [PubMed]
- Rahi, D.K.; Malik, D. Diversity of Mushrooms and Their Metabolites of Nutraceutical and Therapeutic Significance. J. Mycol. 2016, 2016, 7654123. [Google Scholar] [CrossRef]
- Sangtitanu, T.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Peptides obtained from edible mushrooms: Hericium erinaceus offers the ability to scavenge free radicals and induce apoptosis in lung cancer cells in humans. Food Funct. 2020, 11, 4927–4939. [Google Scholar] [CrossRef]
- Chang, H.; Chao, G.; Chen, C.; Mau, J. Non-volatile taste components of Agaricus blazei, Antrodia camphorata and Cordyceps militaris mycelia. Food Chem. 2001, 74, 203–207. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Weng, C.C.; Huang, S.J.; Chen, C.C.; Mau, J.L. Nonvolatile taste components of Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. LWT 2006, 39, 1066–1071. [Google Scholar] [CrossRef]
- Aminuddin, H.; Khan, A.M.; Abidin, H.; Madzlan, K.; Suri, R.; Kamal, M.K.; Hussin, A.; Ayob, K.; Hamid, A.; Kasran, M.; et al. Optimization of submerged culture for the production of Lentinula edodes mycelia biomass and amino acid composition by different temperatures (Pengoptimuman kultur ampaian dalam penghasilan biojisim miselium Lentinula edodes dan komposisi asid amino pada. J. Trop. Agric. Food Sci. 2007, 35, 131–138. [Google Scholar]
- FAO Part I. Amino-Acid Content of Foods. Available online: https://www.fao.org/3/AC854T/AC854T03.htm#chI.I (accessed on 22 June 2023).
- Jeong, S.J.; Stitham, J.; Evans, T.D.; Zhang, X.; Rodriguez-Velez, A.; Yeh, Y.S.; Tao, J.; Takabatake, K.; Epelman, S.; Lodhi, I.J.; et al. Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy-lysosome biogenesis response. Autophagy 2021, 17, 3740–3752. [Google Scholar] [CrossRef]
- Liaud, N.; Giniés, C.; Navarro, D.; Fabre, N.; Crapart, S.; Gimbert, I.H.; Levasseur, A.; Raouche, S.; Sigoillot, J.-C. Exploring fungal biodiversity: Organic acid production by 66 strains of filamentous fungi. Fungal Biol. Biotechnol. 2014, 1, 1. [Google Scholar] [CrossRef]
- Valentão, P.; Lopes, G.; Valente, M.; Barbosa, P.; Andrade, P.B.; Silva, B.M.; Baptista, P.; Seabra, R.M. Quantitation of nine organic acids in wild mushrooms. J. Agric. Food Chem. 2005, 53, 3626–3630. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized Analysis of Organic Acids in Edible Mushrooms from Portugal by Ultra Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Zeisel, S.H. Nutritional Importance of Choline for Brain Development. J. Am. Coll. Nutr. 2004, 23, 621S–626S. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Klatt, K.C.; Caudill, M.A. Choline. Adv. Nutr. 2018, 9, 58–60. [Google Scholar] [CrossRef]
- Baur, A.C.; Kühn, J.; Brandsch, C.; Hirche, F.; Stangl, G.I. Intake of ergosterol increases the vitamin D concentrations in serum and liver of mice. J. Steroid Biochem. Mol. Biol. 2019, 194, 105435. [Google Scholar] [CrossRef]
- Ruess, L.; Häggblom, M.M.; García Zapata, E.J.; Dighton, J. Fatty acids of fungi and nematodes—Possible biomarkers in the soil food chain? Soil Biol. Biochem. 2002, 34, 745–756. [Google Scholar] [CrossRef]
- Zhang, Y.; Mills, G.L.; Nair, M.G. Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J. Agric. Food Chem. 2002, 50, 7581–7585. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.-P.; Zhao, J.; Duan, J.-A.; Tang, Y.-P.; Li, S.-P. Comparison of sterols and fatty acids in two species of Ganoderma. Chem. Cent. J. 2012, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Klug, M.J. Characterization and differentiation of filamentous fungi based on fatty acid composition. Appl. Environ. Microbiol. 1996, 62, 4136–4146. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).