Effects of Different Molecular Weight Oxidized Dextran as Crosslinkers on Stability and Antioxidant Capacity of Curcumin-Loaded Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
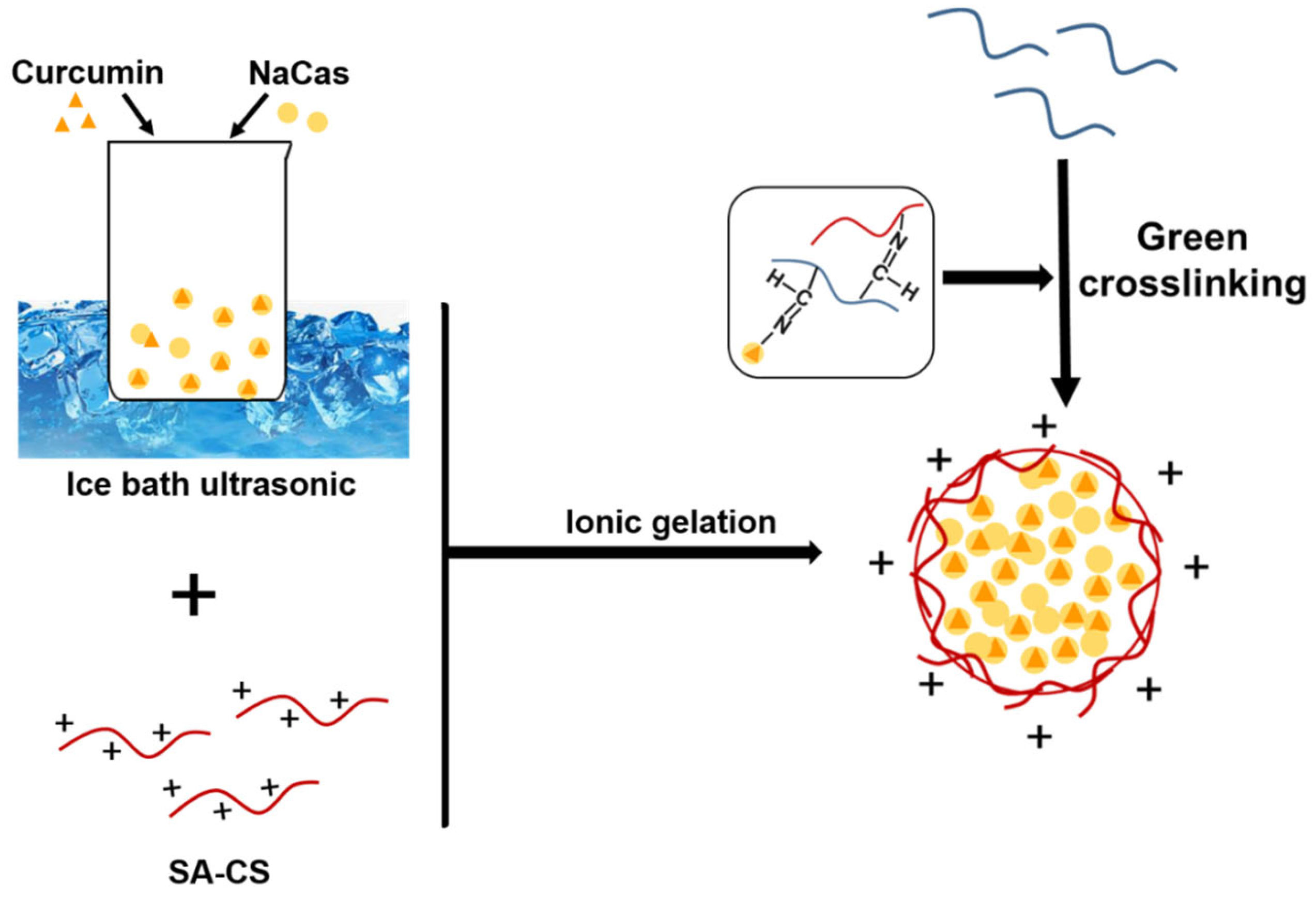
2.2. Preparation of SA-CS
2.3. Preparation of Odex
2.4. Preparation of NPs
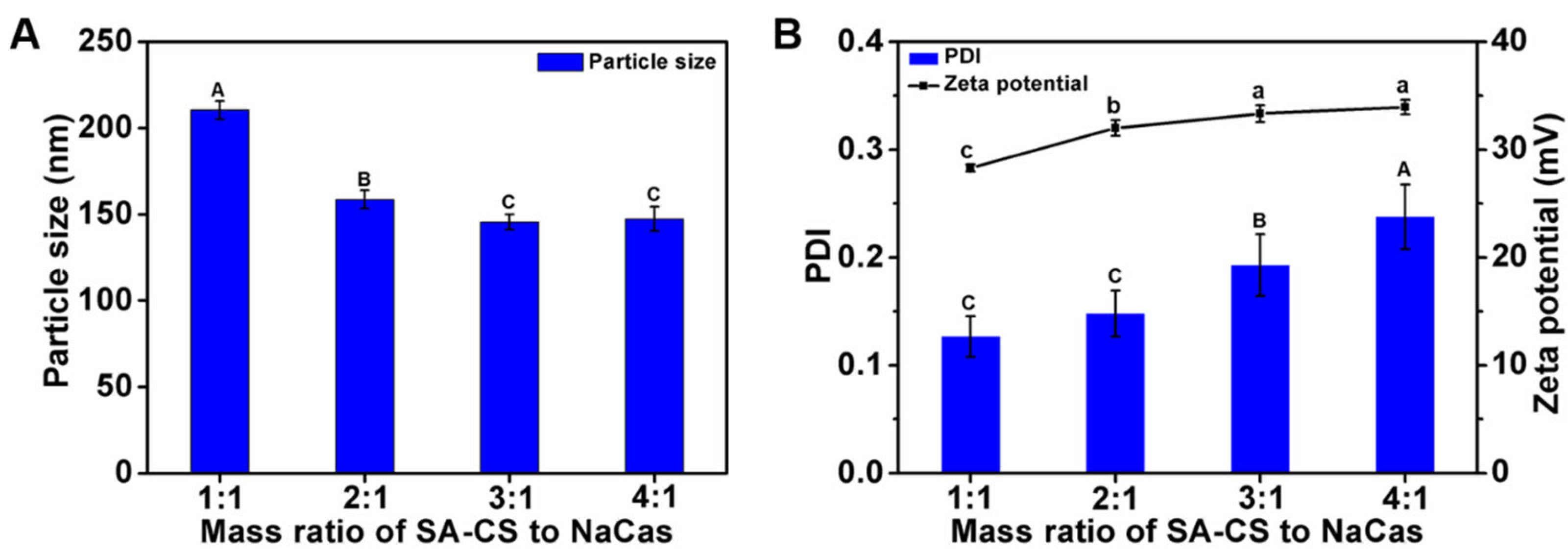
2.4.1. Optimization of the SA-CS/NaCas Mass Ratio
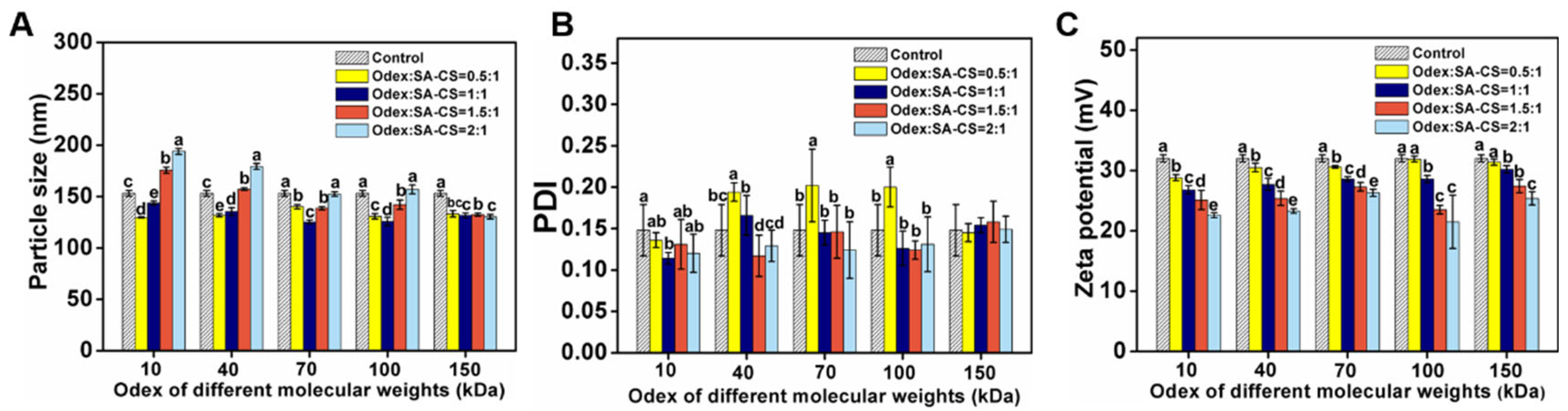
2.4.2. Optimization of the Crosslinking Process
2.5. Curcumin Encapsulation
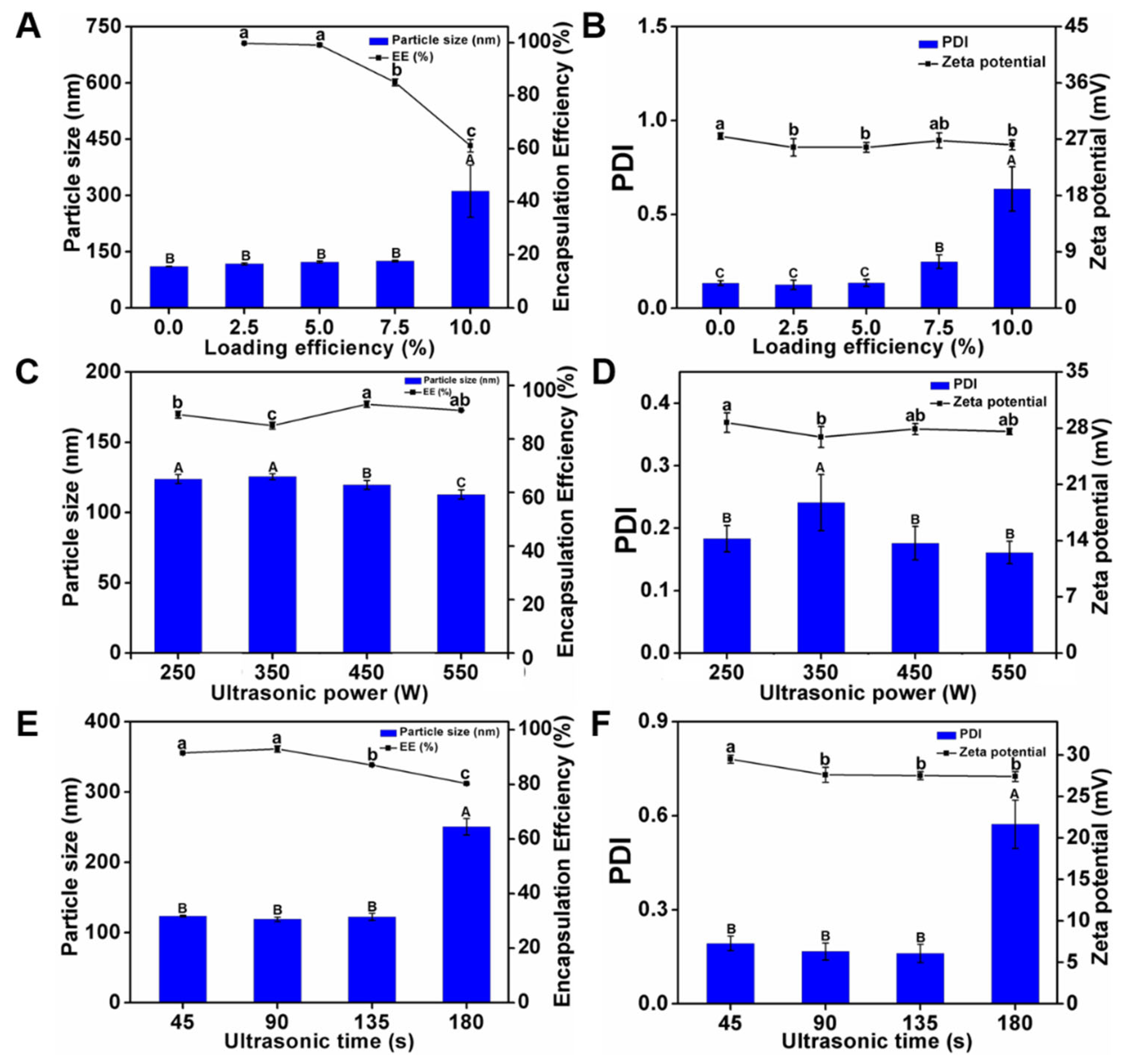
2.5.1. Optimization of Loading Rate
2.5.2. Optimization of Ultrasonic Power
2.5.3. Optimization of Ultrasonication Duration
2.6. FTIR Analysis
2.7. Transmission Electron Microscopy Analysis
2.8. Fluorescence Analysis
2.9. Kinetic Release Profile of Curcumin
2.10. ABTS Free-Radical-Scavenging Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. NPO Preparation
3.1.1. Optimization of the SA-CS/NaCas Mass Ratio
3.1.2. Optimization of the Crosslinking Process
3.2. Encapsulation of Curcumin
3.2.1. Optimization of Drug Loading
3.2.2. Optimization of Ultrasonication Power
3.2.3. Optimization of Ultrasonication Duration
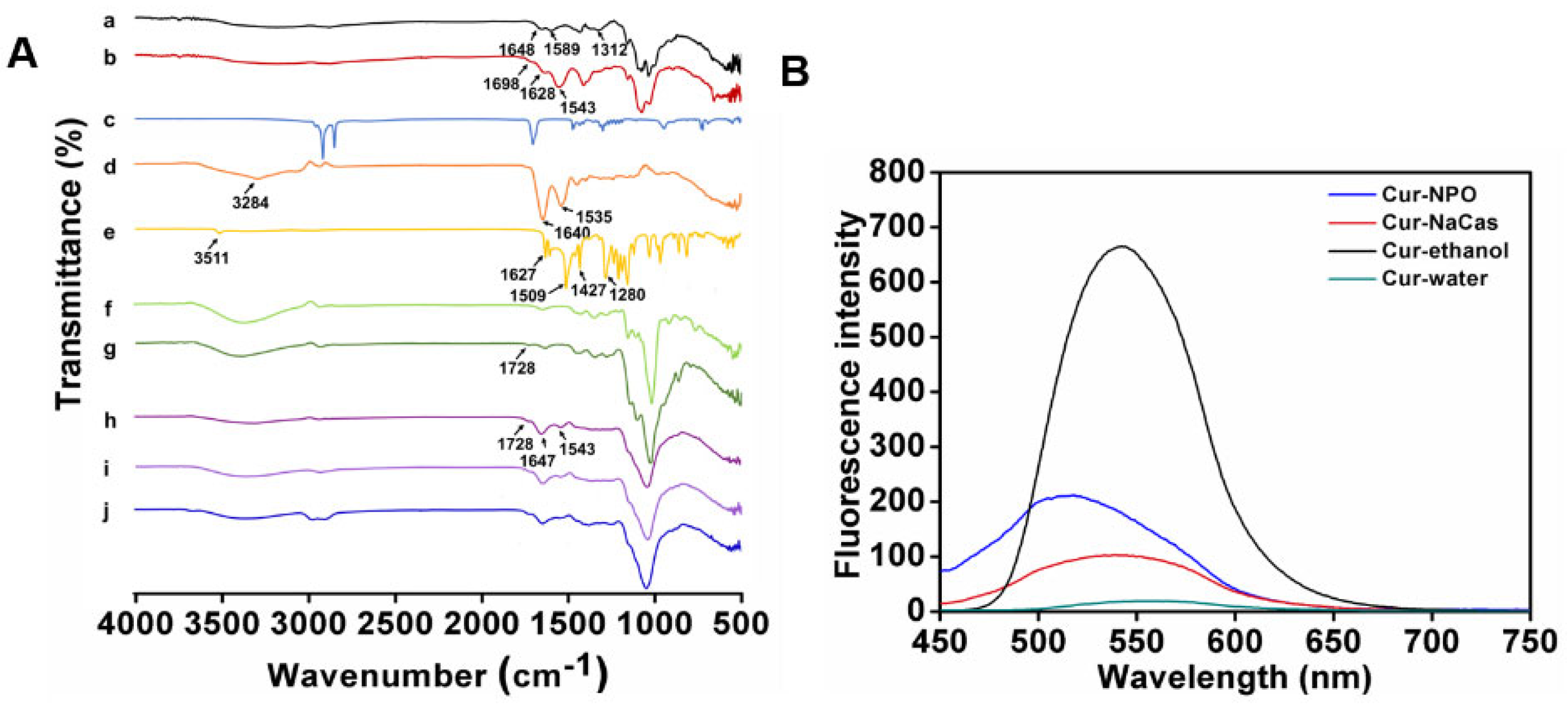
3.3. FTIR Analysis
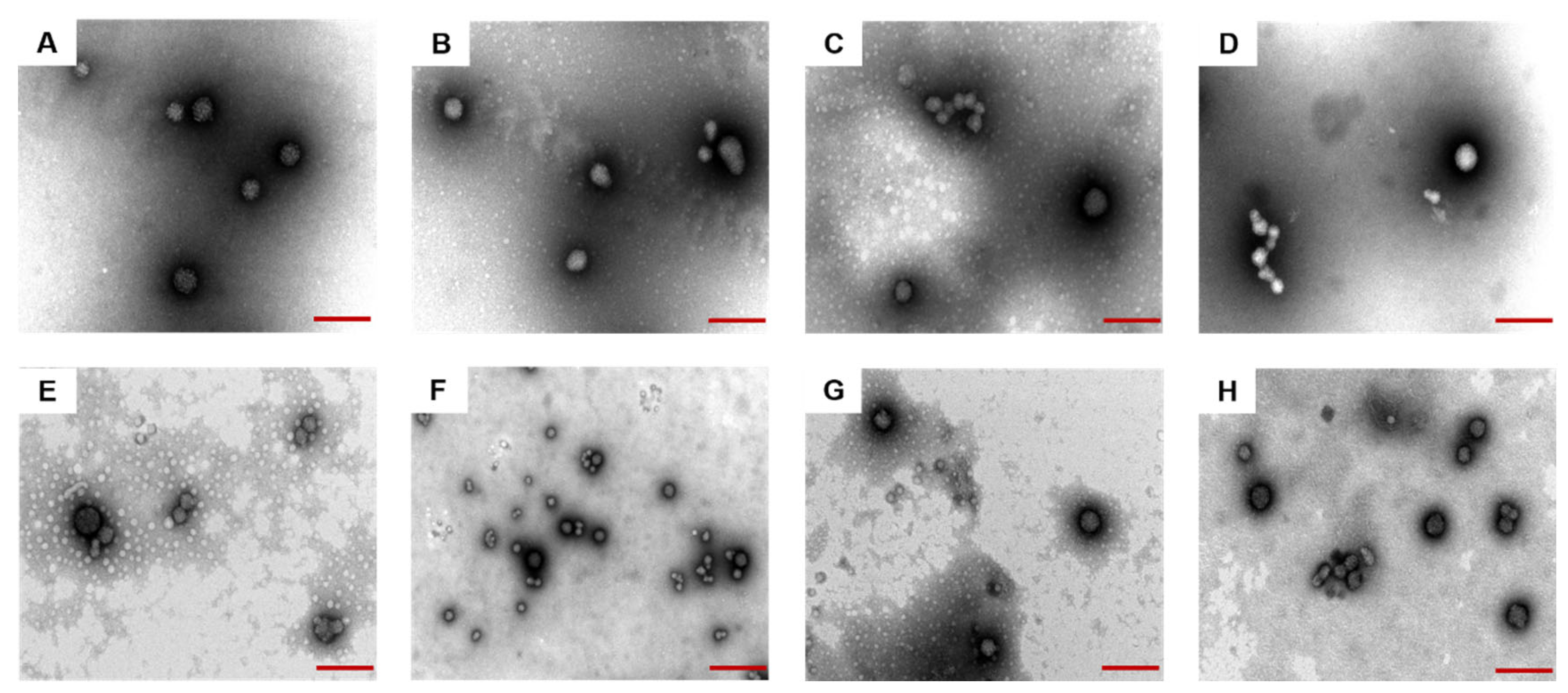
3.4. Morphology of Prepared Nanoparticles
3.5. Fluorescence Analysis
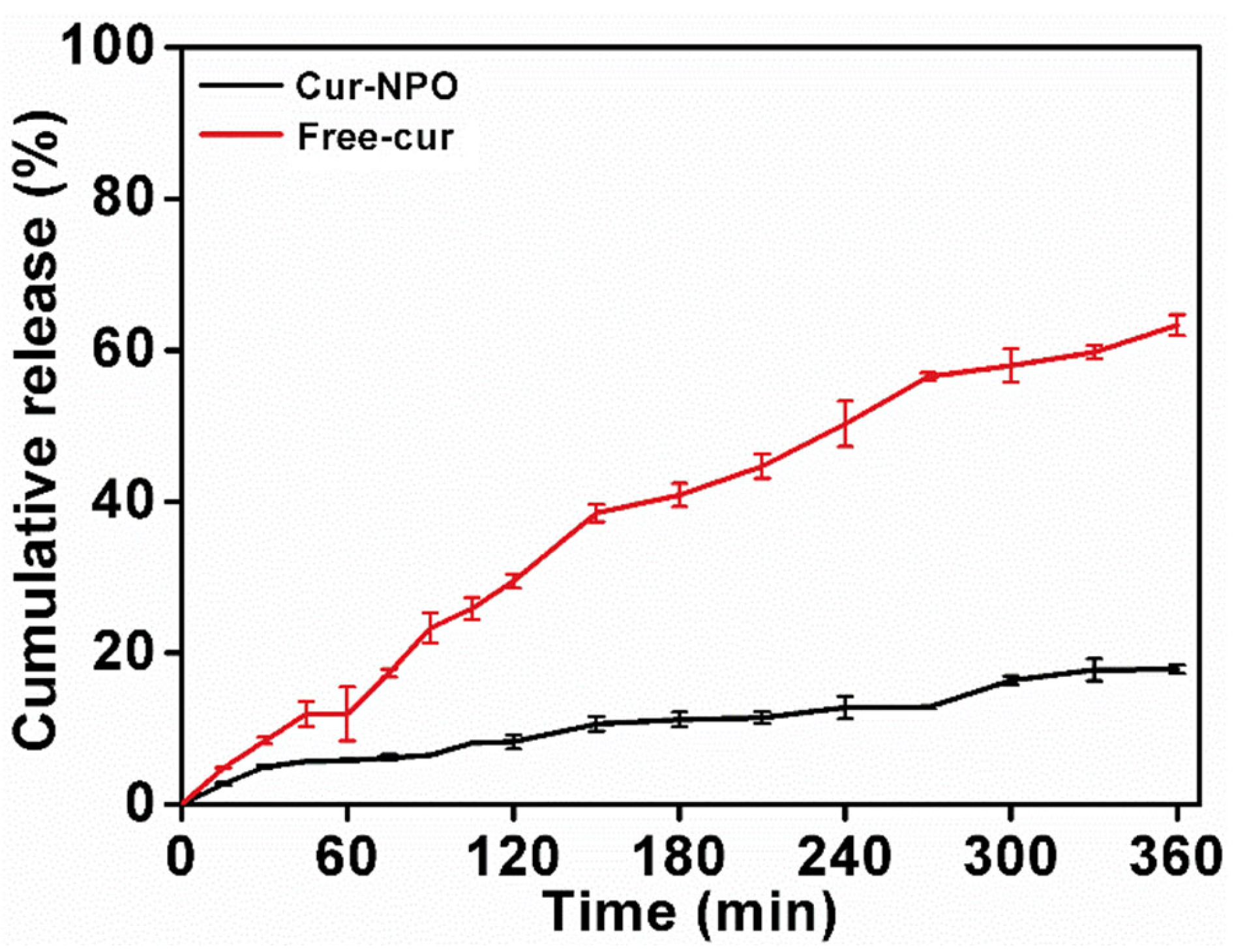
3.6. Kinetic Release Profile of Curcumin
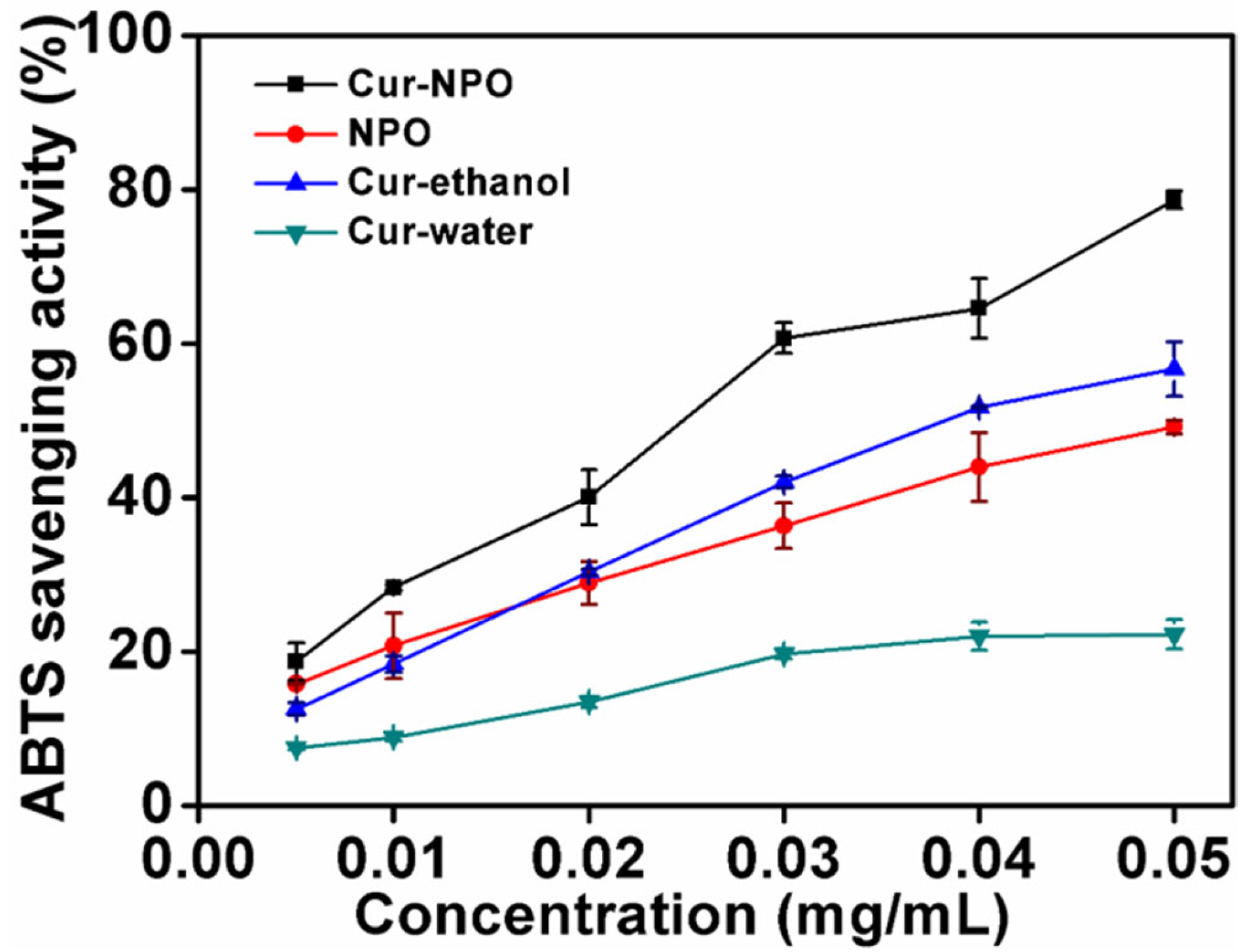
3.7. In Vitro Antioxidant Analysis (ABTS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | chitosan; |
| GI | gastrointestinal; |
| SA-CS | stearic acid-modified chitosan; |
| NaCas | sodium caseinate; |
| NP | SA-CS/NaCas composite nanoparticle; |
| NPO | SA-CS/NaCas/Odex composite nanoparticle; |
| Cur-NPO | SA-CS/NaCas/Odex composite nanoparticle loaded with curcumin; |
| FT-IR | Fourier transform infrared spectroscopy; |
| PDI | polydispersity index; |
| Odex | oxidized dextran |
| ZP | zeta potential |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6)-sulfonic acid |
| SGF | simulated gastric fluid |
| SIF | simulated intestinal fluid |
References
- Xue, J.; Luo, Y. Protein-polysaccharide nanocomplexes as nanocarriers for delivery of curcumin: A comprehensive review on preparation methods and encapsulation mechanisms. J. Future Foods 2023, 3, 99–114. [Google Scholar] [CrossRef]
- Gayathri, K.; Mahendran, B.; Chelliah, S.; Ramasamy, T. Nano formulation approaches for curcumin delivery—A review. J. Drug Deliv. Sci. Technol. 2023, 82, 104326. [Google Scholar] [CrossRef]
- Vauthier, C.; Labarre, D.; Ponchel, G. Design aspects of poly(alkylcyanoacrylate) nanoparticles for drug delivery. J. Drug Target. 2007, 15, 641–663. [Google Scholar] [CrossRef]
- Veneranda, M.; Hu, Q.; Wang, T.; Luo, Y.; Castro, K.; Madariaga, J.M. Formation and characterization of zein-caseinate-pectin complex nanoparticles for encapsulation of eugenol. LWT 2018, 89, 596–603. [Google Scholar] [CrossRef]
- Zhang, H.; Rui, L.; Wang, J.; Cui, S.W.; Wang, S.; Biao, W.; Nan, Z.; Xu, Y.; Jing, L.; Hao, W. Fabrication, characterization, and lipid-lowering effects of naringenin-zein-sodium caseinate-galactosylated chitosan nanoparticles. Int. J. Biol. Macromol. 2023, 230, 123150. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.W.; Rajendran, S.; Joshi, M. Synthesis and characterization of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr. Polym. 2011, 83, 438–446. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qi, J.; Yokoyama, W.; Zhong, F. Physicochemical and morphological properties of size-controlled chitosan-tripolyphosphate nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2015, 465, 137–146. [Google Scholar] [CrossRef]
- Speer, D.P.; Chvapil, M.; Eskelson, C.D.; Ulreich, J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 1980, 14, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, I.; Kulikowska, A.; Janczewska, M.; Michalak, M.; Cymerman, I.A.; Nagalski, A.; Kallinger, P.; Szymanski, W.W.; Ciach, T. Dextran Nanoparticle Synthesis and Properties. PLoS ONE 2016, 11, e0146237. [Google Scholar] [CrossRef]
- Hu, Q.; Bae, M.; Fleming, E.; Lee, J.-Y.; Luo, Y. Biocompatible polymeric nanoparticles with exceptional gastrointestinal stability as oral delivery vehicles for lipophilic bioactives. Food Hydrocoll. 2019, 89, 386–395. [Google Scholar] [CrossRef]
- Shen, D.; Hu, Q.; Sun, J.; Pang, X.; Li, X.; Lu, Y. Effect of oxidized dextran on the stability of gallic acid-modified chitosan-sodium caseinate nanoparticles. Int. J. Biol. Macromol. 2021, 192, 360–368. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Méndez, J.A.; Fernández-Gutiérrez, M.; Vázquez, B.; Román, J.S. Oxidized dextrins as alternative crosslinking agents for polysaccharides: Application to hydrogels of agarose-chitosan. Acta Biomater. 2014, 10, 798–811. [Google Scholar] [CrossRef]
- Yan, S.; Wu, S.; Zhang, J.; Zhang, S.; Huang, Y.; Zhu, H.; Li, Y.; Qi, B. Controlled release of curcumin from gelatin hydrogels by the molecular-weight modulation of an oxidized dextran cross-linker. Food Chem. 2023, 418, 135966. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, V.C.; Melo, K.R.T.; Alves, M.G.C.F.; Medeiros, M.J.C.; Grilo, M.L.P.M.; Almeida-Lima, J.; Pontes, D.L.; Costa, L.S.; Rocha, H.A.O. Dextran: Influence of Molecular Weight in Antioxidant Properties and Immunomodulatory Potential. Int. J. Mol. Sci. 2016, 17, 1340. [Google Scholar] [CrossRef] [PubMed]
- Sojitra, U.V.; Nadar, S.S.; Rathod, V.K. Immobilization of pectinase onto chitosan magnetic nanoparticles by macromolecular cross-linker. Carbohydr. Polym. 2017, 157, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent Insertion of Antioxidant Molecules on Chitosan by a Free Radical Grafting Procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar] [CrossRef]
- Muangsiri, W.; Kirsch, L.E. The protein-binding and drug release properties of macromolecular conjugates containing daptomycin and dextran. Int. J. Pharm. 2006, 315, 30–43. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.J.; Zhong, Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- Shi, L.; Campbell, G.; Jones, W.D.; Campagne, F.; Wen, Z.; Walker, S.J.; Su, Z.; Chu, T.-M.; Goodsaid, F.M.; Pusztai, L.; et al. The MicroArray Quality Control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nat. Biotechnol. 2010, 28, 827–838. [Google Scholar]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of Gallic Acid onto Chitosan Enhances Antioxidant Activities and Alters Rheological Properties of the Copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef]
- Qun, G.; Ajun, W. Effects of molecular weight, degree of acetylation and ionic strength on surface tension of chitosan in dilute solution. Carbohydr. Polym. 2006, 64, 29–36. [Google Scholar] [CrossRef]
- Yu, M.; Wu, J.; Shi, J.; Farokhzad, O.C. Nanotechnology for protein delivery: Overview and perspectives. J. Control. Release 2016, 240, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.A.; Achilias, D.S.; Bikiaris, D.N. Chitosan-g-PEG nanoparticles ionically crosslinked with poly(glutamic acid) and tripolyphosphate as protein delivery systems. Int. J. Pharm. 2012, 430, 318–327. [Google Scholar] [CrossRef]
- Pujana, M.A.; Pérez-Álvarez, L.; Cesteros Iturbe, L.C.; Katime, I. Water dispersible pH-responsive chitosan nanogels modified with biocompatible crosslinking-agents. Polymer 2012, 53, 3107–3116. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Goyal, R.; Gupta, K.C. Surface modification of crosslinked dextran nanoparticles influences transfection efficiency of dextran-polyethylenimine nanocomposites. Soft Matter 2011, 7, 11360–11371. [Google Scholar] [CrossRef]
- Fuentes, M.; Segura, R.L.; Abian, O.; Betancor, L.; Hidalgo, A.; Mateo, C.; Fernandez-Lafuente, R.; Guisan, J.M. Determination of protein-protein interactions through aldehyde-dextran intermolecular cross-linking. Proteomics 2004, 4, 2602–2607. [Google Scholar] [CrossRef]
- Yuan, X.-B.; Li, H.; Zhu, X.-X.; Woo, H.-G. Self-aggregated nanoparticles composed of periodate-oxidized dextran and cholic acid: Preparation, stabilization and in-vitro drug release. J. Chem. Technol. Biotechnol. 2006, 81, 746–754. [Google Scholar] [CrossRef]
- Rodriguez, N.J.; Hu, Q.; Luo, Y. Oxidized Dextran as a Macromolecular Crosslinker Stabilizes the Zein/Caseinate Nanocomplex for the Potential Oral Delivery of Curcumin. Molecules 2019, 24, 4061. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J. Food Eng. 2007, 82, 478–488. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, R.; Mehta, S.K. Formulation of saponin stabilized nanoemulsion by ultrasonic method and its role to protect the degradation of quercitin from UV light. Ultrason. Sonochem. 2016, 31, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sandig, A.G.; Campmany, A.C.C.; Campos, F.F.; Villena, M.J.M.; Naveros, B.C. Transdermal delivery of imipramine and doxepin from newly oil-in-water nanoemulsions for an analgesic and anti-allodynic activity: Development, characterization and in vivo evaluation. Colloids Surf. B Biointerfaces 2013, 103, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, L.; Cai, Q.; Zou, L.; Liu, C.; Liu, W.; McClements, D.J. Utilization of biopolymers to stabilize curcumin nanoparticles prepared by the pH-shift method: Caseinate, whey protein, soy protein and gum Arabic. Food Hydrocoll. 2020, 107, 105963. [Google Scholar] [CrossRef]
- Dang, L.H.; Vu, M.T.; Chen, J.; Nguyen, C.K.; Bach, L.G.; Tran, N.Q.; Le, V.T. Effect of Ultrasonication on Self-Assembled Nanostructures Formed by Amphiphilic Positive-Charged Copolymers and Negative-Charged Drug. ACS Omega 2019, 4, 4540–4552. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, T.; Hu, Q.; Luo, Y. Low density lipoprotein/pectin complex nanogels as potential oral delivery vehicles for curcumin. Food Hydrocoll. 2016, 57, 20–29. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Ren, J.; Zhao, M. Effects of Ultrasound Pretreatment on the Enzymatic Hydrolysis of Soy Protein Isolates and on the Emulsifying Properties of Hydrolysates. J. Agric. Food Chem. 2011, 59, 2600–2609. [Google Scholar] [CrossRef]
- Sui, X.; Bi, S.; Qi, B.; Wang, Z.; Zhang, M.; Li, Y.; Jiang, L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocoll. 2017, 63, 727–734. [Google Scholar] [CrossRef]
- Floris, A.; Meloni, M.C.; Lai, F.; Marongiu, F.; Maccioni, A.M.; Sinico, C. Cavitation effect on chitosan nanoparticle size: A possible approach to protect drugs from ultrasonic stress. Carbohydr. Polym. 2013, 94, 619–625. [Google Scholar] [CrossRef]
- Chen, H.; Xu, B.; Zhou, C.; Yagoub, A.E.G.A.; Cai, Z.; Yu, X. Multi-frequency ultrasound-assisted dialysis modulates the self-assembly of alcohol-free zein-sodium caseinate to encapsulate curcumin and fabricate composite nanoparticles. Food Hydrocoll. 2022, 122, 107110. [Google Scholar] [CrossRef]
- Desrumaux, A.; Marcand, J. Formation of sunflower oil emulsions stabilized by whey proteins with high-pressure homogenization (up to 350 MPa): Effect of pressure mulsion characteristics. Int. J. Food Sci. Technol. 2002, 37, 263–269. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Javvaji, V.; Kim, E.; Lee, M.E.; Raghavan, S.R.; Wang, Q.; Payne, G.F. Tyrosinase-mediated grafting and crosslinking of natural phenols confers functional properties to chitosan. Biofabrication 2014, 89, 21–27. [Google Scholar] [CrossRef]
- Luo, Y.; Qin, W. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Zhou, Y.; Guan, M.; Zhou, X.F.; Liu, Y.; Chen, W.L.; Zhang, C.G.; Yuan, Z.Q.; Liu, C.; Zhu, A.J. Low-Density Lipoprotein-Coupled N-Succinyl Chitosan Nanoparticles Co-Delivering SiRNA and Doxorubicin for Hepatocyte-Targeted Therapy. Biomaterials 2014, 35, 5965–5976. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, S.; Fleming, E.; Lee, J.-Y.; Luo, Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int. J. Biol. Macromol. 2020, 151, 747–756. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Q.; Zhou, M.; Xia, Y.; Nieh, M.-P.; Luo, Y. Development of “all natural” layer-by-layer redispersible solid lipid nanoparticles by nano spray drying technology. Eur. J. Pharm. Biopharm. 2016, 107, 273–285. [Google Scholar] [CrossRef]
- Jamwal, S.; Dautoo, U.K.; Ranote, S.; Dharela, R.; Chauhan, G.S. Enhanced catalytic activity of new acryloyl crosslinked cellulose dialdehyde-nitrilase Schiff base and its reduced form for nitrile hydrolysis. Int. J. Biol. Macromol. 2019, 131, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ying, D.; Cai, Y.; Le, X. Improved antioxidant activity and physicochemical properties of curcumin by adding ovalbumin and its structural characterization. Food Hydrocoll. 2017, 72, 304–311. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Dou, X.W.; Jin, T. Synthesis and self-assembly behavior of amphiphilic diblock copolymer dextran-block-poly(ε-caprolactone) (DEX-b-PCL) in aqueous media. Express Polym. Lett. 2010, 4, 599–610. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced Dispersibility and Bioactivity of Curcumin by Encapsulation in Casein Nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Pei, Y.; Xiong, W.; Zhang, C.; Xu, W.; Liu, S.; Li, B. Curcumin encapsulated in the complex of lysozyme/carboxymethylcellulose and implications for the antioxidant activity of curcumin. Food Res. Int. 2015, 75, 98–105. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence Study of the Curcumin−Casein Micelle Complexation and Its Application as a Drug Nanocarrier to Cancer Cells. Biomacromolecules 2008, 9, 2905–2929. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jie, L.; Xian, C.; Li, R.; Ming, S.; Yuan, W.; Jian, X. Mesona chinensis polysaccharide/zein nanoparticles to improve the bioaccesibility and in vitro bioactivities of curcumin. Carbohydr. Polym. 2022, 295, 119875. [Google Scholar] [CrossRef] [PubMed]
| Samples | Odex/SA-CS (w/w) | pH = 2.0 | |
|---|---|---|---|
| Size (nm) | PDI | ||
| Control | 318.5 ± 24.4 a | 0.424 ± 0.059 a | |
| Odex (10 kDa) | 0.5:1 | 176.3 ± 3.3 d | 0.247 ± 0.005 b |
| 1:1 | 184.3 ± 9.6 d | 0.221 ± 0.026 c | |
| 1.5:1 | 236.9 ± 12.2 c | 0.246 ± 0.023 c | |
| 2:1 | 239.4 ± 12.1 b | 0.225 ± 0.043 c | |
| Odex (40 kDa) | 0.5:1 | 185.2 ± 7.8 d | 0.340 ± 0.032 b |
| 1:1 | 175.9 ± 4.2 d | 0.264 ± 0.011 c | |
| 1.5:1 | 204.4 ± 6.7 c | 0.236 ± 0.011 c | |
| 2:1 | 221.2 ± 7.3 b | 0.271 ± 0.034 c | |
| Odex (70 kDa) | 0.5:1 | 211.0 ± 9.0 b | 0.369 ± 0.011 b |
| 1:1 | 184.9 ± 1.8 c | 0.338 ± 0.028 bc | |
| 1.5:1 | 187.5 ± 4.1 c | 0.304 ± 0.030 c | |
| 2:1 | 190.0 ± 6.9 c | 0.254 ± 0.015 d | |
| Odex (100 kDa) | 0.5:1 | 199.1 ± 7.6 c | 0.346 ± 0.015 b |
| 1:1 | 178.8 ± 3.3 d | 0.350 ± 0.015 b | |
| 1.5:1 | 186.6 ± 4.0 cd | 0.277 ± 0.012 c | |
| 2:1 | 213.5 ± 2.6 b | 0.307 ± 0.036 c | |
| Odex (150 kDa) | 0.5:1 | 182.5 ± 1.0 b | 0.287 ± 0.023 b |
| 1:1 | 175.4 ± 2.9 b | 0.286 ± 0.033 b | |
| 1.5:1 | 178.8 ± 2.4 b | 0.251 ± 0.018 b | |
| 2:1 | 172.3 ± 2.0 b | 0.263 ± 0.022 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, D.; Chen, H.; Li, M.; Yu, L.; Li, X.; Liu, H.; Hu, Q.; Lu, Y. Effects of Different Molecular Weight Oxidized Dextran as Crosslinkers on Stability and Antioxidant Capacity of Curcumin-Loaded Nanoparticles. Foods 2023, 12, 2533. https://doi.org/10.3390/foods12132533
Shen D, Chen H, Li M, Yu L, Li X, Liu H, Hu Q, Lu Y. Effects of Different Molecular Weight Oxidized Dextran as Crosslinkers on Stability and Antioxidant Capacity of Curcumin-Loaded Nanoparticles. Foods. 2023; 12(13):2533. https://doi.org/10.3390/foods12132533
Chicago/Turabian StyleShen, Dongyan, Hongzhou Chen, Mingwei Li, Ling Yu, Xiangfei Li, Huawei Liu, Qiaobin Hu, and Yingjian Lu. 2023. "Effects of Different Molecular Weight Oxidized Dextran as Crosslinkers on Stability and Antioxidant Capacity of Curcumin-Loaded Nanoparticles" Foods 12, no. 13: 2533. https://doi.org/10.3390/foods12132533
APA StyleShen, D., Chen, H., Li, M., Yu, L., Li, X., Liu, H., Hu, Q., & Lu, Y. (2023). Effects of Different Molecular Weight Oxidized Dextran as Crosslinkers on Stability and Antioxidant Capacity of Curcumin-Loaded Nanoparticles. Foods, 12(13), 2533. https://doi.org/10.3390/foods12132533





