A Method to Reduce the Occurrence of Egg Translucency and Its Effect on Bacterial Invasion
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Experimental Materials and Feeding Management
2.3. Sample Collection
2.4. Serum Biochemical Indexes
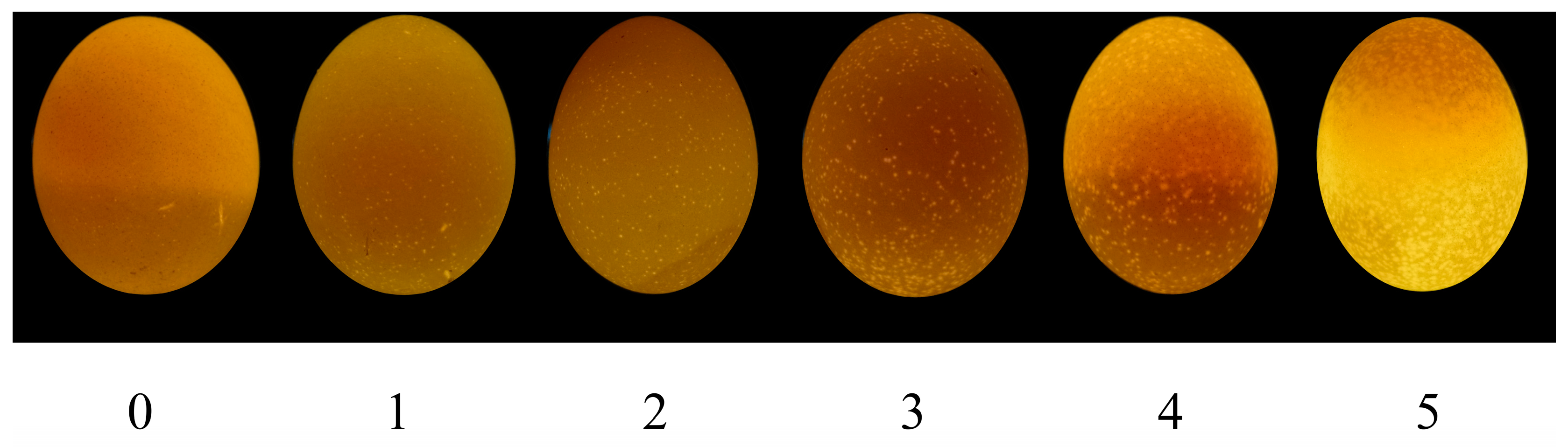
2.5. Grading of Translucent Eggs
2.6. Eggshell Ultrastructure
2.7. Egg Quality
2.8. Determination of E. coli Invasion Rate
2.9. Statistical Analyses
3. Results
3.1. State of Chronic Heat Stress in Different Months
3.2. Effect of Adding MDCP on Blood Heat Stress Indexes
3.3. Effect of Adding MDCP on Eggshell Translucency
3.4. Correlation between Eggshell Translucency and Structure
3.5. Effect of Adding MDCP on Eggshell Structure
3.6. Effects of Adding MDCP on Egg Quality
3.7. Effect of Eggshell Translucency on Bacterial Invasion Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donald, K.L.; Nancy, R.R. Egg Protein as a Source of Power, Strength, and Energy. Nutr. Today 2009, 44, 43–48. [Google Scholar] [CrossRef]
- Sharaf, E.A.; Ibrahim, S.A.; Tahergorabi, R. Egg quality and safety with an overview of edible coating application for egg preservation. Food Chem. 2019, 296, 29–39. [Google Scholar] [CrossRef]
- Wang, D.H.; Chen, H.; Zhou, R.Y.; Huang, C.X.; Gao, H.X.; Fan, B.L.; Liu, G.J.; Ning, Z.H. Study of measurement methods on phenotype of translucent eggs. Poult. Sci. 2019, 98, 6677–6683. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ning, Z. Research progress on bird eggshell quality defects a review. Poult. Sci. 2022, 102, 102283. [Google Scholar] [CrossRef]
- Holst, W.F.; Almquist, H.J.; Lorenz, F.W. A Study of Shell Texture of the Hen’s Egg. Poult. Sci. 1932, 11, 144–149. [Google Scholar] [CrossRef]
- Baker, R.C.; Curtiss, R. Individual Hen Differences in Egg Shell Mottling and the Relationship of Shell Mottling to Clutch Size, Internal Quality and Weight Loss. Poult. Sci. 1957, 36, 904–908. [Google Scholar] [CrossRef]
- Denison, J.W. The Effect of Mechanical Disturbance of the Egg Cuticle on Shell Mottling. Poult. Sci. 1967, 46, 771–772. [Google Scholar] [CrossRef]
- Tyler, C.; Geake, F.H. The effect of water on egg shell strength including a study of the translucent areas of the shell. Br. Poult. Sci. 1964, 5, 277–284. [Google Scholar] [CrossRef]
- Lucas, J.L.; Marcos, H.R. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Marsden, A.; Morris, T.R.; Cromarty, A.S. Effects of constant environmental temperatures on the performance of laying pullets. Br. Poult. Sci. 1987, 28, 361–380. [Google Scholar] [CrossRef]
- Lin, H.; Jiao, H.; Buyse, J.; Decuypere, E. Strategies for preventing heat stress in poultry. World’s Poult. Sci. J. 2006, 62, 71–85. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Gao, P.; Xie, X.; Li, D.; Yu, D.; Yu, M. Effect of curcumin on laying performance, egg quality, endocrine hormones, and immune activity in heat-stressed hens. Poult. Sci. 2020, 99, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef]
- Zaboli, G.; Huang, X.; Feng, X.; Ahn, D.U. How can heat stress affect chicken meat quality?—A review. Poult. Sci. 2019, 98, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Chousalkar, K.K.; Flynn, P.; Sutherland, M.; Roberts, J.R.; Cheetham, B.F. Recovery of Salmonella and Escherichia coli from commercial egg shells and effect of translucency on bacterial penetration in eggs. Int. J. Food Microbiol. 2010, 142, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, W.; Li, X.; Chen, X.; Xu, G.; Zheng, J. Effects of chicken breeds, age, storage time and storage conditions on the quality of egg cuticle. China Poult. 2018, 40, 35–39. (In Chinese) [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Guo, Y.; Li, W.; Song, J.; Xu, G.; Yang, N.; Zheng, J. Impact of cuticle quality and eggshell thickness on egg antibacterial efficiency. Poult. Sci. 2019, 98, 940–948. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, J.K.; Sun, W.Q.; Chen, Z.Y.; Wu, S.R.; Ren, Z.Z.; Yang, X.J. Effect of inorganic phosphate supplementation on egg production in Hy-Line Brown layers fed 2000 FTU/kg phytase. Animals 2020, 14, 2246–2252. [Google Scholar] [CrossRef]
- Beate, C.B.; Elizabeth, B.; Julie, S.; Neil, L.S.; Fiona, P.; Karen, W.; Yoshiko, O.; Amelia, S.; Samuel, N. Use of Multidimensional Clinical Profiles (MDCP) to Characterize Pediatric Patients with Selected Functional Gastrointestinal Disorders (FGID). Gastroenterology 2017, 152, S650–S651. [Google Scholar] [CrossRef]
- Ling, L.; Yongqing, G.; Zhaohai, B.; Yubo, C.; Yan, T.; Zongyong, W.; Yaoji, L.; Zhiguo, W.; Lin, M. Reducing phosphorus excretion and loss potential by using a soluble supplement source for swine and poultry. J. Clean. Prod. 2019, 237, 117654. [Google Scholar] [CrossRef]
- Gibson, K.B.; Vo, D.T.; Nguyen, T.Q. An investigation of dehazing effects on image and video coding. IEEE Trans. Image Process. 2012, 21, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N. Environment: The disappearing nutrient. Nature 2009, 461, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Wu, L.; Younes, M.; Hincke, M. Biotechnological Applications of Eggshell: Recent Advances. Front. Bioeng. Biotechnol. 2021, 9, 675364. [Google Scholar] [CrossRef]
- Bar, A.; Hurwitz, S. Egg shell quality, medullary bone ash, intestinal calcium and phosphorus absorption, and calcium-binding protein in phosphorus-deficient hens. Poult. Sci. 1984, 63, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.R.; Charraga, S.; Avila-Gonzalez, E. Evaluation of a new generation phytase on phytate phosphorus release for egg production and tibia strength in hens fed a corn-soybean meal diet. Poult. Sci. 2019, 98, 2087–2093. [Google Scholar] [CrossRef]
- Pongmanee, K.; Kuhn, I.; Korver, D.R. Effects of phytase supplementation on eggshell and bone quality, and phosphorus and calcium digestibility in laying hens from 25 to 37 wk of age. Poult. Sci. 2020, 99, 2595–2607. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Jin, Y.; Wang, S.; Huang, X.; Li, K.; Xia, W.; Ruan, D.; Wang, S.; Chen, W.; et al. Age-related changes in eggshell physical properties, ultrastructure, calcium metabolism-related serum indices, and gene expression in eggshell gland during eggshell formation in commercial laying ducks. Poult. Sci. 2022, 101, 101573. [Google Scholar] [CrossRef]
- Beshir, M.; Ramsey, J.D. Heat stress indices: A review paper. Int. J. Ind. Ergon. 1988, 3, 89–102. [Google Scholar] [CrossRef]
- Aamir, N.; Fahar, I.; Guanghui, L.; Barbara, K.; Jiang, W.; Wenchao, L.; Yi, Z.; Yasir, N.; Kongquan, L.; Mei, X.; et al. Heat stress in poultry production; Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef]
- Bonato, M.; Malecki, I.A.; Rybnik-Trzaskowska, P.K.; Cornwallis, C.K.; Cloete, S.W. Predicting ejaculate quality and libido in male ostriches: Effect of season and age. Anim. Reprod. Sci. 2014, 151, 49–55. [Google Scholar] [CrossRef]
- Ibtisham, F.; An, L.; Li, T.; Niu, Y.; Xiao, M.; Zhang, L.; Jia, R. Growth Patterns of Two Chinese Native Goose Breeds. Braz. J. Poult. Sci. 2017, 19, 203–209. [Google Scholar] [CrossRef]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Ijaz, A.; Sohail, A.; Shabbir, M.Z.; Rehman, H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012, 91, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Goel, A. Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1136–1145. [Google Scholar] [CrossRef]
- Galarza-Seeber, R.; Latorre, J.D.; Bielke, L.R.; Kuttappan, V.A.; Wolfenden, A.D.; Hernandez-Velasco, X.; Merino-Guzman, R.; Vicente, J.L.; Donoghue, A.; Cross, D.; et al. Leaky Gut and Mycotoxins: Aflatoxin B1 Does Not Increase Gut Permeability in Broiler Chickens. Front. Vet. Sci. 2016, 3, 10. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Altan, O.; Pabuccuoglu, A.; Altan, A.; Konyalioglu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003, 44, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, Y.K.; Kim, S.H.; Lee, K.W. The Impact of Temperature and Humidity on the Performance and Physiology of Laying Hens. Animals 2020, 11, 56. [Google Scholar] [CrossRef]
- Mack, L.A.; Felver-Gant, J.N.; Dennis, R.L.; Cheng, H.W. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013, 92, 285–294. [Google Scholar] [CrossRef]
- Buyse, J.; Decuypere, E.; Berghman, L.; Kuhn, E.R.; Vandesande, F. Effect of dietary protein content on episodic growth hormone secretion and on heat production of male broiler chickens. Br. Poult. Sci. 1992, 33, 1101–1109. [Google Scholar] [CrossRef]
- Elnagar, S.A.; Scheideler, S.E.; Beck, M.M. Reproductive hormones, hepatic deiodinase messenger ribonucleic acid, and vasoactive intestinal polypeptide-immunoreactive cells in hypothalamus in the heat stress-induced or chemically induced hypothyroid laying hen. Poult. Sci. 2010, 89, 2001–2009. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus). Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2004, 139, 737–744. [Google Scholar] [CrossRef]
- Star, L.; Kemp, B.; van den Anker, I.; Parmentier, H.K. Effect of single or combined climatic and hygienic stress in four layers lines: 1. Performance. Poult. Sci. 2008, 87, 1022–1030. [Google Scholar] [CrossRef]
- Shini, S.; Kaiser, P.; Shini, A.; Bryden, W.L. Biological response of chickens (Gallus gallus domesticus) induced by corticosterone and a bacterial endotoxin. Comparative biochemistry and physiology. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Post, J.; Rebel, J.M.; ter Huurne, A.A. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult. Sci. 2003, 82, 1313–1318. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sa, L.R.; Ferreira, A.J.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef]
- Cusack, M.; Fraser, A.C.; Stachel, T. Magnesium and phosphorus distribution in the avian eggshell. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2003, 134, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Yang, Y.; Yuan, J.; Wang, Z.; Guo, Y. Effect of dietary nonphytate phosphorus on laying performance and small intestinal epithelial phosphate transporter expression in Dwarf pink-shell laying hens. J. Anim. Sci. Biotechnol. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Talbot, C.; Tyler, C. A study of the fundamental cause of natural translucent areas in egg shells. Br. Poult. Sci. 1974, 15, 197–204. [Google Scholar] [CrossRef]
- Wang, D.H.; Li, Y.J.; Liu, L.; Liu, J.S.; Bao, M.; Yang, N.; Zhuo-Cheng, H.; Ning, Z.H. Traits of eggshells and shell membranes of translucent eggs. Poult. Sci. 2017, 96, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yan, L.; Zhang, T.; Yin, H.; Liu, J.; Wang, J. Effect of 25-hydroxyvitamin D and essential oil complex on productive performance, egg quality, and uterus antioxidant capacity of laying hens. Poult. Sci. 2021, 100, 101410. [Google Scholar] [CrossRef]
- Zhang, Y.; Tong, S.; Ge, M.; Jing, B.; Hou, S.; Tan, F.; Chen, Y.; Guo, Y.; Wu, L. The influence of relative humidity on the heterogeneous oxidation of sulfur dioxide by ozone on calcium carbonate particles. Sci. Total Environ. 2018, 633, 1253–1262. [Google Scholar] [CrossRef]
- Fu, L.; Wu, G.; Sun, C.; Zheng, J.; Li, G.; Xu, G. Study on the cause of translucent eggshells. China Poult. 2019, 41, 6–11. (In Chinese) [Google Scholar] [CrossRef]
- Abdulla, R.; Sanny, S.; Derman, E. Stability studies of immobilized lipase on rice husk and eggshell membrane. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 12032. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Gao, Q.; Fan, C.; Xia, A.; Zeng, D.; Ning, Z.H. Effect of dietary blend oil on production performance and egg quality of laying hens. China Poult. 2020, 42, 36–39. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, C.T.; Fouad, A.M.; Chen, W.; Ruan, D.; Wang, S.; Xia, W.G. Impact of Heat Stress on Meat, Egg Quality, Immunity and Fertility in Poultry and Nutritional Factors That Overcome These Effects: A Review. Int. J. Poult. Sci. 2016, 15, 81. [Google Scholar] [CrossRef]
- Negoiță, I.; Bălăceanu, R.; Dojană, N. Calcium Intake Level as Factor of Influencing of the Eggshell Qualities in Laying Hens. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2017, 74, 74–79. [Google Scholar] [CrossRef]
- Fathi, M.M.; Galal, A.; Ali, U.M.; Abou-Emera, O.K. Physical and mechanical properties of eggshell as affected by chicken breed and flock age. Br. Poult. Sci. 2019, 60, 506–512. [Google Scholar] [CrossRef]
- Franco-Jimenez, D.; Scheideler, S.; Kittok, R.; Brown-Brandl, T.; Robeson, L.; Taira, H.; Beck, M. Differential effects of heat stress in three strains of laying hens. J. Appl. Poult. Res. 2007, 16, 628–634. [Google Scholar] [CrossRef]
- Mashaly, M.M.; Hendricks, G.R.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef]
- Bain, M.M.; McDade, K.; Burchmore, R.; Law, A.; Wilson, P.W.; Schmutz, M.; Preisinger, R.; Dunn, I.C. Enhancing the egg’s natural defence against bacterial penetration by increasing cuticle deposition. Anim. Genet. 2013, 44, 661–668. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, X.; Shi, X.; Li, X.; Li, C.; Li, J.; Xu, G.; Yang, N.; Zheng, J. Acetic acid, vinegar, and citric acid as washing materials for cuticle removal to improve hatching performance of quail eggs. Poult. Sci. 2020, 99, 3865–3876. [Google Scholar] [CrossRef]
- Bain, M.M.; Zheng, J.; Zigler, M.; Whenham, N.; Quinlan-Pluck, F.; Jones, A.C.; Roberts, M.; Icken, W.; Olori, V.E.; Dunn, I.C. Cuticle deposition improves the biosecurity of eggs through the laying cycle and can be measured on hatching eggs without compromising embryonic development. Poult. Sci. 2019, 98, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Van De Merwe, W.P.; Czege, J.; Milham, M.E.; Bronk, B.V. Rapid optically based measurements of diameter and length for spherical or rod-shaped bacteria in vivo. Appl. Opt. 2004, 43, 5295–5302. [Google Scholar] [CrossRef] [PubMed]





| Items | Items | ||
|---|---|---|---|
| Ingredients (%) | Nutrient levels 3 | ||
| Corn | 62.50 | Metabolic energy (MJ/Kg) | 11.42 |
| Soybean meal | 26.20 | Total methionine | 0.42 |
| Soy oil | 1.00 | Crude protein | 16.04 |
| Limestone | 9.58 | Total lysine | 0.81 |
| Sodium chloride | 0.30 | Total threonine | 0.60 |
| Choline chloride | 0.10 | Ca | 3.64 |
| Vitamin premix 1 | 0.02 | Available phosphorus | 0.35 |
| Trace mineral premix 2 | 0.30 | ||
| Item | Group | Month | p-Value | ||
|---|---|---|---|---|---|
| 6 | 8 | 10 | |||
| T3 (nmol/L) | CON | 5.19 ± 0.28 | 4.93 ± 0.25 b | 5.48 ± 0.25 b | 0.37 |
| MDCP | 5.70 ± 0.31 B | 7.05 ± 0.50 A,a | 6.55 ± 0.33 AB,a | 0.03 | |
| p-value | 0.23 | <0.01 | 0.01 | ||
| T4 (nmol/L) | CON | 60.66 ± 1.81 A | 55.49 ± 2.23 AB,b | 51.71 ± 1.92 B | <0.01 |
| MDCP | 61.66 ± 2.47 AB | 65.74 ± 3.23 A,a | 55.06 ± 2.36 B | <0.01 | |
| p-value | 0.75 | 0.01 | 0.28 | ||
| CORT (ng/mL) | CON | 82.53 ± 3.26 | 90.03 ± 2.84 | 81.37 ± 3.25 | 0.27 |
| MDCP | 83.63 ± 2.43 B | 93.44 ± 3.84 A | 84.82 ± 3.42 AB | <0.01 | |
| p-value | 0.79 | 0.48 | 0.47 | ||
| Items | Month | CON | MDCP | p-Value | ||
|---|---|---|---|---|---|---|
| Mean ± SEM | CV/% | Mean ± SEM | CV/% | |||
| MSH (μm) | 6 | 29.58 ± 0.97 a | 17.95 | 29.00 ± 1.07 a | 20.17 | 0.67 |
| 8 | 26.06 ± 0.64 b | 13.39 | 25.08 ± 0.53 b | 11.48 | 0.98 | |
| 10 | 21.70 ± 0.57 B,c | 14.29 | 25.24 ± 0.44 A,b | 9.55 | <0.01 | |
| p-value | <0.01 | <0.01 | ||||
| MSW (μm) | 6 | 27.31 ± 0.74 a | 14.90 | 28.33 ± 0.89 | 17.26 | 0.34 |
| 8 | 26.74 ± 0.72 a | 14.85 | 25.98 ± 0.75 | 15.86 | 0.43 | |
| 10 | 23.48 ± 0.86 B,b | 20.06 | 27.30 ± 0.75 A | 15.02 | <0.01 | |
| p-value | <0.01 | 0.12 | ||||
| MSA (μm2) | 6 | 533.88 ± 30.07 a | 30.85 | 533.74 ± 33.57 | 34.45 | 0.10 |
| 8 | 466.67 ± 16.05 b | 18.84 | 461.44 ± 13.67 | 16.22 | 0.79 | |
| 10 | 375.74 ± 17.64 B,c | 25.72 | 471.45 ± 12.55 A | 14.58 | <0.01 | |
| p-value | <0.01 | 0.06 | ||||
| SMT (μm) | 6 | 60.92 ± 1.47 a | 13.30 | 65.10 ± 1.57 a | 13.20 | 0.06 |
| 8 | 54.82 ± 1.64 b | 16.36 | 54.58 ± 1.15 b | 20.67 | 0.91 | |
| 10 | 56.10 ± 2.04 ab | 18.89 | 60.04 ± 1.65 ab | 23.31 | 0.14 | |
| p-value | 0.04 | 0.02 | ||||
| Items | Month | Group | p-Value | |
|---|---|---|---|---|
| CON | MDCP | |||
| EW (g) | 6 | 48.62 ± 0.64 b | 47.92 ± 0.57 c | 0.42 |
| 7 | 51.88 ± 0.80 a | 50.60 ± 0.62 b | 0.21 | |
| 8 | 50.91 ± 0.66 a | 50.89 ± 0.81 b | 0.99 | |
| 9 | 51.92 ± 0.51 a | 52.67 ± 0.52 a | 0.31 | |
| 10 | 52.08 ± 0.73 a | 53.57 ± 0.50 a | 0.10 | |
| p-value | <0.01 | 0.02 | ||
| ESS (kg/cm2) | 6 | 3.15 ± 0.10 | 3.03 ± 0.07 | 0.34 |
| 7 | 2.98 ± 0.09 | 3.05 ± 0.13 | 0.67 | |
| 8 | 2.97 ± 0.10 | 2.92 ± 0.08 | 0.69 | |
| 9 | 2.92 ± 0.10 | 3.03 ± 0.09 | 0.43 | |
| 10 | 3.04 ± 0.08 | 2.98 ± 0.09 | 0.64 | |
| p-value | 0.39 | 0.70 | ||
| HU | 6 | 69.37 ± 2.00 bc | 64.75 ± 1.40 b | 0.06 |
| 7 | 66.19 ± 1.78 c | 69.96 ± 2.13 ab | 0.18 | |
| 8 | 64.91 ± 1.98 c | 66.83 ± 2.05 ab | 0.50 | |
| 9 | 72.60 ± 2.00 ab | 71.86 ± 1.68 a | 0.78 | |
| 10 | 76.94 ± 2.01 a | 72.20 ± 1.65 a | 0.07 | |
| p-value | <0.01 | <0.01 | ||
| EST (μm) | 6 | 349.56 ± 6.14 | 340.77 ± 6.07 | 0.31 |
| 7 | 332.31 ± 5.42 | 331.74 ± 6.21 | 0.95 | |
| 8 | 342.47 ± 6.22 | 339.73 ± 5.86 | 0.73 | |
| 9 | 343.68 ± 5.48 | 343.38 ± 5.40 | 0.97 | |
| 10 | 345.82 ± 6.08 | 334.85 ± 6.04 | 0.21 | |
| p-value | 0.76 | 0.97 | ||
| YC | 6 | 6.38 ± 0.12 b | 6.37 ± 0.13 b | 0.95 |
| 7 | 6.48 ± 0.10 b | 6.47 ± 0.09 b | 0.94 | |
| 8 | 6.30 ± 0.16 b | 6.17 ± 0.12 b | 0.51 | |
| 9 | 5.30 ± 0.14 c | 5.37 ± 0.16 c | 0.75 | |
| 10 | 7.20 ± 0.13 a | 7.39 ± 0.09 a | 0.24 | |
| p-value | 0.05 | 0.10 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Liang, Q.; Wang, E.; Jiang, C.; Zeng, L.; Chen, R.; Li, J.; Xu, G.; Zheng, J. A Method to Reduce the Occurrence of Egg Translucency and Its Effect on Bacterial Invasion. Foods 2023, 12, 2538. https://doi.org/10.3390/foods12132538
Shi X, Liang Q, Wang E, Jiang C, Zeng L, Chen R, Li J, Xu G, Zheng J. A Method to Reduce the Occurrence of Egg Translucency and Its Effect on Bacterial Invasion. Foods. 2023; 12(13):2538. https://doi.org/10.3390/foods12132538
Chicago/Turabian StyleShi, Xuefeng, Qianni Liang, Enling Wang, Caiyun Jiang, Lingsen Zeng, Ruochen Chen, Junying Li, Guiyun Xu, and Jiangxia Zheng. 2023. "A Method to Reduce the Occurrence of Egg Translucency and Its Effect on Bacterial Invasion" Foods 12, no. 13: 2538. https://doi.org/10.3390/foods12132538
APA StyleShi, X., Liang, Q., Wang, E., Jiang, C., Zeng, L., Chen, R., Li, J., Xu, G., & Zheng, J. (2023). A Method to Reduce the Occurrence of Egg Translucency and Its Effect on Bacterial Invasion. Foods, 12(13), 2538. https://doi.org/10.3390/foods12132538






