The Hydrolysis of Pigment-Protein Phycoerythrin by Bromelain Enhances the Color Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
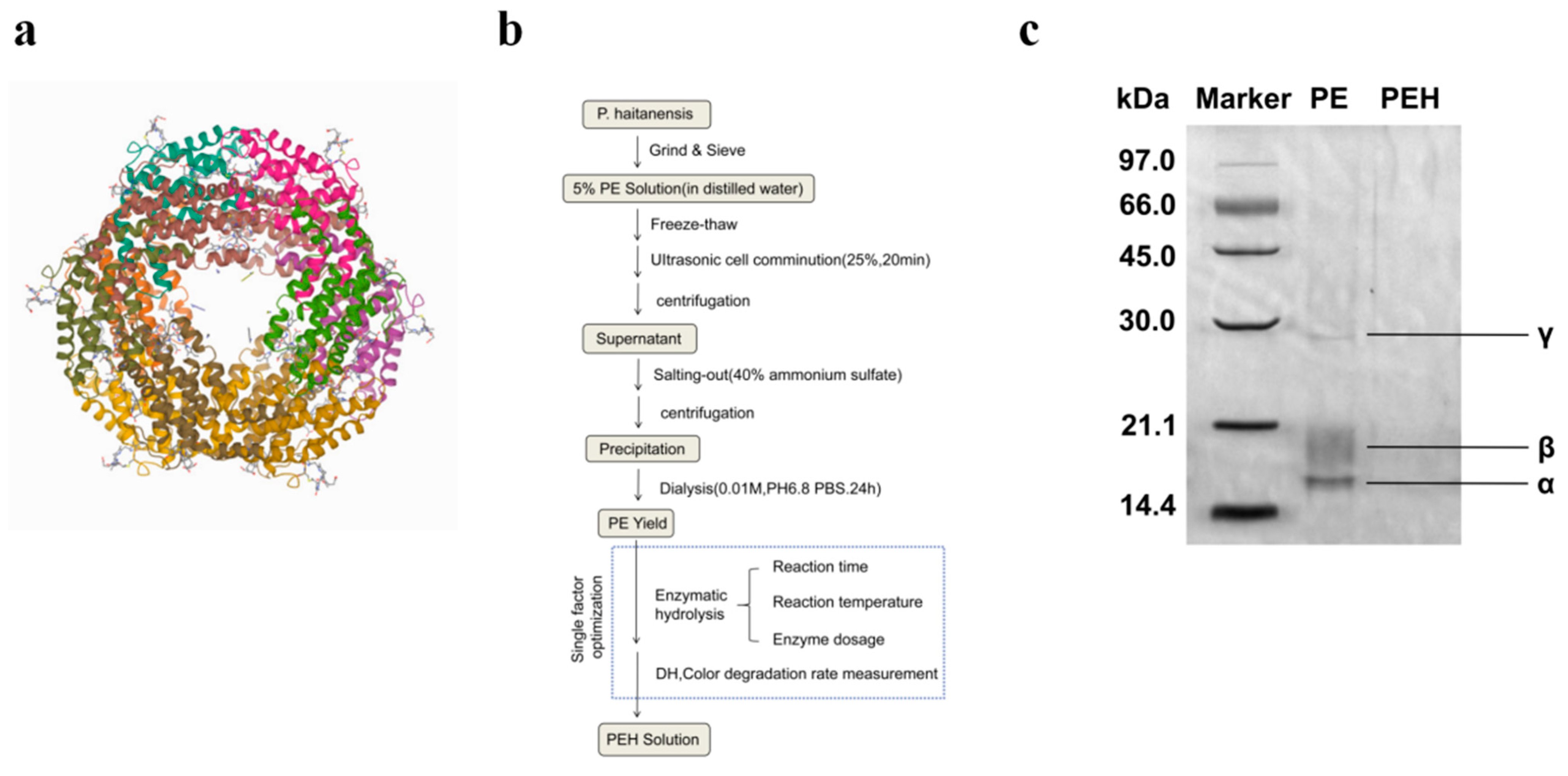
2.2. Preparation of PE
2.3. Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Optimization of Enzymatic Hydrolysis Conditions
2.5. Measurement of Degree of Hydrolysis (DH)
2.6. Detection of Color Degradation Rate
2.7. Characterization of PE and PEH
2.8. Analysis of Spectroscopic Properties
2.9. Scanning Electron Microscope (SEM)
2.10. Antioxidant Capacity Analysis
2.10.1. Radical Scavenging Ability of PE and PEH—DPPH Assay
2.10.2. Radical Scavenging Ability of PE and PEH—ABTS Assay
2.11. Effect of Temperature, Light, and Metal Ion on the Stability of PEH
2.12. Measurement of Color
2.13. Statistical Analysis
3. Results and Discussion
3.1. Preparation of PE and PEH
3.2. Optimization of Enzymatic Hydrolysis Conditions of PE by Bromelain
3.2.1. The Effect of Reaction Time on Enzymatic Hydrolysis
3.2.2. Influence of Reaction Temperature on Enzymatic Hydrolysis
3.2.3. The Effect of Enzyme Dosage on Enzymatic Hydrolysis
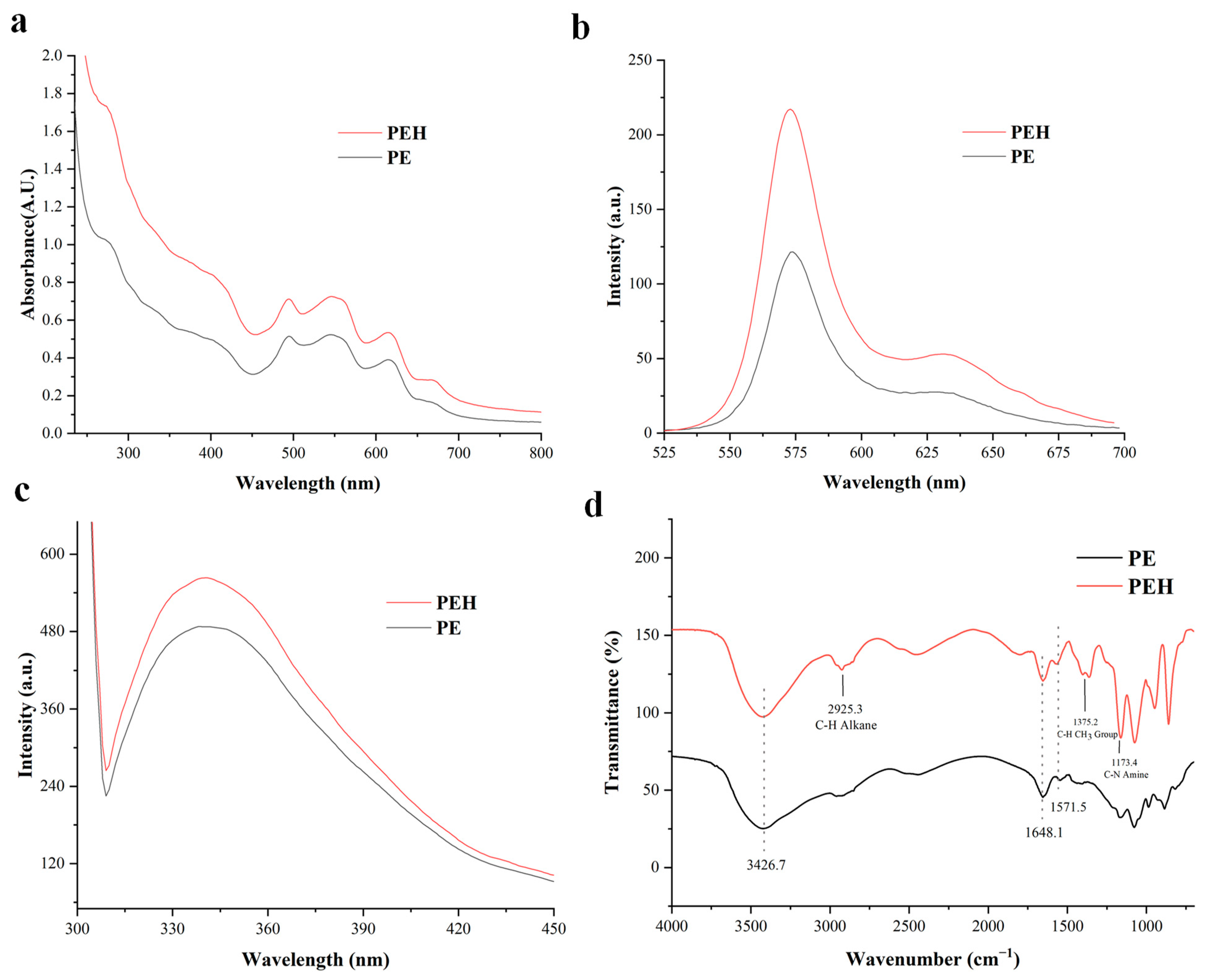
3.3. UV/Vis Absorption and Fluorescence Spectroscopy of PE and PEH
3.4. FTIR Analysis of PE and PEH
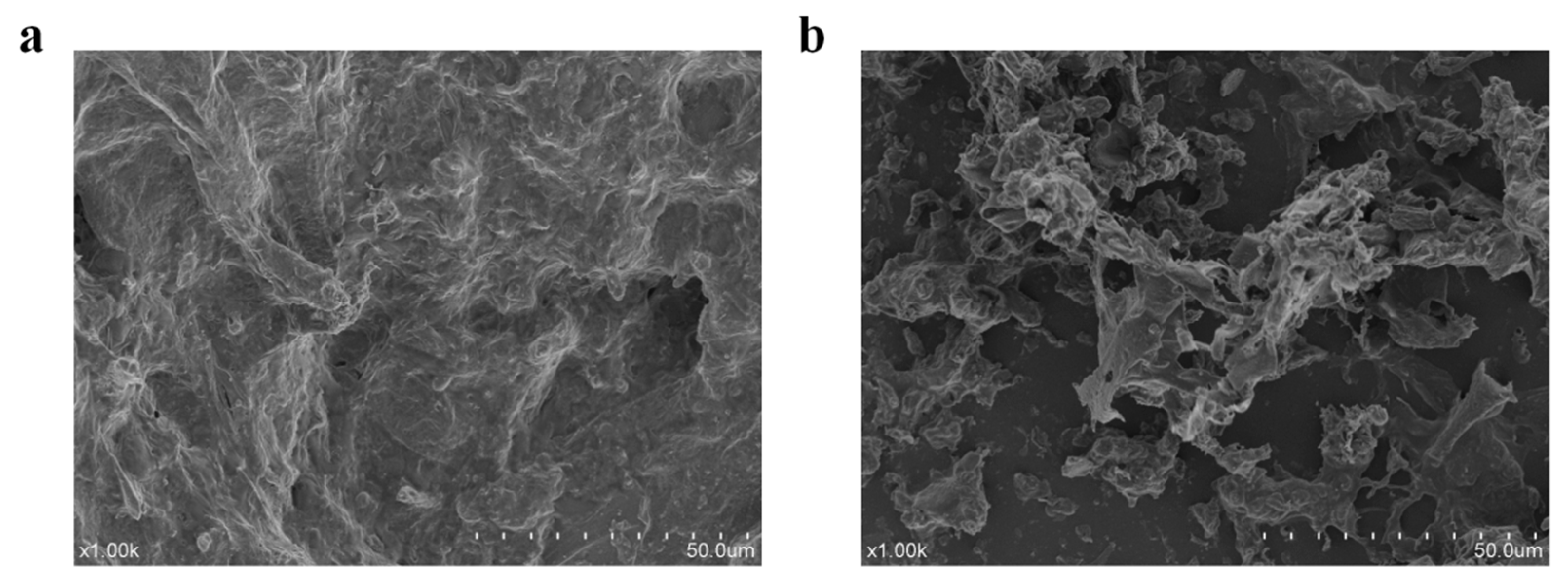
3.5. SEM Morphology
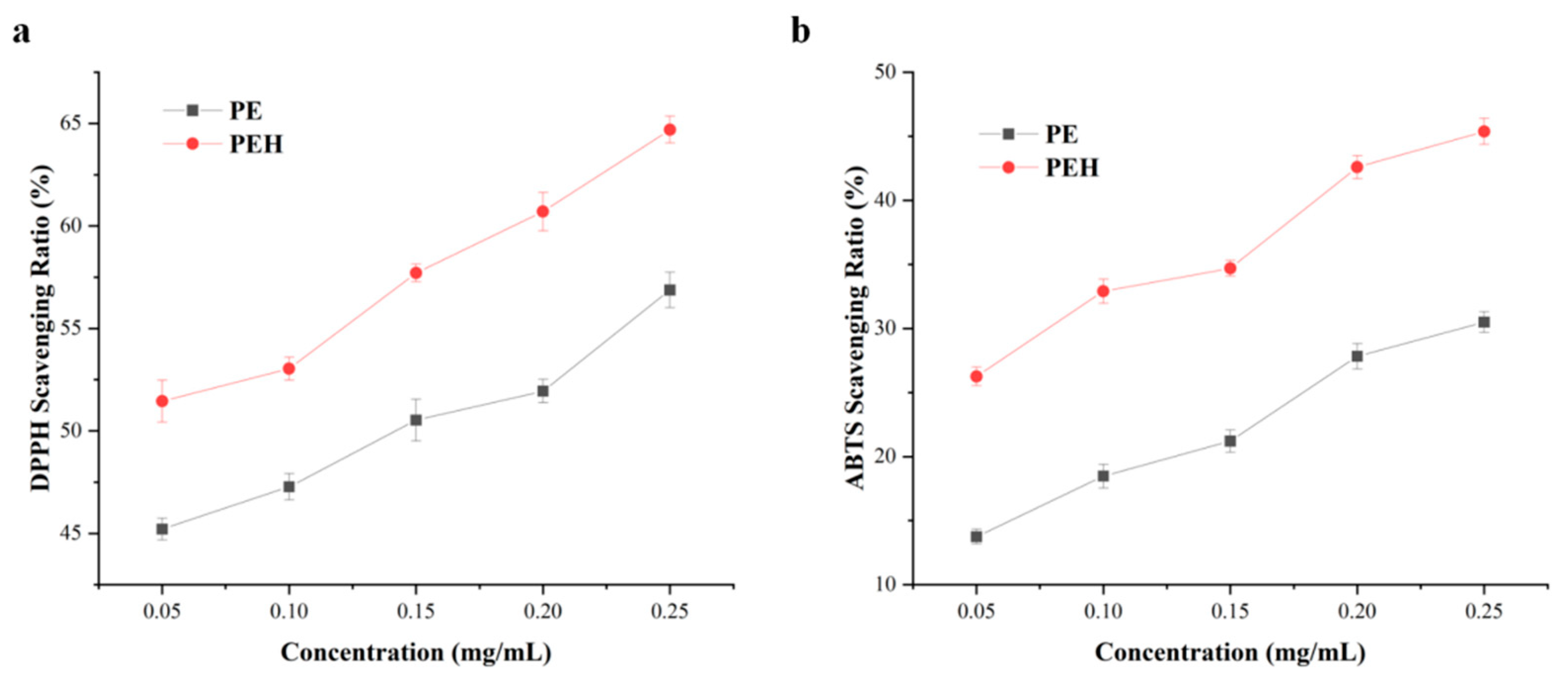
3.6. Antioxidant Capacity
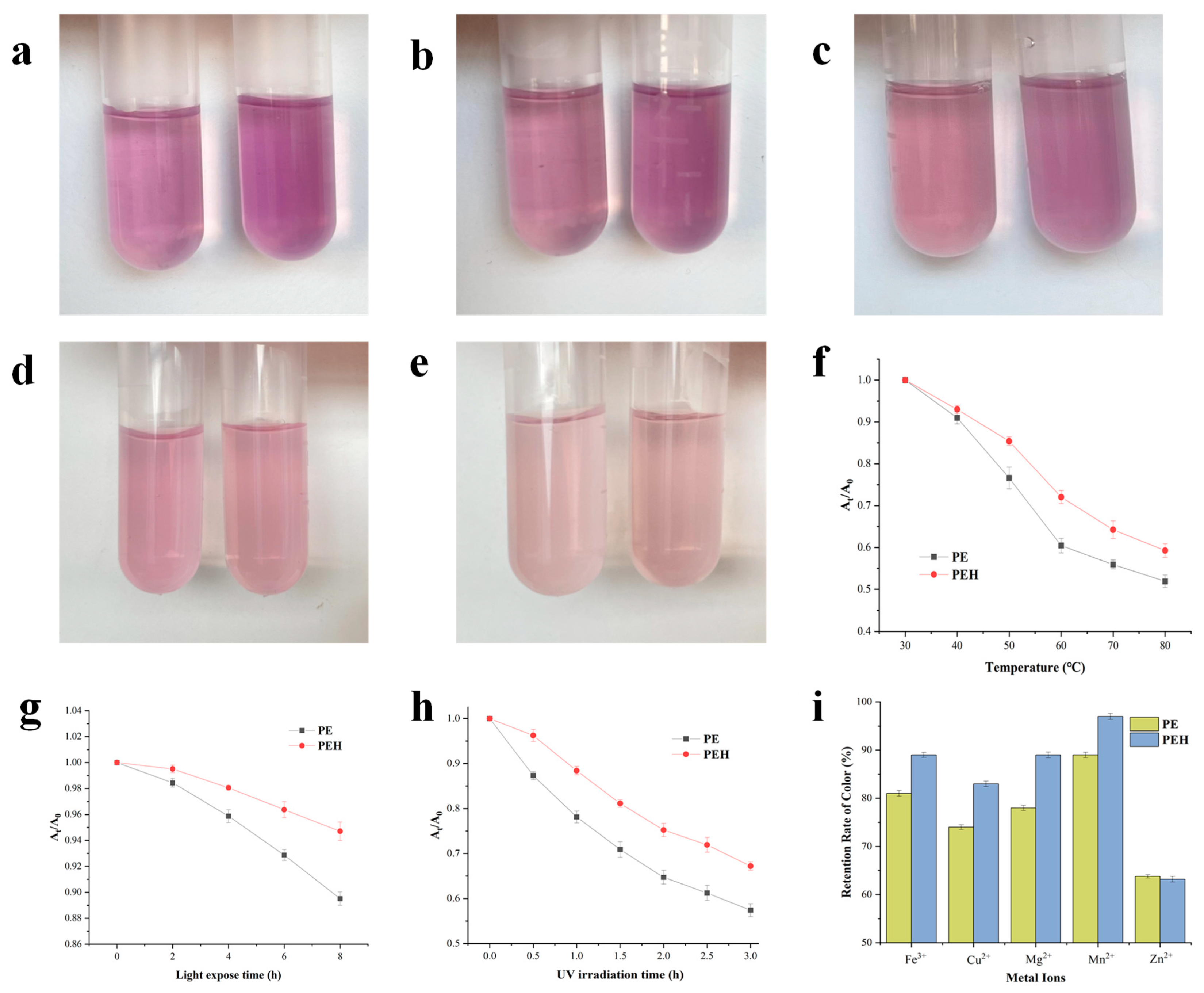
3.7. Color Stability against Environmental Conditions
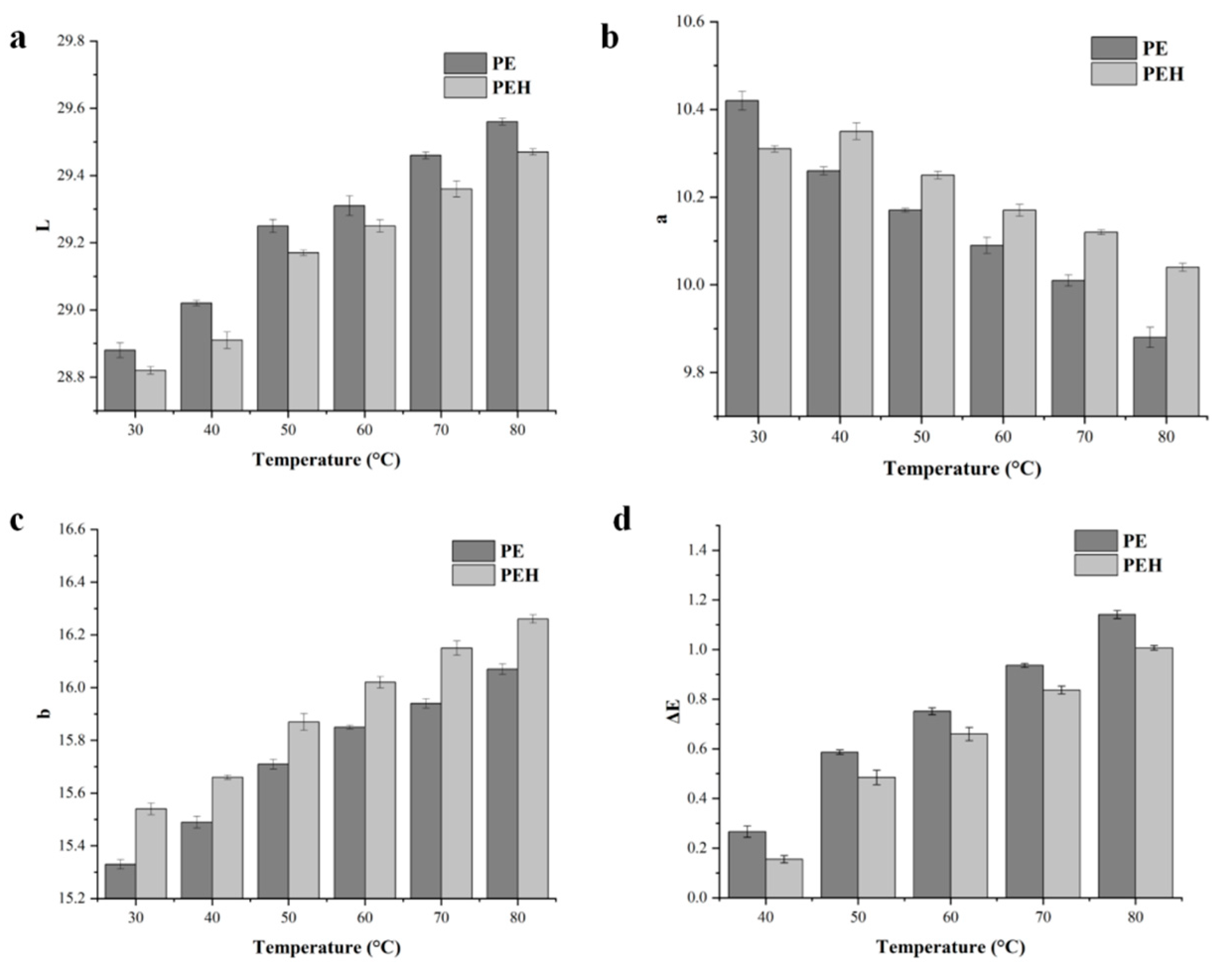
3.8. Aberration Analysis of PE and PEH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gantt, E. Phycobilisomes. Annu. Rev. Plant Physiol. 1981, 32, 327–347. [Google Scholar] [CrossRef]
- Qiang, X.; Wang, L.J.; Niu, J.F.; Gong, X.Z.; Wang, G.C. Phycobiliprotein as fluorescent probe and photosensitizer: A systematic review. Int. J. Biol. Macromol. 2021, 193, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Sintra, T.E.; Bagagem, S.S.; Ghazizadeh Ahsaie, F.; Fernandes, A.; Martins, M.; Macário, I.P.E.; Pereira, J.L.; Gonçalves, F.J.M.; Pazuki, G.; Coutinho, J.A.P.; et al. Sequential recovery of C-phycocyanin and chlorophylls from Anabaena cylindrica. Sep. Purif. Technol. 2021, 255, 117538. [Google Scholar] [CrossRef]
- Swanson, R.V.; Glazer, A.N. Separation of phycobiliprotein subunits by reverse-phase high-pressure liquid chromatography. Anal. Biochem. 1990, 188, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Fernandes e Silva, E.; Figueira, F.S.; Lettnin, A.P.; Salgado, M.T.S.F.; Lopes, A.C.; Rehbein, F.; Kalil, S.J.; Votto, A.P.S. Modulation of reactive oxygen levels and gene expression in sensitive and resistant tumoral cells by C-phyocyanin. Mol. Biol. Rep. 2018, 46, 1349–1356. [Google Scholar] [CrossRef]
- Bito, T.; Teng, F.; Watanabe, F. Bioactive Compounds of Edible Purple Laver Porphyra sp. [Nori]. J. Agric. Food Chem. 2017, 65, 10685–10692. [Google Scholar] [CrossRef]
- Simovic, A.; Combet, S.; Cirkovic Velickovic, T.; Nikolic, M.; Minic, S. Probing the stability of the food colourant R-phycoerythrin from dried Nori flakes. Food Chem. 2022, 374, 131780. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Vanjari, P.; Raghavarao, K.S.M.S. Synergistic method for extraction of high purity Allophycocyanin from dry biomass of Arthrospira platensis and utilization of spent biomass for recovery of carotenoids. Sep. Purif. Technol. 2019, 225, 97–111. [Google Scholar] [CrossRef]
- Munier, M.; Dumay, J.; Morancais, M.; Jaouen, P.; Fleurence, J. Variation in the biochemical composition of the edible seaweed grateloupia turuturu Yamada harvested from two sampling sites on the Brittany coast [France]: The influence of storage method on the extraction of the seaweed pigment R-phycoerythrin. J. Chem. 2013, 2013, 568548. [Google Scholar] [CrossRef] [Green Version]
- Benucci, I.; Liburdi, K.; Garzillo, A.M.V.; Esti, M. Bromelain from pineapple stem in alcoholic–acidic buffers for wine application. Food Chem. 2011, 124, 1349–1353. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Shi, B.; Dia, V.P. Alcalase and bromelain hydrolysis affected physicochemical and functional properties and biological activities of legume proteins. Food Struct. 2021, 27, 100178. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Borrajo, P.; Pateiro, M.; Lorenzo, J.M.; Franco, D. Antioxidant activity and peptidomic analysis of porcine liver hydrolysates using alcalase, bromelain, flavourzyme and papain enzymes. Food Res. Int. 2020, 137, 109389. [Google Scholar] [CrossRef] [PubMed]
- Punampalam, R.; Kong, S.K.; Sit, N.W. Evaluation of antioxidant properties of phycobiliproteins and phenolic compounds extracted from Bangia atropurpurea. Malays. J. Fundam. Appl. 2018, 14, 289–297. [Google Scholar] [CrossRef]
- Ardiles, P.; Cerezal-Mezquita, P.; Salinas-Fuentes, F.; Rdenes, D.; Ruiz-Domínguez, M.C. Biochemical composition and phycoerythrin extraction from red microalgae: A comparative study using green extraction technologies. Processes 2020, 8, 1628. [Google Scholar] [CrossRef]
- Omotoyinbo, O.V.; Fatoki, T.; Sanni, D.M. In Silico evaluation of bromelain from stem and fruit of pineapple (Ananas comosus). J. Agric. Sci. 2018, 4, 32–40. [Google Scholar]
- Yang, R.; Liu, M.Y.; Liu, Y.X.; Meng, D.M.; Ma, T.H.; Wang, C.T.; Liu, J.; Wang, Q.E.; Zhou, Z.K. The structure and stability analysis of the pea seed legumin glycosylated by oligochitosan. J. Sci. Food Agric. 2021, 101, 1065–1075. [Google Scholar] [CrossRef]
- Dan, M.L.; Shen, J.; Zhao, G.H.; Wang, D.M. Complete conversion of agarose into water soluble agaro-oligosaccharides by microwave assisted hydrothermal hydrolysis. Food Chem. 2022, 395, 133622. [Google Scholar] [CrossRef]
- Zheng, J.X.; Yin, H.; Shen, C.C.; Zhang, L.; Lu, J. Functional and structural properties of Spirulina phycocyanin modified by ultra-high-pressure composite glycation. Food Chem. 2019, 306, 125615. [Google Scholar] [CrossRef]
- Tabassum, R.; Ashfaq, M.; Tahir, T.; Oku, H. DPPH and Nitric Oxide Free Radical Scavenging Potential of Phenyl Quinoline Derivatives and Their Transition Metal Complexes. J. Mol. Struct. 2022, 134058. [Google Scholar] [CrossRef]
- Dang, D.S.; Buhler, J.F.; Stafford, C.D.; Taylor, M.J.; Shippen, J.E.; Dai, X.; England, E.M.; Matarneh, S.K. Nix Pro 2 and Color Muse as potential colorimeters for evaluating color in foods. LWT-Food Sci. Technol. 2021, 147, 111648. [Google Scholar] [CrossRef]
- Cotabarren, J.; Rosso, A.M.; Tellechea, M.; García-Pardo, J.; Rivera, J.L.; Obregón, W.D.; Parisi, M.G. Adding value to the chia [Salvia hispanica L.] expeller: Production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with Papain. Food Chem. 2019, 274, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Siddiqi, R.A.; Sogi, D.S. Statistical optimization of enzymatic hydrolysis of rice bran protein concentrate for enhanced hydrolysate production by papain. LWT-Food Sci. Technol. 2019, 99, 77–83. [Google Scholar] [CrossRef]
- Amini, A.; Masoumi-Moghaddam, S.; Morris, D.L. Utility of Bromelain and N-Acetylcysteine in Treatment of Peritoneal Dissemination of Gastrointestinal Mucin-Producing Malignancies. Anticancer Res. 2016, 32, 3224. [Google Scholar]
- Zhang, H.; Jia, H.X.; Xiong, P.P.; Yao, G.Y.; He, M.X. Transcriptome and enzyme activity analyses of tolerance mechanisms in pearl oyster [Pinctada fucata] under high-temperature stress. Aquaculture 2022, 550, 737888. [Google Scholar] [CrossRef]
- Nelson, G.; Herbert, O. Liquifying cod frames under acidic conditions with a fungal enzyme. J. Food Process Pres. 2007, 18, 87–101. [Google Scholar]
- Tong, X.; Prasanna, G.; Zhang, N.; Jing, P. Spectroscopic and molecular docking studies on the interaction of phycocyanobilin with peptide moieties of C-phycocyanin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 236, 118316. [Google Scholar] [CrossRef]
- Zong, Y.T.; Xiao, Q.; Malik, Z.; Su, Y.; Wang, Y.F.; Lu, S.G. Crop straw-derived biochar alleviated cadmium and copper phytotoxicity by reducing bioavailability and accumulation in a field experiment of rice-rape-corn rotation system. Chemosphere 2021, 280, 130830. [Google Scholar] [CrossRef]
- Dywili, N.R.; Ntziouni, A.; Ikpo, C.; Ndipingwi, M.; Hlongwa, N.W.; Yonkeu, A.; Masikini, M.; Kordatos, K.; Iwuoha, E.I. Graphene Oxide Decorated Nanometal-Poly [Anilino-Dodecylbenzene Sulfonic Acid] for Application in High Performance Supercapacitors. Micromachines 2019, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Guleken, Z.; Bulut, H.; Gültekin, G.İ.; Arıkan, S.; Yaylım, İ.; Hakan, M.T.; Sönmez, D.; Tarhan, N.; Depciuch, J. Assessment of structural protein expression by FTIR and biochemical assays as biomarkers of metabolites response in gastric and colon cancer. Talanta 2021, 231, 122353. [Google Scholar] [CrossRef]
- Nurlina, N.; Syahbanu, I.; Tamnasi, M.T.; Nabela, C.; Furnata, M.D. Ekstraksi dan penentuan gugus fungsi asam humat dari pupuk kotoran sapi. Indones. J. Pure Appl. Chem. 2018, 1, 30. [Google Scholar] [CrossRef]
- Sukwong, P.; Sunwoo, I.Y.; Nguyen, T.H.; Jeong, G.-T.; Kim, S.-K. R-phycoerythrin, R-phycocyanin and ABE production from Gelidium amansii by Clostridium acetobutylicum. Process Biochem. 2019, 81, 139–147. [Google Scholar] [CrossRef]
- Wu, X.; Liu, A.; Wang, W.; Ye, R. Improved mechanical properties and thermal-stability of collagen fiber based film by crosslinking with casein, keratin or SPI: Effect of crosslinking process and concentrations of proteins. Int. J. Biol. Macromol. 2017, 109, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- José, V.; Maria, B.; Agueda, M.; Isabel, A.; Ricardo, P.M. Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology. Mar. Drugs 2017, 15, 10–14. [Google Scholar]
- Ali, I.; Rafique, R.; Khan, K.M.; Chigurupati, S.; Ji, X.; Wadood, A.; Rehman, A.U.; Salar, U.; Iqbal, M.S.; Taha, M.; et al. Potent α-amylase inhibitors and radical [DPPH and ABTS] scavengers based on benzofuran-2-yl[phenyl]methanone derivatives: Syntheses, in vitro, kinetics, and in silico studies. Bioorg Chem. 2020, 104, 104238. [Google Scholar] [CrossRef] [PubMed]
- Azarian, B.; Sajedin, S.M.; Azimi, A.; Raigani, M.; Vaziri, B.; Davami, F. Proteomics Profiling of Chimeric-Truncated Tissue Plasminogen activator Producing- Chinese Hamster Ovary Cells Cultivated in a Chemically Defined Medium Supplemented with Protein Hydrolysates. Iran. Biomed. J. 2017, 21, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Yona, D.; Park, M. Seasonal variation of phycoerythrin chromophores of Synechococcus spp. in the East Sea/Japan Sea. Indones. J. Nat. Pigment. 2019, 1, 6. [Google Scholar] [CrossRef]
- Suh, J.-M.; Kim, M.; Yoo, J.; Han, J.; Paulina, C.; Lim, M.H. Intercommunication between metal ions and amyloidogenic peptides or proteins in protein misfolding disorders. Coordin. Chem. Rev. 2023, 478, 214978. [Google Scholar] [CrossRef]
- Meng, D.M.; Zhang, L.Q.; Wang, Q.E.; Zhang, Y.D.; Sun, Y.F.; Zhang, H.L.; Wang, Z.W.; Zhou, Z.K.; Yang, R. Self-Assembly of Phycoerythrin with Oligochitosan by Electrostatic Interaction for Stabilization of Phycoerythrin. J. Agric. Food Chem. 2021, 69, 12818–12827. [Google Scholar] [CrossRef]
- Dale, G.L. Rapid production of quasi-stable antibody-phycoerythrin conjugates for use in flow cytometry. Cytometry 2015, 33, 482–486. [Google Scholar] [CrossRef]

| Sample | β-Sheet | Random | α-Helix | Turn | β-Anti |
|---|---|---|---|---|---|
| PE | 44.8 ± 0.32 | 16.12 ± 0.36 | 7.18 ± 0.21 | 29.65 ± 0.57 | 2.25 ± 0.28 |
| PEH | 35.4 ± 0.45 | 7.63 ± 0.33 | 16.33 ± 0.46 | 33.27 ± 0.31 | 7.37 ± 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Cui, Y.; Wang, R.; Ma, J.; Sun, H.; Cheng, L.; Yang, R. The Hydrolysis of Pigment-Protein Phycoerythrin by Bromelain Enhances the Color Stability. Foods 2023, 12, 2574. https://doi.org/10.3390/foods12132574
Sun Y, Cui Y, Wang R, Ma J, Sun H, Cheng L, Yang R. The Hydrolysis of Pigment-Protein Phycoerythrin by Bromelain Enhances the Color Stability. Foods. 2023; 12(13):2574. https://doi.org/10.3390/foods12132574
Chicago/Turabian StyleSun, Yifei, Yuanmeng Cui, Ruhua Wang, Junrui Ma, Haili Sun, Lei Cheng, and Rui Yang. 2023. "The Hydrolysis of Pigment-Protein Phycoerythrin by Bromelain Enhances the Color Stability" Foods 12, no. 13: 2574. https://doi.org/10.3390/foods12132574
APA StyleSun, Y., Cui, Y., Wang, R., Ma, J., Sun, H., Cheng, L., & Yang, R. (2023). The Hydrolysis of Pigment-Protein Phycoerythrin by Bromelain Enhances the Color Stability. Foods, 12(13), 2574. https://doi.org/10.3390/foods12132574








